Summary
Nitric oxide (NO) is implicated in a wide range of biological processes, including innate immunity against pathogens, signal transduction, and protection against oxidative stress. However, its possible roles in beneficial host-microbe associations are less well recognized. During the early stages of the squid-vibrio symbiosis, the bacterial symbiont Vibrio fischeri encounters host-derived NO, which has been hypothesized to serve as specificity determinant. We demonstrate here that the flavohemoglobin, Hmp, of V. fischeri protects against NO, both in culture and during colonization of the squid host. Transcriptional analyses indicate that hmp expression is highly responsive to NO, principally through the repressor, NsrR. Hmp protects V. fischeri from NO inhibition of aerobic respiration, and removes NO under both oxic and anoxic conditions. A Δhmp mutant of V. fischeri initiates squid colonization less effectively than wild type, but is rescued by the presence of an NO synthase inhibitor. The hmp promoter is activated during the initial stage of colonization, during which the Δhmp strain fails to form normal-sized aggregates of colonizing cells. Taken together, these results suggest that the sensing of host-derived NO by NsrR, and the subsequent removal of NO by Hmp, influence aggregate size and, thereby, V. fischeri colonization efficiency.
Keywords: nitric oxide, Hmp, NsrR, colonization
Introduction
Nitric oxide (NO) is a small, freely diffusible molecule that is implicated in a wide range of biological processes including innate immunity, signal transduction, and protection against oxidative stress (Fang, 2004) Due to its reactivity towards diverse cellular constituents such as [Fe-S] clusters and heme, NO is used by many eukaryotes as an antimicrobial agent. In addition, when combined with reactive oxygen species (ROS), which are produced by NADPH oxidase during the “oxidative burst”, NO can also form other radicals of even greater cytotoxicity (Fang, 2004). Therefore, NO and ROS are regarded as early and effective host immune responses to invasive microorganisms. In contrast, at low (nM) concentrations, NO is an important signaling molecule that triggers specific physiological responses in mammalian cells, mainly by interacting with the heme-NO/Oxygen-binding (HNOX) domain of the soluble guanylate cyclase (sGC) (Cary et al., 2006). Similarly, in prokaryotes, low concentrations of NO are sensed by certain transcriptional regulators (for example, NsrR and NorR), resulting in the initiation of specific cellular responses (Spiro, 2007). Moreover, the wide distribution of homologues of the eukaryotic HNOX domain among prokaryotic lineages (Iyer et al., 2003) suggests that NO sensing via HNOX proteins may be important in bacteria as well, including V. fischeri (Wang et al., 2010).
Bacteria have evolved specific mechanisms to detoxify NO, whether it is endogenously generated during nitrite reduction, or exogenously imposed by host defenses. In Escherichia coli, flavohemoglobin (Hmp) detoxifies NO both aerobically as an NO dioxygenase or O2 nitroxylase and, to a lesser extent, anaerobically as a NO reductase (Poole & Hughes, 2000). Hmp plays a critical role during pathogenesis, for example, in intraphagosomal survival and virulence of Salmonella typhimurium in mice (Stevanin et al., 2002; Bang et al., 2006), in protecting the respiration of Erwinia chrysanthemi from NO inhibition (Boccara et al., 2005), and in resistance of Staphylococcus aureus to host innate immunity (Richardson et al., 2006).
While hmp expression is generally up-regulated by NO and nitrosating agents such as S-nitrosoglutathione, the underlying mechanism can vary. For instance, E. coli NsrR, an [Fe-S]-containing regulatory protein, represses hmp transcription in the absence of NO (Spiro, 2007), while hmp expression in Vibrio cholerae is predicted to be regulated by NorR, a sigma 54-dependent activator (Rodionov et al., 2005). NO induces hmp expression either by attacking the [Fe-S] cluster in NsrR (Tucker et al., 2010), or nitrosylating the non-heme iron in NorR, which relieves intramolecular repression and leads to activation of gene expression (Arai et al., 2005; D’Autreaux et al., 2005). NorR can also activate the expression of genes encoding another important NO-detoxifying activity, the flavorubredoxin NorV (FlRd) and its redox partner NorW in E. coli (Hutchings et al., 2002; Gardner et al., 2002). Interestingly, cytochrome c nitrite reductase (NrfA), also works in combination with NorVW to protect S. typhimurium against NO killing in anoxic environments (Mills et al., 2008). Thus, synthesis of one or more of these different NO detoxification systems enables a bacterium to survive high concentrations of NO over a range of oxygen concentrations.
Recent studies have revealed the existence of functional commonalities in the mechanisms underlying pathogenic and beneficial host-microbe associations (Hooper et al., 2001; Rawls et al., 2004; Koropatnick et al., 2004). However, in contrast to the well-described role of NO in defending against pathogens, its function(s) in beneficial interactions are poorly understood. NO is known to be present in nodules of the model legume Medicago truncatula during its interactions with symbiont Sinorhizobium meliloti (Mathieu et al., 1998; Baudouin et al., 2006); however, only recently has an hmp mutant been shown to display a reduced nitrogen-fixation efficiency in planta (Meilhoc et al., 2010). The role(s) of NO in the symbiosis between the marine luminous bacterium Vibrio fischeri and the Hawaiian bobtail squid, Euprymna scolopes has been better described (Davidson et al., 2004; Wang et al., 2010). In this symbiosis, V. fischeri resides along the apical surfaces of epithelia lining the crypts of the host’s light-emitting organ, populating it as a monospecific culture. The symbiont’s bioluminescence is exploited by the squid in an anti-predatory behavior called counterillumination; in return, the squid provides nutrients to the symbiont (Graf & Ruby, 1998; Wier et al., 2010). Because the nascent light organ of a newly hatched squid is free of symbionts, each generation of juveniles must become inoculated by V. fischeri present in the ambient seawater. During initiation of the symbiosis, V. fischeri cells gather as an aggregate in mucus shed by the epithelial surface of the organ. After 2 to 4 hours, the symbionts disaggregate and migrate toward and into pores on the organ’s surface, moving through ducts to the deep crypts, where the symbiont population is established within 12 hours (Nyholm & McFall-Ngai, 2004). At the aggregation stage, the bacteria are first exposed to a relatively low level of host-derived NO produced by vesicles embedded in the secreted mucus, while later they again encounter NO at higher levels as they traverse the light-organ ducts (Davidson et al., 2004), where ROS are also produced (Small & McFall-Ngai, 1999). Given its antimicrobial and signaling properties, this host-derived NO has been proposed to act as both a specificity determinant and a symbiotic signal (Nyholm & McFall-Ngai, 2004; Wang et al., 2010). Interestingly, while the location and relative intensity of NO exposure have been well described only during the first few hours to days of the symbiosis, the presence of NO synthase (NOS), and likely, NO in the symbiont-containing tissues continue throughout the life of the host (Davidson et al., 2004).
Analysis of the V. fischeri genome sequence (Ruby et al., 2005) has revealed the presence of genes encoding homologues of Hmp (VF_2316) and NorVW (VF_1782-1781), as well as the regulators NsrR (VF_2315) and NorR (VF_1783), and a bacterial homologue of HNOX (VF_A0071). Thus, we hypothesize that V. fischeri both senses and detoxifies host-derived NO encountered in the early stages of the symbiosis. Previously, we have established that the V. fischeri HNOX protein senses NO, differentially regulates the expression of the Fur regulon, and modulates colonization proficiency (Wang et al., 2010). Here, we report Hmp is required for NO resistance both in pure culture and during animal colonization: specifically, (i) NO induces expression of V. fischeri hmp, mainly through NsrR; (ii) Hmp removes NO under both oxic and anoxic conditions; and, (iii) hmp expression is activated at the aggregation stage of colonization, and the capacity to remove NO by Hmp controls the size and symbiotic efficiency of these bacterial aggregates.
