Abstract
Background
Several nutritional and physiological factors have been linked to depression in adults including low folate and vitamin B-12 and elevated total homocysteine (tHcy) levels.
Methods
Nationally representative data on US adults (aged 20–85 years, n=2,524) from the National Health and Nutrition Examination Survey of the period 2005–06 were used. Depressive symptoms were measured with the Patient Health Questionnaire (PHQ) and elevated symptoms were defined as PHQ total score≥10. Serum folate, vitamin B-12 and tHcy were mainly expressed as tertiles. Age, sex, race/ethnicity, education, poverty income ratio, marital status, smoking status, physical activity, body mass index and selected nutrient intakes (average of two 24-hr recalls) were considered as potential confounders. Multiple ordinary least square (OLS), logistic and zero-inflated Poisson regression models were conducted in the main analysis.
Results
Overall, mean PHQ score was significantly higher among women compared to men. Elevated depressive symptoms (PHQ≥10) were inversely associated with folate status particularly among women [Fully adjusted odds ratio (Tertiles T3 vs. T1)=0.37 (95% CI = 0.17–0.86)], but not significantly related to tHcy or vitamin B-12. No interaction was noted between the three exposures in affecting depressive symptoms. In older adults (≥50 years) and both sexes combined, total homocysteine was positively associated with elevated depressive symptoms [Fully adjusted odds ratio (Tertiles T2 vs. T1)=3.01 (95% CI = 1.01–9.03)], though no significant dose-response relationship was found.
Conclusions
Future interventions aiming at improving mental health outcomes among US adults should take into account dietary and other factors that would increase levels of serum folate.
Keywords: Depression, folate, vitamin B-12, homocysteine, adults
INTRODUCTION
Prevalence rates of depression are approximately 12% and 24% among US men and women, respectively. (1) Emerging evidence points to low levels of folate and vitamin B-12 and high levels of tHcy as risk factors for elevated depressive symptoms and depression diagnosis (2–3), although a recent review and meta-analysis concluded that research was still needed to strengthen evidence through large population-based cohort studies, particularly for young and middle-aged adults. (4) Moreover, a number of reports suggest that folate supplementation may also enhance the effectiveness of certain anti-depressant regimens. (5–6)
Folate is a group of water-soluble naturally occurring compounds found mostly in green vegetables, peanuts, legumes, strawberries, and orange juice, predominantly as polyglutamates.(7) When foods are fortified, folic acid is added in the pteroylmonoglutamate form. Upon absorption, folate circulates freely or bound to albumin as a monoglutamate and uses an active transport system to enter the cerebrospinal fluid (CSF). This raises its concentration in the CSF to three times that in plasma. Both dietary and supplemental intakes may affect serum level of folate, which decreases after diminished intake within 1 to 6 months period. (8)
Although DNA biosynthesis is the most essential function of folate, its role in the central nervous system (CNS) differs as it is mostly involved in neuroprotection, with several related pathways. The first pathway leads to the synthesis of neurotransmitters through increased production of tetrahydro biopterins (BH4), a co-factor needed to convert phenylalanine into tyrosine and to hydroxylate tyrosine and tryptophan. (9) These reactions are limiting steps for the synthesis of dopamine, noradrenalin and serotonin, three major neurotransmitters in the CNS. In fact, reduced excretion of BH4 was observed among depressed patients, (10–11) a marker of poor BH4 bioavailability.
Another pathway is the series of methylation reactions (MR) within the CNS that help reduce blood total homocysteine (tHcy) levels, a putative toxic metabolite to the vascular system and the CNS. (3) In animal models, tHcy damages nerve cells by enhancing toxicity of β-amyloid and copper, (12), and leads to the production of reactive oxygen species, DNA damage and apoptosis i.e. programmed cell death. (13) tHcy might affect certain parts of the brain to a greater extent than others, and studies have linked tHcy to brain atrophy, possibly in areas related to increased depressive symptoms. (14–18) To prevent build-up of tHcy, methylation reactions are required. One reaction (MR1) requires Vitamin B-12 to transfer a methyl group from 5-methyltetrahydrofolate to tHcy, producing methionine and controlling the production of S-adenosylmethionine, a universal methyl donor of the brain and in other tissues, including those involving serotonin and monoamine neurotransmitter (10, 19). S-adenosylmethionine has been reported to have anti-depressant properties. (20) In physiological folate and vitamin B-12 deficiency, the conversion of tHcy to methionine is hampered. (13) An alternative MR (MR2), not requiring Vitamin B-12 or 5-methyltetrahydrofolate, the betaine:Hcy methyltransferase reaction, is only available in the peripheral nervous system. Thus, in the CNS, MR1 is one of few possible pathways to reduce the tHcy level in the brain. A third metabolic reaction requires Vitamin B-6 for cystationine-β-synthetase which condenses tHcy with serine converting it to cystathionine, leading to the formation of glutathione a major antioxidant. (3)
All these metabolic reactions suggest a possible link between levels of folate, vitamin B-12, tHcy and depression. Recent observational studies have shown that high levels of tHcy (21–27) and low levels of folate and vitamin B-12 (23, 25, 27–45) are associated with risk of depression or elevated depressive symptoms in adults. However, studies examining the impact of low folate, vitamin B-12, and elevated tHcy status simultaneously on depressive symptoms did not examine interaction between those three risk factors and have had inconsistent findings as to the individual associations. In fact, while a cross-sectional study of Chinese older adults residing in Southeast Singapore (n=669, age:55 years or older) had shown that both low levels of folate and vitamin B-12 were associated with depression independently of elevated tHcy level (31), another prospective cohort study with a cross-sectional component among 732 Korean older adults (age:65 years or older) indicated that all three exposures, namely lower folate, lower vitamin B-12 and higher tHcy, were significant risk factors for late-life depression. (26) Hence, it is important to consider potential interactive effects between those exposures, particularly due to the fact that vitamin B-12 and folate act together to re-methylate tHcy into methionine, and therefore preventing build-up of brain neurotoxicity which potentially can cause atrophy in brain areas associated with depressive symptoms.
