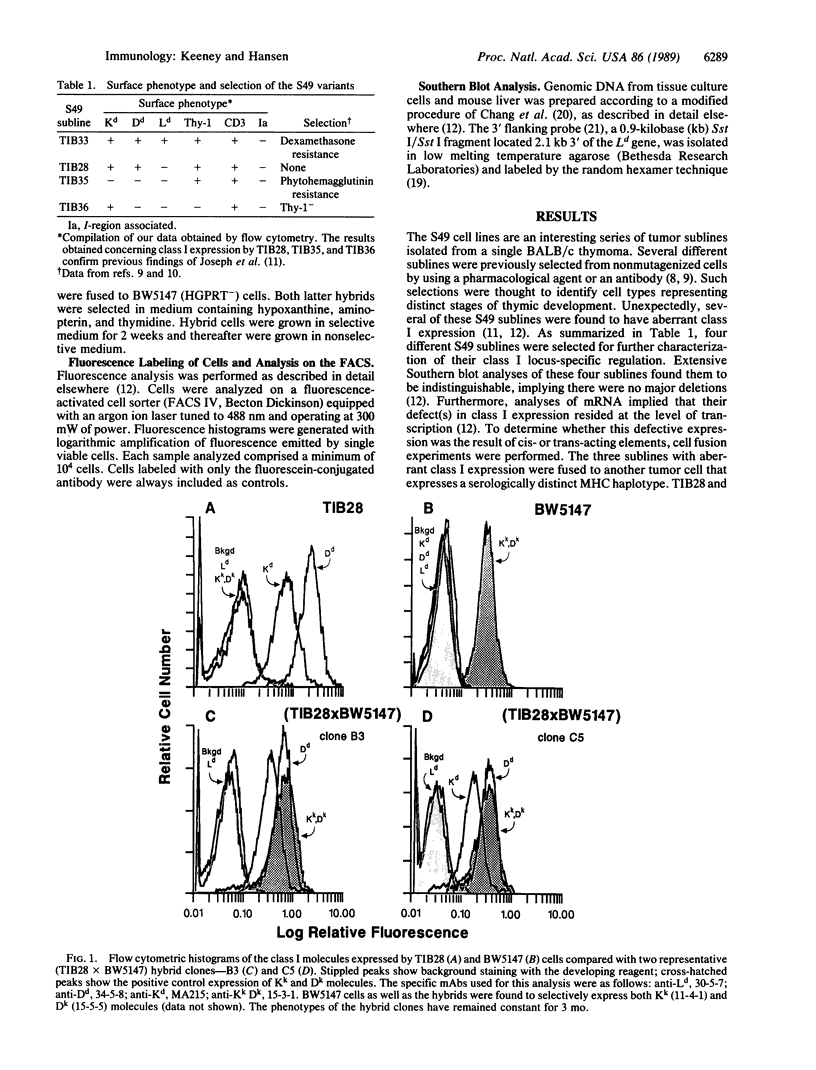
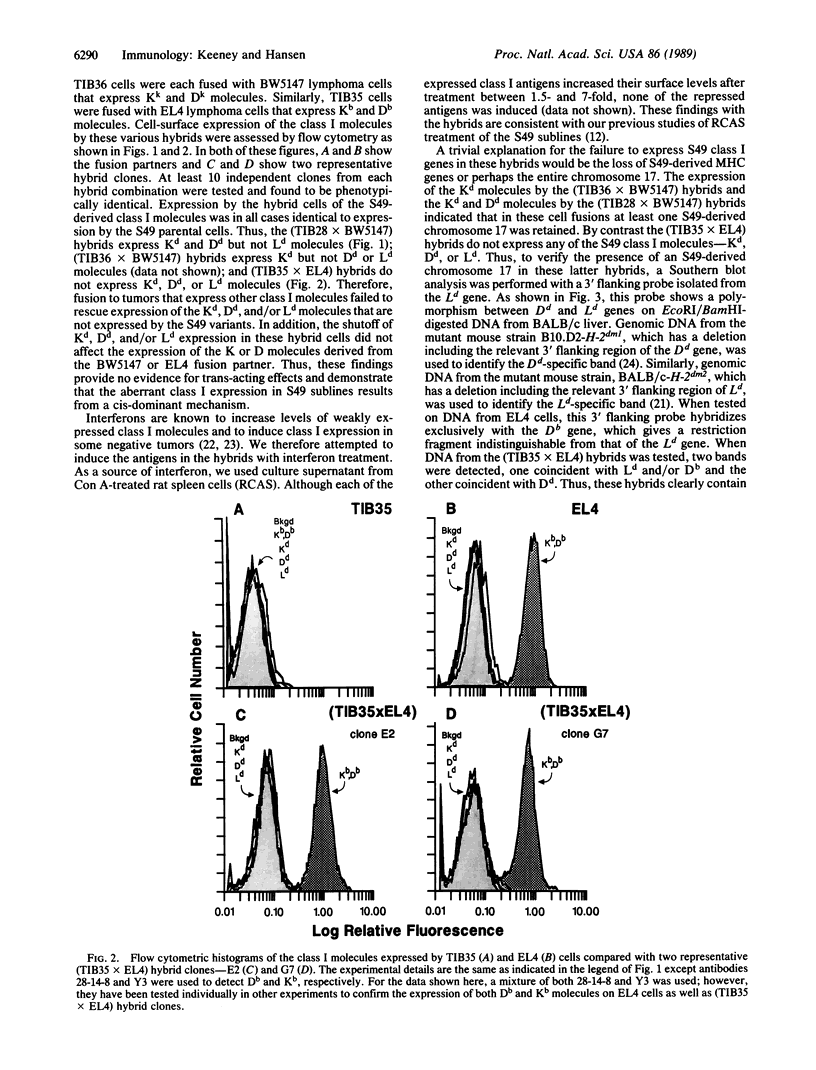
Abstract
Several tumors have been reported to down-regulate expression of their class I major histocompatibility molecules, potentially altering their immune recognition. To investigate this phenomenon, we are using various sublines isolated from the S49 lymphoma of the BALB/c mouse strain. These S49 tumor sublines were previously found to have shut off expression of their Kd, Dd, and/or Ld class I molecules in a locus-specific manner. Extensive Southern blot analyses indicated that there were no major chromosome aberrancies in these S49 sublines, and analyses of steady-state class I mRNA suggested that a form of transcriptional regulation was responsible for their variant class I expression. In this report, we characterize the nature of this locus-specific regulation of class I in S49 cells by producing somatic cell hybrids. Three phenotypically distinct S49 sublines were each fused to tumor cells with normal class I expression, and several of the resulting hybrids were analyzed. In every case, the class I molecules expressed by the hybrids were an exact composite of the two fusion partners. Thus, these fusions failed to rescue expression of the Kd, Dd, and/or Ld molecules shut off within each of the S49 tumor sublines. These findings indicate that this locus-specific shutoff of class I expression results from a cis-acting defect and not trans-acting factors. Because the analysis of each of three phenotypically different S49 cells implicated a form of cis-dominant regulation, we hypothesize that a common mechanism generating homologous mutations in class I genes is operative in S49 tumor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W., Goodfellow P. N. Antigen expression by somatic cell hybrids of a murine embryonal carcinoma cell with thymocytes and L cells. Somatic Cell Genet. 1980 Mar;6(2):271–284. doi: 10.1007/BF01538801. [DOI] [PubMed] [Google Scholar]
- Bahler D. W., Frelinger J. G., Harwell L. W., Lord E. M. Reduced tumorigenicity of a spontaneous mouse lung carcinoma following H-2 gene transfection. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4562–4566. doi: 10.1073/pnas.84.13.