Results
V. fischeri hmp expression responds to NO through its negative regulator NsrR
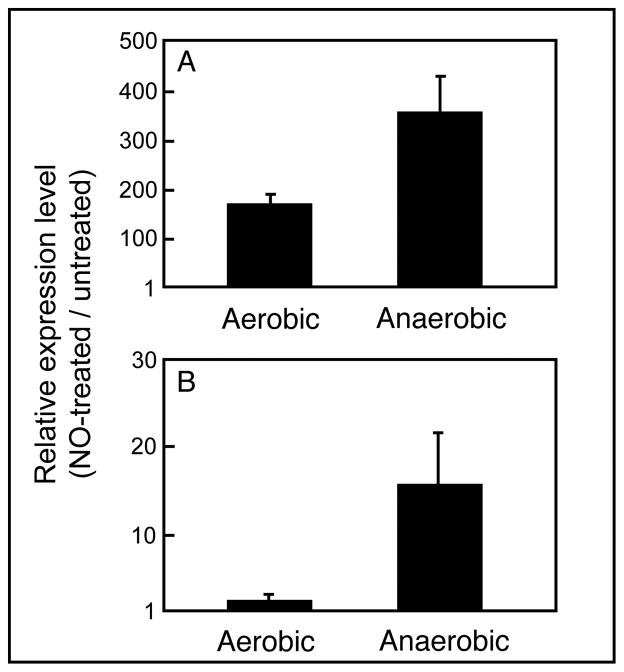
A previous whole-genome transcriptomic study showed that the V. fischeri hmp gene was up-regulated >120 fold in response to NO (Wang et al., 2010). In the present study, when wild type V. fischeri was exposed aerobically to 80 μM of the NO-generator DEA-NONOate, hmp was induced >170 fold (Fig. 1A) as measured by quantitative real-time PCR (qRT-PCR), confirming the previous study’s microarray result. An even stronger response (>300 fold) was observed under anoxic conditions, perhaps because the NO being generated was not scavenged by oxygen (Gilberthorpe et al., 2007). In contrast, only in the absence of oxygen did NO significantly induce the expression of norV (Fig. 1B).
Fig. 1.
Expression of V. fischeri hmp responds to NO under both oxic and anoxic conditions, while norV responds only when oxygen is absent. Wild-type cells were grown in minimal-salts medium plus GlcNAc, either in the presence or absence of O2. The relative expression levels (NO-treated/untreated) of either hmp (A) or norV (B) transcripts were determined by qRT-PCR. Data points are the means (±1 SEM) calculated from three biological replicate experiments.
A bioinformatic study of the V. fischeri genome sequence predicted the presence of a binding site for NsrR upstream of hmp (Rodionov et al., 2005). Thus, we hypothesized that the V. fischeri NsrR is an NO-responsive regulator of hmp expression. To test this hypothesis, we constructed a deletion mutant of nsrR (ΔnsrR) by allelic exchange, and used qRT-PCR to measure the relative expression levels of hmp under different conditions. Under normal growth conditions, hmp was constitutively expressed at a higher level (230 to 250 fold) in the ΔnsrR mutant, irrespective of the presence or absence of oxygen (Table 1). This observation strongly supports the role of NsrR as a negative regulator when NO is absent. When nsrR was deleted, hmp expression was constitutively high, and no longer responded to NO; that is, the hmp-expression levels in NO-treated and untreated ΔnsrR cells were within a factor of 2 of each other (Table 1). Thus, NsrR appears to be the major NO-responsive regulator for hmp transcription under both aerobic and anaerobic growth conditions.
Table 1.
NsrR is the major NO-responsive negative regulator of hmp.
| Comparison | Relativehmpexpression* | |
|---|---|---|
| Aerobic | Anaerobic | |
| ΔnsrR/WT† | 231 ± 33 | 250 ± 44 |
| ΔnsrR+NO/WT+NO‡ | 1.0 ± 0.2 | 1.6 ± 0.2 |
| ΔnsrR+NO/ΔnsrR | 0.9 ± 0.1 | 2.3 ± 0.3 |
The ratio of expression levels of hmp transcripts in each pair of strains being compared as measured by qRT-PCR.
WT; wild type
Cultures were exposed to NO by adding either 80 μM (aerobic conditions) or 5 μM (anaerobic conditions) DEA-NONOate.
Hmp ameliorates the toxic effects of NO under both oxic and anoxic culture conditions
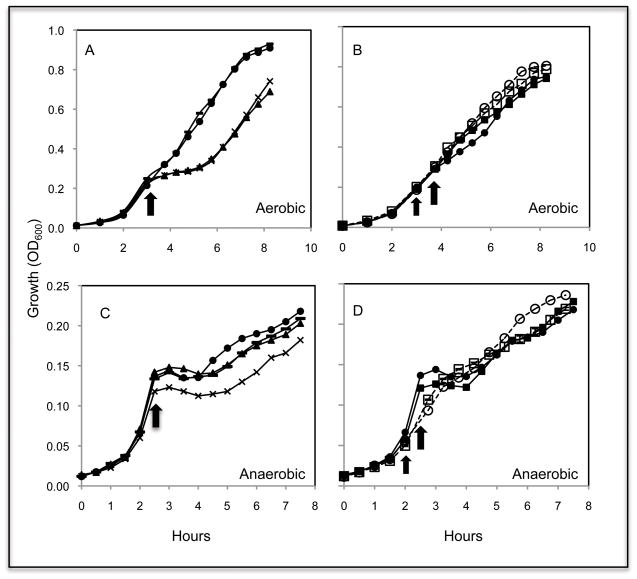
Because hmp expression is highly responsive to NO, both in the presence and absence of oxygen, while norV was significantly induced only under anoxic conditions (Fig. 1), we asked what the physiological roles of the two systems were during NO stress. Four deletion mutants (ΔnsrR, Δhmp, ΔnorV and Δhmp-norV) had the same aerobic growth rate as wild type (data not shown). In contrast, the addition of an NO-generator (100 μM DEA-NONOate) during early-exponential growth (OD600 0.2 to 0.4) resulted in an immediate growth arrest only in the Δhmp and Δhmp-norV strains (Fig. 2A). After 2 hrs, both these strains had recovered and begun growing like wild type, suggesting that, at this concentration, the effect of NO is simply bacteriostatic. A determination of the total colony forming units (CFUs) of cultures during the NO exposure supported this conclusion (Fig. S1). Consistent with the previous observation that NorVW contributes only to anaerobic NO detoxification in E. coli and S. typhimurium (Gardner et al., 2002; Mills et al., 2008), an imposed aerobic NO challenge did not halt the growth of the V. fischeri ΔnorV mutant (Fig. 2A).
Fig. 2.
Hmp and NorV confer protection from NO inhibition on growth, depending on the oxygen availability. Growth in minimal-salts medium plus GlcNAc, was monitored at OD600. Aerobic cultures (A and B) were challenged with 100 μM of the NO-donor DEA-NONOate (thick arrow), when growth reached early log phase. To pre-adapt the cells (B), a pretreatment of 40 μM DEA-NONOate was added (thin arrow) 45 min before the challenge. Similarly, anaerobic cultures (C and D) were challenged with 40 μM DEA-NONOate (thick arrow). To pre-adapt the cells (D), 5 μM DEA-NONOate was added (thin arrow) 45 min before the challenge. Growth, either without (filled symbols) or with (open symbols) pretreatment, was monitored for the wild-type (circle), Δhmp (triangle), ΔnorV (dash line), Δhmp-norV (cross) and ΔnsrR (square) strains. Experiments were repeated three times with similar results. One representative experiment is shown here. Data points are the means calculated from the three technical replicates of that experiment.
Based on the negative regulation of hmp by NsrR (Table 1), and its likely mechanism of NO-sensing mechanism (Tucker et al., 2010), we made two predictions: (i) pretreatment of wild-type cells with a low dose (e.g., 40 μM) of NO will relieve hmp repression, thereby resulting in a greater resistance to subsequent NO challenge; and, (ii) because hmp expression is constitutive in the ΔnsrR mutant (Table 1), this strain will be ‘blind” to the pretreatment, but still able to cope with the NO stress. As predicted, aerobically grown wild-type cells pretreated with NO were resistant to NO challenge while, without the pretreatment, they exhibited a short (approximately 1 hr) delay before recovering (Fig. 2B). In contrast, the ΔnsrR mutant was resistant to NO challenge, even in the absence of pretreatment (Fig. 2B). These data indicate that hmp expression levels correlate directly with the capacity for aerobic NO resistance.
No difference in anaerobic growth was observed with any of the deletion mutants in the absence of NO challenge (Fig. S2). However, in contrast to the growth profile obtained under oxic conditions (Fig. 2A), both the Δhmp and ΔnorV strains were arrested for 2 hrs after the challenge, and growth of the double mutant (Δhmp-norV) was even more severely inhibited by NO addition (Fig. 2C). The delayed recovery of the three mutants suggests that, as with E. coli and S. typhimurium (Mills et al., 2008), in V. fischeri both Hmp and NorV contribute to removing NO under anoxic conditions.
The protective effect of NO pretreatment was also evident under anoxic growth conditions. In contrast to the 1.5-hr lag observed in wild-type cells without pretreatment, a pre-exposure to 5 μM DEA-NONOate, while initially leading to a partial growth retardation, did rescue cells from the strong growth inhibition caused by a subsequent, stronger NO challenge (Fig. 2D). This rescue probably results from the increased expression of hmp and norV (Fig. 1). Interestingly, in contrast to the full resistance seen under oxic conditions (Fig. 2B), the ΔnsrR mutant remained sensitive to an anaerobic challenge by NO (Fig. 2D). This observation suggests that Hmp itself, even when constitutively expressed, is not sufficient for removal of NO in the absence of oxygen. Thus, while Hmp may be the major NO scavenging mechanism in the presence of oxygen, Hmp and NorV apparently work in combination under anoxic conditions.
