Abstract
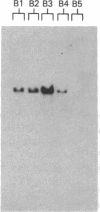
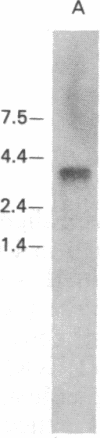
We have cloned and completely sequenced a gene encoding the heavy chain of Dictyostelium myosin I. Like the myosin I molecules from Acanthamoeba, the Dictyostelium myosin I heavy chain is composed of a globular head domain fused to a 45-kDa glycine-, proline-, and alanine-rich carboxyl-terminal domain, rather than the coiled-coil rod domain of conventional myosins. Comparisons of the Dictyostelium myosin I heavy-chain amino acid sequence with those of the Acanthamoeba myosins I reveal that they are highly similar throughout, including the unconventional carboxyl-terminal domains. The Dictyostelium myosin I gene is expressed in growing cells as a 3600-nucleotide mRNA. Measurements of the steady-state level of this mRNA at different times during starvation-induced aggregation and development are consistent with a role for myosin I in chemotaxis and aggregation. Generation of Dictyostelium cells lacking myosin I by gene disruption and/or antisense RNA production should provide a way to test directly the role of this nonfilamentous myosin in cell motility. These experiments will be simplified by the fact that Southern blot analyses of Dictyostelium genomic DNA are consistent with there being a single myosin I heavy-chain gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Pollard T. D. Propulsion of organelles isolated from Acanthamoeba along actin filaments by myosin-I. Nature. 1986 Aug 21;322(6081):754–756. doi: 10.1038/322754a0. [DOI] [PubMed] [Google Scholar]
- Albanesi J. P., Fujisaki H., Hammer J. A., 3rd, Korn E. D., Jones R., Sheetz M. P. Monomeric Acanthamoeba myosins I support movement in vitro. J Biol Chem. 1985 Jul 25;260(15):8649–8652. [PubMed] [Google Scholar]
- Bretscher M. S., Bretcher M. S. Fibroblasts on the move. J Cell Biol. 1988 Feb;106(2):235–237. doi: 10.1083/jcb.106.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté G. P., Albanesi J. P., Ueno T., Hammer J. A., 3rd, Korn E. D. Purification from Dictyostelium discoideum of a low-molecular-weight myosin that resembles myosin I from Acanthamoeba castellanii. J Biol Chem. 1985 Apr 25;260(8):4543–4546. [PubMed] [Google Scholar]
- De Lozanne A., Spudich J. A. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987 May 29;236(4805):1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Fujisaki H., Albanesi J. P., Korn E. D. Experimental evidence for the contractile activities of Acanthamoeba myosins IA and IB. J Biol Chem. 1985 Sep 15;260(20):11183–11189. [PubMed] [Google Scholar]
- Hammer J. A., 3rd, Bowers B., Paterson B. M., Korn E. D. Complete nucleotide sequence and deduced polypeptide sequence of a nonmuscle myosin heavy chain gene from Acanthamoeba: evidence of a hinge in the rodlike tail. J Cell Biol. 1987 Aug;105(2):913–925. doi: 10.1083/jcb.105.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G., Korn E. D., Hammer J. A., 3rd The heavy chain of Acanthamoeba myosin IB is a fusion of myosin-like and non-myosin-like sequences. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6720–6724. doi: 10.1073/pnas.84.19.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Intervening sequences in a Dictyostelium gene that encodes a low abundance class mRNA. Nucleic Acids Res. 1980 Dec 11;8(23):5599–5610. doi: 10.1093/nar/8.23.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R. Methods for manipulating and investigating developmental timing in Dictyostelium discoideum. Methods Cell Biol. 1987;28:413–431. doi: 10.1016/s0091-679x(08)61660-x. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Warrick H. M., De Lozanne A., Leinwand L. A., Spudich J. A. Conserved protein domains in a myosin heavy chain gene from Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9433–9437. doi: 10.1073/pnas.83.24.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D., Soll D. R., Knecht D., Loomis W. F., De Lozanne A., Spudich J. Cell motility and chemotaxis in Dictyostelium amebae lacking myosin heavy chain. Dev Biol. 1988 Jul;128(1):164–177. doi: 10.1016/0012-1606(88)90279-5. [DOI] [PubMed] [Google Scholar]