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldacci P., Transy C., Cochet M., Penit C., Israel A., Kourilsky P. A trans-acting mechanism represses the expression of the major transplantation antigens in mouse hybrid thymoma cell lines. J Exp Med. 1986 Sep 1;164(3):677–694. doi: 10.1084/jem.164.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham F. J., Quintero M. A., Goodfellow P. N. Human-mouse hybrids with an embryonal carcinoma phenotype continue to transcribe HLA-A,B,C. EMBO J. 1983;2(11):1963–1968. doi: 10.1002/j.1460-2075.1983.tb01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Chang H., Dmitrovsky E., Hieter P. A., Mitchell K., Leder P., Turoczi L., Kirsch I. R., Hollis G. F. Identification of three new Ig lambda-like genes in man. J Exp Med. 1986 Feb 1;163(2):425–435. doi: 10.1084/jem.163.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmür R., Solter D., Knowles B. B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980 Jun 1;151(6):1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby R. A., Osborne B. A., Simpson E., Herzenberg L. A. Hybrid cell lines with T-cell characteristics. Nature. 1977 Jun 23;267(5613):707–708. doi: 10.1038/267707a0. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Characterization of a progressive tumor from C3H fibroblasts transformed in vitro with SV40 virus. Immunoresistance in vivo correlates with phenotypic loss of H-2Kk. J Immunol. 1982 Sep;129(3):1306–1312. [PubMed] [Google Scholar]
- Green W. R., Phillips J. D. Differential induction of H-2K vs H-2D class I major histocompatibility complex antigen expression by murine recombinant interferon-gamma. J Immunol. 1986 Aug 1;137(3):814–818. [PubMed] [Google Scholar]
- Green W. R., Rich R. F., Beadling C. Differential induction of H-2K versus H-2D class I major histocompatibility antigens by recombinant gamma interferon. Lack of Kk augmentation in a leukemia virus-induced tumor is due to a cis-dominant effect. J Exp Med. 1988 May 1;167(5):1616–1624. doi: 10.1084/jem.167.5.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkrug K. J., Cory J. M., Stimpfling J. H. Monoclonal antibodies defining mouse tissue antigens encoded by the H-2 region. Immunogenetics. 1987;25(2):136–139. doi: 10.1007/BF00364282. [DOI] [PubMed] [Google Scholar]