Hmp protects aerobic respiration from NO inhibition
Owing to its reactivity with the heme groups of proteins, NO transiently and reversibly inhibits the activity of bacterial oxidases by competing with oxygen for binding (Stevanin et al., 2000; Borisov et al., 2004). To ask whether V. fischeri Hmp protects aerobic respiration from this inhibition, we determined the NO concentration that gave rise to half-maximal inhibition of oxygen consumption by cell suspensions (Fig. S3). For wild-type V. fischeri, this concentration was 2.4 ± 0.08 μM, while the Δhmp strain (0.58 ± 0.02 μM) was 4-fold more sensitive. This increased sensitivity, which is close to that reported for an E. coli hmp mutant (Stevanin et al., 2000), supports the conclusion that Hmp protects V. fischeri from NO-dependent inhibition of respiration.
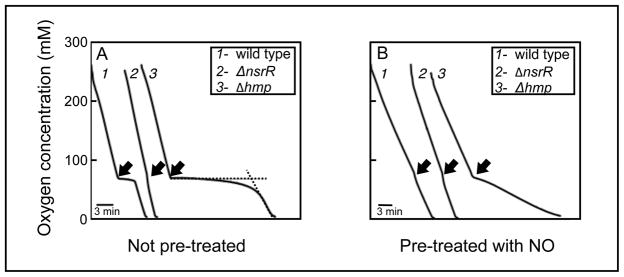
We next compared the inhibitory effect of NO on respiration in wild-type, Δhmp, ΔnorV, Δhmp-norV and ΔnsrR strains. Before exposure to NO, the wild-type, Δhmp and ΔnsrR strains consumed oxygen at a similar rate (Fig. 3A); however, the addition of NO immediately inhibited oxygen uptake by wild-type and Δhmp strains (traces 1 and 3, Fig. 3A). In contrast, respiration by the ΔnsrR strain, in which hmp expression is constitutive (Table 1), was resistant to NO exposure (trace 2, Fig. 3A). In addition, the period of inhibition for both the Δhmp and Δhmp-norV strains was 5-fold longer than that of the wild-type and ΔnorV strains (Table 2), further implicating Hmp as the major contributor to NO removal under these conditions. Of note, when either wild-type or Δhmp cells carried pYLW29, which encodes a wild-type copy of hmp, they became hyper-resistant to NO inhibition (Table 2), possibly due either to the high (10–15) copy number of the plasmid or to NsrR titration by the native hmp promoter in the plasmid construct (Dunn, et al., 2010). As expected, because hmp expression is highly responsive to NO (Fig. 1), pretreatment of either wild-type or ΔnorV strains with NO resulted in loss of sensitivity of respiration to NO (trace 1, Fig. 3B; Table 2). Surprisingly, pretreatment with NO allowed both the Δhmp and Δhmp-norV strains to continue to consume oxygen, albeit at a reduced rate (trace 3, Fig. 3B; Table 2). This intriguing result is consistent with NO-mediated induction (via NsrR) of the NO-resistant alternative oxidase (Dunn et al., 2010).
Fig. 3.
V. fischeri Hmp protects aerobic respiration from NO inhibition. Measurements of oxygen consumption by wild type (trace 1), ΔnsrR (trace 2) and Δhmp (trace 3) were made before and after addition (arrow) of the NO-generator Proli-NONOate (arrow). The measurements were performed on cells either without (A) or with (B) pretreatment with 80 μM DEA-NONOate. The dashed lines in trace 3 of panel A define how the period of inhibition was derived. The experiment was repeated three times with similar results. One representative experiment is shown here.
Table 2.
Extent of inhibition of oxygen consumption by NO challenge.
| Strain | Period of inhibition (min)* | |
|---|---|---|
| Without pretreatment | With NO pretreatment | |
| WT | 3.1 ± 0.5 | < 0.5 |
| Δhmp | 14.9 ± 0.5 | partial inhibition† |
| ΔnorV | 3.1 ± 0.1 | < 0.5 |
| Δhmp-norV | 15.3 ± 0.1 | partial inhibition |
| ΔnsrR | < 0.5 | < 0.5 |
| WT+pVSV105 | 3.8 ± 0.1 | ND‡ |
| WT+pYLW29 | < 0.5 | ND |
| Δhmp+pVSV105 | 16.3 ± 1.3 | ND |
| Δhmp+pYLW29 | < 0.5 | ND |
Mean (± SEM) extent of inhibition by NO challenge (from three repeated experiments); Wild type (WT) and mutant strain were each tested either after receiving an NO pretreatment or after no pretreatment
Partial inhibition means that oxygen consumption was not inhibited, but continued at a reduced linear rate.
ND, not determined.
Hmp confers colonization efficiency in the squid-vibrio symbiosis
We hypothesized that Hmp and/or NorV serve to detoxify host-derived NO during the establishment of symbiosis by V. fischeri. Using inoculation conditions under which approximately 50% of juvenile squids were colonized by wild-type, only 40%, 22% and 17% of the animals were colonized by the ΔnorV, Δhmp and Δhmp-norV strains, respectively (Table 3), suggesting a lower colonization efficiency. In addition, the Δhmp-norV and Δhmp strains consistently took longer to initiate symbiotic bioluminescence and colonization compared to either the wild-type or ΔnorV strains (Table 3). Thus, while both Hmp and NorV may function in colonization, the contribution of Hmp appears more important. We further compared the infectivity of wild-type and Δhmp strains by determining the inoculum dose that resulted in colonization of 50% of the animals (ID50). In this assay, about 670 CFU/ml of wild type were required to colonize half of a cohort of squids, while the ID50 of Δhmp was ~ 2-fold higher (Fig. S4). Interestingly, the ΔnsrR mutant did not colonize more effectively than wild type in the assay (Table 3), suggesting that a constitutive expression of hmp early in colonization does not confer a significant colonization advantage.
Table 3.
Colonization characteristics of mutants defective in NO detoxification
| Strain | Single-strain colonization* | Competitive colonization as RCI (WT/mutant) | ||
|---|---|---|---|---|
| Percentage of squids becoming luminous | Hours until colonization | |||
| luminous | detected | No treatment | + NOS inhibitor‡ | |
| WT | 50 ± 15 | 9.3 ± 0.1 | --- | --- |
| ΔnorV | 40 ± 5 | 9.7 ± 0.1 | 1.1 ± 0.2 | ND¶ |
| Δhmp | 22 ± 5 | 10.2 ± 0.2 | 2.6 ± 0.5 | 0.9 ± 0.1 |
| Δhmp-norV | 17 ± 3 | 12.0 ± 1.2 | 3.4 ± 0.3 | 1.0 ± 0.1 |
| ΔnsrR | 60 ± 20 | 9.5 ± 0.2 | 1.1 ± 0.1 | ND |
More effective colonization is indicated by a higher percentage of squid becoming luminous at 24 hrs, and by a more rapid onset of colonization as indicated by onset of detectable squid bioluminescence; values that are significantly different from wild type (WT) are in bold font; each experiment was repeated 2–3 times with similar results
A relative colonization index (RCI) >1.0 indicates that the WT outcompetes the mutant after 24 hrs; RCI values that are significantly different from 1.0 are in bold font; each experiment was repeated at least 3 times with similar results. Mean RCI (±1 SEM) from the three experiments is shown.
Addition of the NOS inhibitor S-methyl-L-thiocitrulline (SMTC)
ND, not determined
The relative symbiotic proficiency of each of the mutants was also compared to wild type using a competitive-colonization assay. When juvenile squids were exposed to a two-strain inoculum, wild type out-competed the Δhmp or Δhmp-norV strains by 3–4 fold (Table 3). In the natural environment, where juvenile squids are likely to be colonized by only one or a few V.fischeri cells (Wollenberg & Ruby, 2009; Lee & Ruby, 1994), a 3- to 4-fold advantage would result in a significant increase in fitness over time. Just as the ΔnsrR strain did not show higher efficiency in the single-strain colonization, it also did not out-compete wild type (Table 3, Fig. S5). If the diminished competitiveness of the Δhmp or Δhmp-norV strains is due to their reduced ability to withstand host-derived NO stress, we reasoned that decreasing host-derived NO production would protect these two mutants. This hypothesis was tested by performing competition experiments in the presence of S-methyl-L-thiocitrulline (SMTC), a nitric-oxide synthase (NOS) inhibitor that effectively reduces NO levels in the light organ of juvenile squid (M. Altura, pers. comm.). Consistent with the prediction, SMTC treatment enhanced the relative colonization competence of the two mutants(Table 3). Similarly, genetic complementation of the Δhmp strain with a wild-type copy of hmp (on pYLW29) also reversed its colonization defect (data not shown). Thus, the ability to remove NO stress during the establishment of the symbiosis contributes to colonization efficiency, and is evident within the first 24 hours.
The hmp promoter responds to NO in host mucus through NsrR
Because hmp expression increases in response to NO in culture (Fig. 1), we asked whether the bacteria encounter sufficient NO in the mucus outside the light organ to activate this response during the initial stage of the colonization. We introduced a plasmid (pYLW45) containing: i) the hmp promoter fused to a promoterless gfp; and ii) a constitutively expressed rfp, into both the wild-type and the ΔnsrR strains. The appearance of fluorescence in these strains was monitored by a microplate reader and laser-scanning confocal microscopy. Cells were monitored first in seawater to determine whether the constructs would faithfully report the presence of NO, and then in mucus as the bacteria initiated symbiosis. In the wild type containing pYLW45, GFP expression was detected when 10 to 100 μM DEA-NONOate was added to seawater, with the response peaking between 2.5 to 3 hrs (Fig. S6), consistent with the reported maturation time of GFP (Ward, 2005). In the ΔnsrR mutant suspended in seawater, the gfp reporter was constitutively expressed, and at higher levels than those observed in wild type (data not shown).
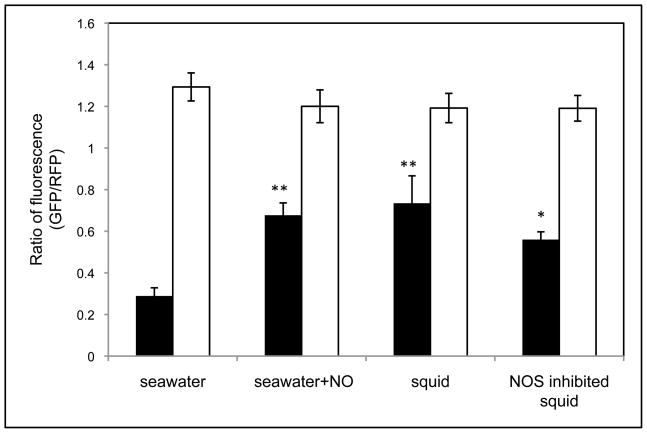
When observed by confocal microscopy, significantly higher GFP fluorescence in wild type cells was detected when the NO generator DEA-NONOate was added to seawater (Fig. 4). As predicted, the reporter was constitutively expressed in ΔnsrR cells, irrespective of the presence or absence of the NO generator (Fig. 4). When the two strains carried the vector control (pVSV209) instead, only RFP was detected (data not shown).
Fig. 4.
The hmp promoter responds to host-derived NO during the aggregation stage of the symbiosis. Cells of wild type (black bars) or ΔnsrR (white bars), carrying the hmp promoter-reporter plasmid pYLW45, were added to seawater, or used to inoculate newly hatched squids. To induce the hmp promoter in the seawater experiment, 80μM DEA-NONOate was added to seawater. To lower the intrinsic level of NO production in the mucus, some squids were pretreated with 100 μM NOS inhibitor SMTC for 2 to 3 hrs before inoculation with V. fischeri. Between 3 to 4 hrs after inoculation, the levels of induced GFP fluorescence and constitutively expressed RFP fluorescence in both strains were monitored using confocal microscopy. The ratio of GFP/RFP fluorescence generated under different conditions was calculated. Significant differences between the ratio found in seawater and the other treatments are indicated: *, p<0.05; **, p<0.01. The experiments were repeated three times with similar results. Data points are means (±1 SEM) calculated from three biological replicates.
Similarly, hmp promoter activity was induced as the wild-type V. fischeri cells initially encountered juvenile squid (Fig. 4). Within the first 3 to 4 hrs of aggregating in host mucus, wild-type cells carrying pYLW45 began producing GFP, and the fluorescence intensity appeared to be at a similar level as that observed in the same strain after exposure to NO in seawater. Again, the reporter gfp was expressed at high levels in ΔnsrR cells, regardless of the test conditions (Fig. 4). In aggregates formed by the wild-type and ΔnsrR strains carrying the vector control (pVSV209), only RFP fluorescence was detected (data not shown). Thus, the hmp promoter of V. fischeri is activated during the aggregation stage of colonization, and NsrR is involved in its de-repression. Of note, pre-treating squids with 100 μM of the NOS inhibitor SMTC lowered the GFP intensity in wild-type cells, although not significantly, perhaps because the treatment did not lower the concentration of NO below the sensing sensitivity level of NsrR.
Hmp protects against NO in the mucus and affects the size of aggregates
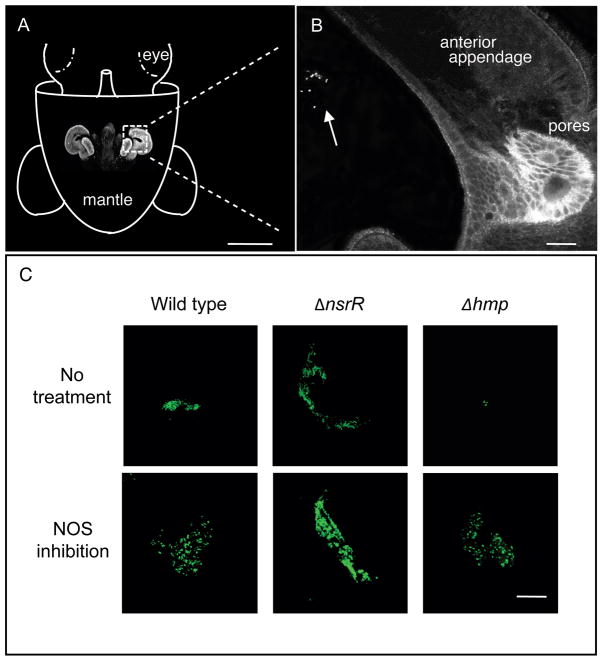
Because V. fischeri detects NO in the mucus (Fig. 4), and high levels of NO limit the size of its aggregates (Davidson et al., 2004), we wondered whether removal of NO by Hmp contributes to the ability of V. fischeri to form normal-sized aggregates. It seemed reasonable to predict that the ΔnsrR mutant would form a larger aggregate than wild type, while an aggregate formed by the Δhmp mutant would be smaller than that of wild type. If we observed the area of the host’s mantle cavity in which the symbiont aggregates (Fig. 5A), we found that the ΔnsrR strain had formed large and tight aggregates, which were easily detected as bright green patches, 2 to 3.5 hrs after inoculation (Fig. 5B). In contrast, the Δhmp strain formed much smaller aggregates of only a few cells. Over the next few hours, the size of wild-type and Δhmp aggregates increased, although the former remained generally larger (data not shown).
Fig. 5.
The size of aggregates formed by V. fischeri during squid colonization correlates with the potential of the bacteria to remove NO. (A) Orientation figure indicating the position of the juvenile’s nascent light organ. A confocal image of the light organ is placed on the cartoon in its approximate position within the mantle cavity (scale bar = 300 μm). (B) Enlarged confocal micrograph of the region of the light organ located within the dotted square of A. An aggregate of V. fischeri cells is indicated by the arrow (scale bar = 10 μm). (C) The bacterial aggregates formed in host-derived mucus by wild-type, ΔnsrR or Δhmp strains carrying GFP-encoded pVSV102, and observed using confocal microscopy (at 400X) between 2.5 and 4 hrs after inoculation (scale bar = 20 μm). To lower the intrinsic level of NO production in the mucus, some squids were pretreated with the nitric-oxide synthesis inhibitor SMTC for 2 to 3 hrs before inoculation with V. fischeri. The experiments were repeated three times with similar results.
Pre-treating juvenile squids with the NOS inhibitor SMTC for 2 to 3 hrs before inoculation ameliorated these differences between the strains (Fig. 5B). Specifically, Δhmp now formed larger aggregates, further suggesting that ambient NO levels somehow limit aggregation. In contrast, ΔnsrR cells appeared blind to NO levels in the mucus, forming aggregates of similar size and appearance in both non-treated and SMTC-treated squids (Fig. 5B). Intriguingly, when wild type and one of the two mutants were differentially marked with either constitutively expressed gfp (pVSV102) or constitutively expressed rfp (pVSV208), and co-inoculated at a 1:1 ratio, wild type did not appear to dominate Δhmp in the aggregation, nor did ΔnsrR dominate over wild type (data not shown). One possibility is that the strain with the higher level of Hmp effectively removed the ambient NO, compensating for the other strain thereby masking the difference seen in single-strain colonization experiments. Taken together, these data support the conclusion that the capacity to remove NO by Hmp controls the size of aggregates in the early stages of the symbiosis.