- Hauptfeld V., Nahm M., Hauptfeld M., Shreffler D. C. Monoclonal antibodies to mouse MHC antigens. I. Serologic characterization of ten anti-H-2 and anti-Ia reagents. Immunogenetics. 1984;19(2):169–173. doi: 10.1007/BF00387861. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Jones B., Janeway C. A., Jr Cooperative interaction of B lymphocytes with antigen-specific helper T lymphocytes is MHC restricted. Nature. 1981 Aug 6;292(5823):547–549. doi: 10.1038/292547a0. [DOI] [PubMed] [Google Scholar]
- Joseph L. J., Levy E., Ozato K., Hochman J., Shearer G. M. Differential lack of class I H-2d antigen expression by sublines of the BALB/c S49 T cell lymphoma. J Immunol. 1986 Dec 15;137(12):4016–4020. [PubMed] [Google Scholar]
- Klyczek K. K., Murasko D. M., Blank K. J. Interferon-gamma, interferon-alpha/beta, and tumor necrosis factor differentially affect major histocompatibility complex class I expression in murine leukemia virus-induced tumor cell lines. J Immunol. 1987 Oct 15;139(8):2641–2648. [PubMed] [Google Scholar]
- Ozato K., Hansen T. H., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. II. Antibodies to the H-2Ld antigen, the products of a third polymorphic locus of the mouse major histocompatibility complex. J Immunol. 1980 Dec;125(6):2473–2477. [PubMed] [Google Scholar]
- Ozato K., Mayer N. M., Sachs D. H. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982 Sep;34(3):113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Ralph P., Hyman R., Epstein R., Nakoinz I., Cohn M. Independence of theta and TL surface antigens and killing by thymidine, cortisol, phytohemagglutinin, and cyclic AMP in a murine lymphoma. Biochem Biophys Res Commun. 1973 Dec 19;55(4):1085–1091. doi: 10.1016/s0006-291x(73)80006-3. [DOI] [PubMed] [Google Scholar]
- Rubocki R. J., Hansen T. H., Lee D. R. Molecular studies of murine mutant BALB/c-H-2dm2 define a deletion of several class I genes including the entire H-2Ld gene. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9606–9610. doi: 10.1073/pnas.83.24.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Howell D. N., Cresswell P. Genes regulating HLA class I antigen expression in T-B lymphoblast hybrids. Immunogenetics. 1985;21(3):235–246. doi: 10.1007/BF00375376. [DOI] [PubMed] [Google Scholar]
- Sun Y. H., Goodenow R. S., Hood L. Molecular basis of the dm1 mutation in the major histocompatibility complex of the mouse: a D/L hybrid gene. J Exp Med. 1985 Nov 1;162(5):1588–1602. doi: 10.1084/jem.162.5.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]