Discussion
In this study, we have used the squid-vibrio symbiosis to establish that: (i) V. fischeri Hmp is an NO-inducible protein that confers protection against NO both in vitro and during host colonization; (ii) NsrR is the major NO-responsive regulator of hmp expression; and, (iii) early during symbiosis, exposure to NO in host mucus serves as a signal that may prepare the colonizing bacteria for a stronger NO stress encountered as the symbioint migrates through the light-organ duct (Davidson et al., 2004). To our knowledge, this is the first report of the physiological function of Hmp in the Vibrionaceae; more significantly, it demonstrates a novel process by which a bacterial symbiont both responds to and copes with NO during its initial contact with the host.
Genes encoding NO-detoxification systems are widely distributed among bacteria (Rodionov et al., 2005), and allow the cells to survive NO that is either endogenously generated, or imposed externally by host tissues. Interestingly, while the organization of the genes encoding the major NO-detoxification systems (i.e., hmp and norVW) is conserved among Vibrio species, there are apparent differences in the architecture of their regulatory networks. For instance, in Vibrio cholerae, hmp expression is predicted to be controlled by the positive regulator NorR, but not by the repressor NsrR (Rodionov et al., 2005). Thus, within the Vibrionaceae, the induction of hmp expression by NO may involve different regulators, indicating the need to look beyond the well-studied enteric bacteria for novel regulatory mechanisms. For example, hmp was recently reported to be a novel target for HapR regulation in V. cholerae (Tsou et al., 2009), suggesting that quorum-sensing may play an unanticipated role in this bacterium’s NO-stress response.
The hmp promoter region of V. fischeri has predicted binding sites both for NsrR, the repressor considered in this study, and for NarP (Rodionov et al., 2005). NarQP is a two-component sensor-regulator that senses and responds to the presence of nitrate and nitrite in E. coli (Stewart, 2003). While it is not known whether V. fischeri encounters these anionic forms of nitrogen during the initiation of colonization, after the association is established, the symbionts apparently generate energy by anaerobic nitrite respiration as they proliferate in the light-organ crypts (Wier et al., 2010). Interestingly, hmp expression in V. fischeri was up-regulated about 10-fold during nitrate respiration (pers. comm., J. Schwartzman), possibly through NarQP modulation or NsrR that senses NO from nitrate metabolism. It is tempting to hypothesize that NarQP-mediated hmp activation prepares the bacterium for detoxification of any endogenously produced NO during its respiration of nitrate.
Because cytochrome c nitrite reductase (Nrf) has been implicated in the respiratory detoxification of NO by E. coli and S. typhimurium (Poock et al., 2002; Mills et al., 2008), we also asked whether the V. fischeri Nrf plays a similar role. Mutants deleted for the entire nrf operon (VF_1548-VF_1555) alone, or in combination with either hmp or norV, were constructed. Interestingly, the Δnrf strain behaved similarly to wild type in the anoxic challenge experiment described above, while the Δhmp-nrf and ΔnorV-nrf strains reacted like the Δhmp and ΔnorV mutants, respectively (data not shown). It is possible that the presence of at least one NO-detoxification system (i.e., Hmp or NorV) masked the activity of Nrf as a NO reductase in V. fischeri; alternatively, nrf may simply not be expressed under the growth conditions used. In any case, our inability to obtain a Δhmp-norV-nrf triple mutant (even in the absence of added NO) may indicate the need by V. fischeri to maintain at least one intact NO mechanism for response to NO stress.
Apart from hmp, two other genes, nnrS and aox, are predicted to belong to the NsrR regulon in V. fischeri (Rodionov et al., 2005). NnrS is believed to be involved in denitrification (Bartnikas et al., 2002), although its physiological role remains unknown. Recently we reported that Aox, the alternative oxidase, is an NO-resistant oxidase in V. fischeri, (Dunn et al., 2010). Not surprisingly, in wild-type cells, the expression of aox, like hmp, is highly up-regulated in response to NO (Wang et al., 2010; Dunn et al., 2010). Thus, we predicted that the ability of NO-pretreatment to protect respiration in both Δhmp and Δhmp-norV strains (Fig. 3B; Table 3) was due to de-repression of aox. Indeed, while the Δhmp strain was only partially inhibited by NO after pretreatment, oxygen consumption by a Δhmp-aox mutant ceased completely for about 9–10 min before returning to normal (data not shown). This observation suggests that Aox continues to consume oxygen in the presence of NO (trace 3, Fig. 3B; Dunn et al., 2010), supporting its role as an inducible NO-resistant oxidase.
Because NsrR represses two enzymes that provide resistance to NO (Wang et al., 2010; Dunn et al., 2010), and the ΔnsrR strain appeared to be more resistant to NO challenge under culture conditions, we were surprised to find that the ΔnsrR strain did not out-compete wild-type bacteria in a competitive colonization of juvenile squid. One possible explanation is that the ΔnsrR strain becomes more sensitive to the oxidative stress (Gilberthorpe et al., 2007) that is encountered during the establishment of symbiosis (Weis et al., 1996; Small & McFall-Ngai, 1999; Visick & Ruby, 1998). Specifically, although Hmp plays an important role in NO resistance, it is also a potent generator of ROS such as superoxide anion (O2−) (Bang et al., 2006; Gilberthorpe et al., 2007). Thus, an Hmp over-producing strain like ΔnsrR might be more susceptible to oxidative killing, either directly by O2− or its derivative hydrogen peroxide (H2O2), or indirectly by providing H2O2 for halide peroxidase-mediated synthesis of highly toxic hypochlorous acid (Small & McFall-Ngai, 1999). Thus, although the V. fischeri ΔnsrR strain has an increased resistance to host-derived NO stress, it may be exposed to a more severe oxidative killing by H2O2 and hypochlorous acid produced by the duct epithelium of the light organ (Weis et al., 1996).
In the first few hours of the squid-vibrio symbiosis, where both host-derived NO and a respiratory burst are simultaneously present (Davidson et al., 2004; Small & McFall-Ngai, 1999), NO may serve not only as a specificity determinant, but also as an inducer of oxidative-stress protection in the symbiont. For instance, in response to NO induction, V. fischeri suppresses both iron uptake/utilization and cystine transport, probably to limit potential oxidative damage (Wang et al., 2010).
Because the presence of NO in the light organ tissues continues throughout the life of the host (Davidson et al., 2004; Weir et al., 2010), we are currently developing techniques for raising juvenile squids to adulthood, allowing an evaluation of the long-term role of V. fischeri Hmp. Based on the studies described here, we propose the following model for NO-signaling during the initiation of symbiosis: as they first encounter NO during their aggregation outside the light organ, V. fischeri cells de-repress hmp, thereby preparing themselves to survive the high concentrations of NO encountered during their subsequent migration along the ducts that lead to the light organ’s crypts (Davidson et al., 2004). At the same time, exposure to NO present in the mucus activates HNOX signaling, temporarily suppressing the iron uptake/utilization capacity of the bacteria (Wang et al., 2010) until after the host’s ROS production has been curtailed (Small-Howard, 2004). Safely within the crypts, the symbiont population reaches a high enough density that it induces luminescence, lowers oxygen tension, and reduces host-produced NO via anaerobic detoxification mechanisms such as Hmp and NorVW. Thus, the initial exposure to NO in the mucus apparently serves as a “pretreatment” that results in better survival of the oxidative stress conditions within the ducts. In this way, host-derived NO may serve as a signal that is exploited by V. fischeri to help it endure the hostile conditions (both NO and oxidative stress) encountered during the establishment of its symbiosis.
Experimental procedures
Strains, media and growth conditions
The strains, plasmids and PCR primers used in this study are listed in Table S1. Cultures of E. coli were incubated at 37° C in either LB (Miller, 1992) or BHI (Difco Laboratories) media. When needed, chloramphenicol (Cam) and kanamycin (Kan) were added to LB medium at 20 and 50 μg/ml, respectively. Erythromycin (Erm) was added to BHI medium at 150 μg/ml. Cultures of V. fischeri were grown at 28° C in LBS medium (Bose et al., 2007), solidified with 1.5% (w/v) Bacto-Agar (Difco Laboratories) as required. Cam, Erm and Kan were added to LBS at 5, 5, and 100 μg/ml, respectively. Before exposure to NO, V. fischeri cells were grown in a minimal-salts medium that contained (per liter) 500 ml of 2X-artificial seawater stock (Boettcher & Ruby, 1990) supplemented with 1 ml of 5.4% K2HPO4, 50 ml of 1M Tris-HCl buffer (pH 7.5), and 449 ml of tap water. Ten millimolar N-acetyl-D-glucosamine (GlcNAc) (MP Biomedicals) was added as the sole carbon and nitrogen source. To achieve anoxic conditions, the headspace of culture tubes was sequentially evacuated and flushed with argon for 5 cycles. Anaerobic cultures were incubated statically at 28° C. Stock solutions of the NO donors DEA-NONOate and Proli-NONOate (Cayman Chemicals), were reconstituted in 10mM NaOH just before use. At 25° C and pH 7.4, the two NO generators release 1.5 and 2.0 equivalents of NO, and have half-lives of 16 min and 5 sec, respectively.
Genetic manipulations
The deletion mutants used in this study were constructed by allelic exchange as previously described (Dunn & Stabb, 2008). Briefly, approximately 1.5 kb of DNA upstream of the start codon of the gene to be deleted was PCR amplified and fused to an approximately 1.5-kb DNA fragment downstream of the stop codon using an engineered restriction site (NheI). This procedure resulted in the replacement of the gene with a 6-bp restriction-enzyme recognition site between the start and stop codons. The hmp promoter-reporter plasmid (pYLW45) was made by cloning approximately 300 bp of DNA upstream of the Hmp start codon into pVSV209 (Table S1) at the SalI-AvrII restriction sites, generating a transcriptional promoter-fusion to a promoterless gfp. We constructed the hmp complementing plasmid (pYLW29) by cloning the wild-type DNA fragment that encompasses 300 bp upstream of the start codon of Hmp and 20 bp downstream of the stop codon into pVSV105 at the XbaI-SacI restriction sites. Plasmids containing the RP4 origin of transfer were introduced into V. fischeri isolates using tri-parental mating as previously described (Stabb, 2002).
Quantitative real-time PCR (qRT-PCR)
V. fischeri strains were grown by shaking at 28 C in LBS medium to an optical density at 600 nm (OD600) of approximately 0.8, as measured with a Biophotometer (Eppendorf). Twenty microliters of the culture were subcultured into 20 ml of mineral-salts medium containing GlcNAc in a 125-ml Erlenmeyer flask. This culture was grown to an OD600 of approximately 0.3, and 10 ml of culture were transferred to each of two flasks. To one flask, DEA-NONOate (in 10mM NaOH) was added to a final concentration of 80 μM, while the other was treated with a corresponding volume of 10mM NaOH as a control. Samples were allowed to grow at 28° C for 30 min with shaking. Twenty milliliters of RNAprotect Bacteria Reagent (Qiagen) were added to both cultures, which were incubated for 5 min at room temperature. Cells were then pelleted by centrifugation at 8000 × g for 30 min at 4° C, and frozen at −80° C before further processing. Total RNA was prepared using the RNeasy Mini Kit (Qiagen). Residual DNA was removed by using the RNase-free DNase Set (Qiagen), with both on- and off-column digestion steps. cDNA was synthesized by AMV reverse transcriptase (Promega) using 3 μg of purified RNA from each sample. qRT-PCR was performed on an iCycler iQ™ real time PCR machine (BioRad), with 400 ng cDNA/reaction in the iQ™ SyBR Green Supermix (BioRad), with an annealing/extension temperature of 59° C. Primers were designed to target an internal fragment (~120–150 bp) of hmp, norV, or a normalizing control gene (DNA polymerase I, VF_0074). PCR efficiency was similar for all the primer sets. The relative expression levels were calculated using the ΔΔCt method (http://pathmicro.med.sc.edu/pcr/realtime-home.htm). Samples from anaerobic cultures were processed following the same procedures as above, except 5 μM DEA-NONOate was added 45 min before fixation with RNAprotect Bacteria Reagent (Qiagen).
Growth during the NO challenge
Three milliliters of each culture of V. fischeri cells were grown to an OD600 of approximately 0.8 in LBS medium by shaking at 28° C. Twenty microliters were subcultured into 20 ml of mineral-salts medium containing GlcNAc in a 125-ml Erlenmeyer flask. Aerobic growth was monitored by measuring the OD600 of the cultures every 30 mins. When the culture reached early exponential phase (OD600 ~0.3), DEA-NONOate was added to a final concentration of 100 μM to produce the NO challenge. For those cultures receiving an aerobic NO pretreatment, 40 μM DEA-NONOate was added 45 min before the NO challenge.
For V. fischeri cultures grown under anoxic conditions, 40 μl of the pre-culture was sub-cultured into 4 ml of minimal-salts medium plus GlcNAc, degassed and sealed in an 11-ml Pyrex culture tube. Every 30 min, the OD600 of the culture was monitored on a Genesys 20 Spectrophotometer (Thermo Scientific) without the need for subsampling. Because all the strains were more susceptible to NO under anoxic conditions (data not shown), DEA-NONOate was added as a challenge at a final concentration of only 40 μM during the early-mid log phase (OD600 = 0.1 to 0.15) of culture. Similarly, for the pretreatment, only 5 μM DEA-NONOate was added, 45 min before the challenge.
NO inhibition of oxygen consumption
A fresh culture (3 ml) of each V. fischeri strain was grown in LBS medium to an OD600 of approximately 0.8 with shaking at 28° C. Thirty microliters of this pre-culture was subcultured into 30 ml of mineral-salts medium containing GlcNAc in a 250-ml Erlenmeyer flask. Cells in the early exponential phase (OD600 ~0.3) were harvested at 6000 rpm for 10 min at 4° C The pellet was washed and resupended in about 500 μl of modified HEPES buffer (50 mM HEPES, pH 7.4, 300 mM NaCl, 5 mM KCl and 1 mM each of CaCl2, MgCl2, NaH2PO4, and D-glucose) (Stevanin et al., 2000). The final volume of the buffer was adjusted to assure that the suspension was of approximately the same cell density for all of the test strains.
Oxygen consumption was measured using a Clark-type oxygen electrode (Hansatech Instruments, Norfolk, England). Specifically, 50 μl of a cell suspension were mixed with 650 μl of modified HEPES buffer and maintained at 25° C in a water-jacketed electrode chamber. Each assay contained approximately 2.8 mg (dry weight) of cells. The sensitivity of E. coli respiration to NO depends on both NO concentration and the oxygen tension at which the NO is added (Stevanin et al., 2000). Therefore, we ran pilot experiments on wild-type V. fischeri cells to determine the optimal testing conditions. In line with previous studies (Stevanin et al., 2000), NO (released from Proli-NONOate; final concentration 5.5 μM) was injected into a culture of respiring cells in the electrode chamber when the oxygen tension was between 40 and 200 μM. The rate of oxygen consumption was measured immediately before and after NO addition. We found that the degree of inhibition was strongly dependent on the oxygen concentration at the time the NO was added: at a higher concentration, the inhibitory effects were less severe (data not shown). We chose to run experiments at an oxygen concentration of 80 μM, where a clear inhibitory effect occurred when the NO generator was added; however, once the inhibition was reversed, oxygen consumption resumed.
The fractional inhibition of respiration (I) was calculated according to the formula I = 1 − (O2 consumption rate after NO addition/rate before addition). I was plotted against NO concentration and, using Kaleidagraph (Synergy Software), the data were fit to the Hill equation: I = [NO]n/(Ki)n + [NO]n, where Ki is the concentration of NO that gives rise to half-maximal inhibition, and n is the Hill coefficient. When respiration was completely inhibited by NO (i.e., there was no further change in the oxygen concentration after NO addition), the length of inhibition was calculated as the period between NO addition and the point obtained by extrapolation of the residual respiration rate after inhibition is relieved (Stevanin et al., 2000). If oxygen consumption still continued after NO addition, but only at a reduced rate, it was recorded as “partial inhibition”. To test the protective effects of pretreating the cells with NO, cultures were exposed to 80 μM DEA-NONOate for 30 min before collection for respiration measurements.
Single-strain colonization assay
Approximately 22 newly hatched juvenile squids were inoculated with ~1500 cells of the different V. fischeri strains per ml. After a 3-hr exposure time, each squid was transferred to 4 ml of filter-sterilized seawater (FSW) in individual scintillation vials. The onset of bioluminescence was monitored in an LKB scintillation counter (PerkinElmer), modified to operate as an automated photometer. Aposymbiotic animals, which were never exposed to any V. fischeri, were included as negative controls. Twenty-four hours post-inoculation, the percentage of luminous squids, and the time each strain took to initiate luminescence, were calculated: the onset of luminescence has been shown to be a good marker for colonization (Ruby & Asato, 1993). Wild-type and mutant strains used in this study produced the same level of luminescence per cell in culture (data not shown).
The minimal infection dose (MID) was determined as previously described (McCann et al., 2003). To calculate the efficiency of infection (ID50), we determined the percentage of animals that became colonized as a function of the inoculum dose. In these calculations, we assumed that the log inoculum is paired with the logit of percent of animals colonized, and applied the log-logistic model to estimate the ID50 values of the wild-type and Δhmp strains.
Competitive colonization assay
Competition experiments between co-inoculated strains were carried out as previously described (Dunn & Stabb, 2008). Wild-type and mutant strains were marked with a stable plasmid carrying either the gfp (pVSV102) or rfp (pVSV208) gene (Table S1). The ratio of CFUs in the mixed inoculum could be easily determined by the color of the colonies after 48 hrs. The relative competitiveness index (RCI) was calculated by dividing the ratio of the CFUs of wild type to the mutant in each light organ by the ratio of these strains in the initial inoculum. An RCI >1 indicates that wild type out-competes the mutant in the colonization of host animals. In each experiment, approximately 30 squids were inoculated, and subsequently scored for bioluminescence as an indicator for colonization at 24 hrs post-inoculation. Only colonized animals were sacrificed for RCI determination. When desired, 100 μM of the NOS inhibitor S-methyl-L-thiocitrulline (SMTC) was added to the seawater throughout the experiments. Supplementation with SMTC did not compromise the host’s capacity to support normal levels of symbiont growth in the light organ (data not shown).
Induction of the hmp promoter in NO-supplemented seawater and during symbiotic aggregation
To determine whether the V. fischeri hmp promoter responds to exogenously added NO in seawater, cultures of either the wild-type or ΔnsrR strains (~108 CFU/ml), carrying either pYLW45 or pVSV209, were exposed to between 10 nM and 100 μM DEA-NONOate. Triplicate 100 μL samples of the NO-treated cell suspensions were aliquoted into a 96-well microplate, and monitored every 30 min for optical density and GFP fluorescence intensity using a microplate reader (Tecan Group Ltd, Mannedorf, Switzerland). Between each data collection point, the plate was incubated at 28° C with shaking. When either strain carried the vector control containing the promoterless gfp (pVSV209), no significant GFP signal was detected. The final level of GFP fluorescence was obtained by first subtracting this vector-control fluorescence (background), and then normalizing by cell density (OD600).
Either wild-type or ΔnsrR cells, each carrying pYLW45, were used to inoculate between 5 and 10 newly hatched juvenile squids at 106 CFU/ml. The level of Induction of the hmp promoter, and the resultant GFP signal, were determined during aggregation as follows. At between 1.5 and 4.5 hrs post-inoculation, squid were washed 3 times in FSW, and anesthetized in a 1:1 solution of 7.5% MgCl2 in FSW (Nyholm et al., 2000). After dissection, fluorescent emission was visualized by LSM 510 confocal laser-scanning microscope (Carl Zeiss Microimaging). As a control, cells were inoculated into FSW (106 CFU/ml), induced by the addition of 80 μM DEA-NONOate and, after 2 to 3 hrs, a 3 μL sample was examined by confocal microscopy. Cells that did not receive any treatment were also examined. The intensity of fluorescence of GFP and constitutively expressed RFP in the aggregating cells was recorded and extracted by the computer software (Zeiss LSM 510 ver. 4.20). Comparisons between the test conditions and the seawater control were performed using Student’s t-test. The statistical analysis was made with Excel software.
Observation of aggregates in the mucus
Five to 10 newly hatched juvenile squids were inoculated with approximately 106 CFU (per ml) of V. fischeri strains that were marked with constitutively expressed gfp (pVSV102) or rfp (pVSV208) to allow their visualization by confocal microscopy during the initiation of colonization. Samples were processed as described above, except that squids were stained with 1 μM CellTracker Orange CMRA (Molecular Probes, Invitrogen) and 10 μg/ml Wheat Germ Agglutinin Alexa Fluor 633 conjugant (WGA-633) (Molecular Probes, Invitrogen) for 20 min before examination. After dissection, squid tissue, secreted mucus and the aggregates formed by V. fischeri were observed. Images were recorded by the Axioplan 2 Imaging system attached to the scope. To study the effects of NOS inhibitor on aggregate size, squids were treated with 100 μM of the NOS inhibitor SMTC for 2–3 hrs before exposure to V. fischeri strains. After inoculation, the presence of SMTC was maintained by addition of the inhibitor to the inoculated seawater. For competitive colonization experiments, juvenile squid were co-inoculated with a 1:1 ratio of differentially marked wild-type and mutant strains at a final concentration of 106 CFU/ml. All other procedures were performed as described above.
Supplementary Material
Acknowledgments
We thank Elizabeth Heath-Heckman for help and advice with the confocal microscopy and data analysis of GFP intensity, and Spencer Nyholm for confocal images. This work was supported by National Science Foundation grant IOS-0817232 (M. M-N. and E. G. R.) and National Institutes of Health grant R01 RR 12294 (E. G. R. and M. M-N.), NSF grant MCB-0702858 (S. S.), and NSF grant MCB-0803181 and the University of Oklahoma (A. K. D.).
References cited
- Arai H, Hayashi M, Kuroi A, Ishii M, Igarashi Y. Transcriptional regulation of the flavohemoglobin gene for aerobic nitric oxide detoxification by the second nitric oxide-responsive regulator of Pseudomonas aeruginosa. J Bacteriol. 2005;187:3960–3968. doi: 10.1128/JB.187.12.3960-3968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC. Maintenance of nitric oxide and redox homeostasis by the Salmonella flavohemoglobin Hmp. J Biol Chem. 2006;281:28039–28047. doi: 10.1074/jbc.M605174200. [DOI] [PubMed] [Google Scholar]
- Bartnikas TB, Wang Y, Bobo T, Veselov A, Scholes CP, Shapleigh JP. Characterization of a member of the NnrR regulon in Rhodobacter sphaeroides 2.4.3 encoding a haem-copper protein. Microbiology. 2002;148:825–833. doi: 10.1099/00221287-148-3-825. [DOI] [PubMed] [Google Scholar]
- Baudouin E, Pieuchot L, Engler G, Pauly N, Puppo A. Nitric oxide Is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol Plant-Microbe Interact. 2006;19:970–975. doi: 10.1094/MPMI-19-0970. [DOI] [PubMed] [Google Scholar]
- Boccara M, Mills CE, Zeier J, Anzi C, Lamb C, Poole RK, Delledonne M. Flavohaemoglobin HmpX from Erwinia chrysanthemi confers nitrosative stress tolerance and affects the plant hypersensitive reaction by intercepting nitric oxide produced by the host. Plant J. 2005;43:226–237. doi: 10.1111/j.1365-313X.2005.02443.x. [DOI] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisov VB, Forte E, Konstantinov AA, Poole RK, Sarti P, Giuffr A. Interaction of the bacterial terminal oxidase cytochrome bd with nitric oxide. FEBS Lett. 2004;576:201–204. doi: 10.1016/j.febslet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Bose JL, Kim U, Bartkowski W, Gunsalus RP, Overley AM, Lyell NL, Visick KL, Stabb EV. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol Microbiol. 2007;65:538–553. doi: 10.1111/j.1365-2958.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Cary SPL, Winger JA, Derbyshire ER, Marletta MA. Nitric oxide signaling: no longer on or off. Trends Biochem Sci. 2006;31:231–239. doi: 10.1016/j.tibs.2006.02.003. [DOI] [PubMed] [Google Scholar]
- D’Autreaux B, Tucker NP, Dixon R, Spiro S. A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature. 2005;437:769–772. doi: 10.1038/nature03953. [DOI] [PubMed] [Google Scholar]
- Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ. NO means ‘yes’ in the squid-vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cellul Microbiol. 2004;6:1139–1151. doi: 10.1111/j.1462-5822.2004.00429.x. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Karr EA, Wang Y, Barron AR, Ruby EG, Stabb EV. The alternative oxidase (AOX) gene in Vibrio fischeri is controlled by NsrR and upregulated in response to nitric oxide. Mol Microbiol. 2010;77:44–55. doi: 10.1111/j.1365-2958.2010.07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Stabb EV. Genetic analysis of trimethylamine N-oxide reductases in the light organ symbiont Vibrio fischeri ES114. J Bacteriol. 2008;190:5814–5823. doi: 10.1128/JB.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Milikan DS, Adin DM, Bose JL, Stabb EV. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl Environ Microbiol. 2006;72:802–810. doi: 10.1128/AEM.72.1.802-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Micro. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Gardner AM, Helmick RA, Gardner PR. Flavorubredoxin, an inducible catalyst for nitric oxide reduction and detoxification in Escherichia coli. J Biol Chem. 2002;277:8172–8177. doi: 10.1074/jbc.M110471200. [DOI] [PubMed] [Google Scholar]
- Gilberthorpe NJ, Lee ME, Stevanin TM, Read RC, Poole RK. NsrR: a key regulator circumventing Salmonella enterica serovar Typhimurium oxidative and nitrosative stress in vitro and in IFN-{gamma}-stimulated J774.2 macrophages. Microbiology. 2007a;153:1756–1771. doi: 10.1099/mic.0.2006/003731-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J, Ruby EG. Host-derived amino acids support the proliferation of symbiotic bacteria. Proc Natl Acad Sci USA. 1998;95:1818–1822. doi: 10.1073/pnas.95.4.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- Hutchings MI, Mandhana N, Spiro S. The NorR Protein of Escherichia coli activates expression of the flavorubredoxin gene norV in response to reactive nitrogen species. J Bacteriol. 2002;184:4640–4643. doi: 10.1128/JB.184.16.4640-4643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer L, Anantharaman V, Aravind L. Ancient conserved domains shared by animal soluble guanylyl cyclases and bacterial signaling proteins. BMC Genomics. 2003;4:5. doi: 10.1186/1471-2164-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropatnick TA, Engle JT, Apicella MA, Stabb EV, Goldman WE, McFall-Ngai MJ. Microbial factor-mediated development in a host-bacterial mutualism. Science. 2004;306:1186–1188. doi: 10.1126/science.1102218. [DOI] [PubMed] [Google Scholar]
- Lee K, Ruby EG. Effects of the squid host on the abundance and distribution of symbiontic Vibrio fischeri in nature. Appl Environ Microbiol. 1994;60:1565–1571. doi: 10.1128/aem.60.5.1565-1571.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu C, Moreau S, Frendo P, Puppo A, Davies MJ. Direct detection of radicals in intact soybean nodules: presence of nitric oxide-leghemoglobin complexes. Free Rad Biol Med. 1998;24:1242–1249. doi: 10.1016/s0891-5849(97)00440-1. [DOI] [PubMed] [Google Scholar]
- McCann J, Stabb EV, Millikan DS, Ruby EG. Population Dynamics of Vibrio fischeri during Infection of Euprymna scolopes. Appl Environ Microbiol. 2003;69:5928–5934. doi: 10.1128/AEM.69.10.5928-5934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhoc E, Cam Y, Skapski A, Bruand C. The response to nitric oxide of the nitrogen-fixing symbiont Sinorhizobium meliloti. Mol Plant Microbe Interact. 2010;23:748–759. doi: 10.1094/MPMI-23-6-0748. [DOI] [PubMed] [Google Scholar]
- Miller JH. A short course in bacterial genetics. Cold Spring Harbor Labratory Press; Cold Spring Harbor, N. Y: 1992. [Google Scholar]
- Mills PC, Rowley G, Spiro S, Hinton JCD, Richardson DJ. A combination of cytochrome c nitrite reductase (NrfA) and flavorubredoxin (NorV) protects Salmonella enterica serovar Typhimurium against killing by NO in anoxic environments. Microbiology. 2008;154:1218–1228. doi: 10.1099/mic.0.2007/014290-0. [DOI] [PubMed] [Google Scholar]
- Nyholm SV, McFall-Ngai MJ. The winnowing: establishing the squid-Vibrio symbiosis. Nat Rev Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- Nyholm SV, Stabb EV, Ruby EG, McFall-Ngai MJ. Establishment of an animal-bacterial association: recruiting symbiotic vibrios from the environment. Proc Natl Acad Sci USA. 2000;97:10231–10235. doi: 10.1073/pnas.97.18.10231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poock SR, Leach ER, Moir JWB, Cole JA, Richardson DJ. Respiratory detoxification of nitric oxide by the cytochromec nitrite reductase of Escherichia coli. J Biol Chem. 2002;277:23664–23669. doi: 10.1074/jbc.M200731200. [DOI] [PubMed] [Google Scholar]
- Poole RK, Hughes MN. New functions for the ancient globin family: bacterial responses to nitric oxide and nitrosative stress. Mol Microbiol. 2000;36:775–783. doi: 10.1046/j.1365-2958.2000.01889.x. [DOI] [PubMed] [Google Scholar]
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AR, Dunman PM, Fang FC. The nitrosative stress response of Staphylococcus aureus is required for resistance to innate immunity. Mol Microbiol. 2006;61:927–939. doi: 10.1111/j.1365-2958.2006.05290.x. [DOI] [PubMed] [Google Scholar]
- Rodionov DA, I, Dubchak L, Arkin AP, Alm EJ, Gelfand MS. Dissimilatory metabolism of nitrogen oxides in bacteria: comparative reconstruction of transcriptional networks. PLoS Comput Biol. 2005a;1:e55. doi: 10.1371/journal.pcbi.0010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby EG, Asato LM. Growth and flagellation of Vibrio fischeri during initiation of the sepiolid squid light organ symbiosis. Arch Microbiol. 1993;159:160–167. doi: 10.1007/BF00250277. [DOI] [PubMed] [Google Scholar]
- Ruby EG, Urbanowski M, Campbell J, Dunn A, Faini M, Gunsalus R, Lostroh P, Lupp C, McCann J, Millikan D, Schaefer A, Stabb E, Stevens A, Visick K, Whistler C, Greenberg EP. Complete genome sequence of Vibrio fischeri: A symbiotic bacterium with pathogenic congeners. Proc Natl Acad Sci USA. 2005;102:3004–3009. doi: 10.1073/pnas.0409900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small-Howard AL. PhD thesis. Los Angeles: University of Southern California; 2004. A halide peroxidase and reactive oxygen species in the mutulistic light organ symbiosis between the sepilolid squid Euprymna scolopes and the luminous bacterium Vibrio fischeri. Faculty of the graduate school. [Google Scholar]
- Small AL, McFall-Ngai MJ. Halide peroxidase in tissues that interact with bacteria in the host squid Euprymna scolopes. J Cellul Biochem. 1999;72:445–457. [PubMed] [Google Scholar]
- Spiro S. Regulators of bacterial responses to nitric oxide. FEMS Microbiol Rev. 2007;31:193–211. doi: 10.1111/j.1574-6976.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- Stabb EV, Ruby EG. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Meth Enzymol. 2002;358:413–426. doi: 10.1016/s0076-6879(02)58106-4. [DOI] [PubMed] [Google Scholar]
- Stevanin TM, Ioannidis N, Mills CE, Kim SO, Hughes MN, Poole RK. Flavohemoglobin Hmp affords inducible protection for Escherichia coli respiration, catalyzed by cytochromes bc or bd, from nitric oxide. J Biol Chem. 2000;275:35868–35875. doi: 10.1074/jbc.M002471200. [DOI] [PubMed] [Google Scholar]
- Stevanin TM, Poole RK, Demoncheaux EAG, Read RC. Flavohemoglobin Hmp protects Salmonella enterica serovar Typhimurium from nitric oxide-related killing by human macrophages. Infect Immun. 2002;70:4399–4405. doi: 10.1128/IAI.70.8.4399-4405.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Nitrate- and nitrite-responsive sensors NarX and NarQ of proteobacteria. Biochem Soc Trans. 2003;31:1–10. doi: 10.1042/bst0310001. [DOI] [PubMed] [Google Scholar]
- Tsou AM, Cai T, Liu Z, Zhu J, Kulkarni RV. Regulatory targets of quorum sensing in Vibrio cholerae: evidence for two distinct HapR-binding motifs. Nucl Acids Res. 2009;37:2747–2756. doi: 10.1093/nar/gkp121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker NP, Le Brun NE, Dixon R, Hutchings MI. There’s NO stopping NsrR, a global regulator of the bacterial NO stress response. Trends Microbiol. 2010;18:149–156. doi: 10.1016/j.tim.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Visick KL, Ruby EG. The periplasmic, group III catalase of Vibrio fischeri Is required for normal symbiotic competence and is induced both by oxidative stress and by approach to stationary phase. J Bacteriol. 1998;180:2087–2092. doi: 10.1128/jb.180.8.2087-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dufour YS, Carlson HK, Donohue TJ, Marletta MA, Ruby EG. H-NOX–mediated nitric oxide sensing modulates symbiotic colonization by Vibrio fischeri. Proc Natl Acad Sci USA. 2010;107:8375–8380. doi: 10.1073/pnas.1003571107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward WW. Biochemical and physical properties of green fluorescent protein. In: Martin Chalfie SRK, editor. Green Fluorescent Protein. 2. NY: John Wiley & Sons, Inc; 2005. pp. 39–65. [PubMed] [Google Scholar]
- Weis V, Small A, McFall-Ngai M. A peroxidase related to the mammalian antimicrobial protein myeloperoxidase in the Euprymna-Vibrio mutualism. Proc Natl Acad Sci USA. 1996;93:13683–13688. doi: 10.1073/pnas.93.24.13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier AM, Nyholm SV, Mandel MJ, Massengo-Tiassé RP, Schaefer AL, Koroleva I, Splinter-BonDurant S, Brown B, Manzella L, Snir E, Almabrazi H, Scheetz TE, Bonaldo MdF, Casavant TL, Soares MB, Cronan JE, Reed JL, Ruby EG, McFall-Ngai MJ. Transcriptional patterns in both host and bacterium underlie a daily rhythm of anatomical and metabolic change in a beneficial symbiosis. Proc Natl Acad Sci USA. 2010;107:2259–2264. doi: 10.1073/pnas.0909712107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg MS, Ruby EG. Population structure of Vibrio fischeri within the light organs of Euprymna scolopes from two different host populations. Appl Environ Microbiol. 2009;75:193–202. doi: 10.1128/AEM.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.