Previous studies present differential results across the sexes for these associations. (5, 27, 37–38, 43, 46–47) Although several previous studies have been conducted using a variety of study designs, including prospective cohort and randomized controlled trials (RCT), most have been conducted in non-US settings and RCTs had relatively small sample sizes. To our knowledge, this is the first nationally representative study conducted among US adults after mandatory fortification of food with folic acid that examines associations of serum folate, vitamin B-12, and tHcy levels with depressive symptoms. This study uses cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) from 2005–06 by assessing interactions (two-way and three-way) among the three exposures (i.e. folate, vitamin B-12 and tHcy) and testing effect modification of the associations by sex.
MATERIALS AND METHODS
Database and study subjects
The NHANES (http://www.cdc.gov/nchs/nhanes.htm) are a series of cross-sectional surveys that provide health and nutrition data on nationally-representative samples of the US civilian population. NHANES has a stratified, multistage probability cluster sampling design. In-home interviews collecting basic socio-demographic data are followed by health examinations in a mobile examination center (MEC) which include various anthropometric, blood pressure and laboratory measurements. Detailed descriptions of study design and data collection procedures have been published elsewhere. (48) Procedures followed were in accordance with ethical standards of the institution or regional committee on human experimentation and approval was obtained from the relevant committee on human subjects welfare. (49)
In this study, we analyzed NHANES data from adults aged 20 to 85 years from 2005–2006. Among a sample of 4,979 subjects (2,387 men and 2,592 women) with complete demographic data (Sample 1), 4,520 had complete dietary data and 4,179 had complete plasma folate, vitamin B-12 and tHcy status and dietary data. Among those, physical activity, anthropometric measures (weight and height) and smoking status were complete for 4,126 subjects (Sample 2). Among participants in Sample 2, those with complete data on depressive symptoms (Sample 3) were considered in our final analysis (n=2,524). This final sample differed significantly from the non-covered group in Sample 1 (both with pseudo-complete data on age, sex, race/ethnicity, educational level and poverty income ratio) by having a higher proportion female (56% vs. 47%) and having a lower mean age (46.1y vs. 47.1y); (p<0.05 based on design-based F-test and non-design based t-test). However, there were no differences in the distribution by race/ethnicity, education and poverty income ratio (PIR).
Outcome assessment
The questionnaire section of NHANES 2005–06 included the Patient Health Questionnaire (PHQ). This set of 10 questions reflects self-reported depressive signs and symptoms that are derived from DSM IV criteria. There are nine signs and symptoms in DSM IV that were scored between zero (not at all) and 3 (nearly every day), and an additional follow-up question to assess overall impairment ascribed to depressive symptoms. The PHQ was validated in primary care settings and shown as a reliable tool for depression diagnosis. (50–51) Summing scores on 10 PHQ items potentially yields a total score between 0 and 30. In our sample, the 90th percentile on total PHQ score corresponded to a value of 10. This cutoff point was also used elsewhere and had a sensitivity of 88% and a specificity of 88% for major depression. (50–51)
Exposure assessment
Serum folate was measured by Bio-Rad Laboratories “Quantaphase II Folate” radioassay kit. A serum or whole blood hemolysate sample is mixed with 125I-folate and cyanide and then boiled to inactivate endogenous folate-binding proteins. After stabilizing the mixture with dithiothreitol, it was cooled and combined with immobilized affinity-purified folate-binding proteins, which adjusted the pH to 9.2. The reaction mixture was incubated at room temperature for 1 hour, centrifuged and decanted. Radioactivity of labeled bound folate to folate-binding protein was counted and concentration of endogenous bound folate was determined by comparing it to standard curves derived from pre-calibrated folate standards. This is possible because of competition between endogenous and labeled folate for the same binding sites on the folate-binding protein. Serum folate in ng/mL was converted to nmol/L by multiplying by 2.265.
The same radioassay kit as above was used to determine vitamin B-12 concentration by combining serum or whole blood hemolysate with 57Co-vitamin B-12 in a solution containing dithiothreitol and cyanide and boiling the mixture to convert various forms of vitamin B-12 to cyanocobalamin. After cooling and addition of porcine intrinsic factor, the mixture was incubated for 1 hour at room temperature, centrifuged and decanted. Radioactivity of labeled bound vitamin B-12 was counted and concentration of endogenous bound vitamin B-12 was determined using standard curves.
Plasma tHcy was measured using “Abbott Homocysteine (HCY) assay”, a fully automated technique developed by Abbott Diagnostics. (52–53) Plasma tHcy was converted to S-adenosyl-homocysteine through addition of dithiothereitol followed by a hydrolase in the presence of adenosine. Later, a monoclonal antibody was added along with a fluorescinated amount of S-adenosyl-homocysteine analog which allows calculation of plasma tHcy concentration using a machine-stored calibration curve. (54–55) This new method was shown to be equivalent to more common techniques such as the High-Performance Liquid Chromatography. (56)
Covariates
Socio-demographic
The following socio-demographic variables were considered a priori based on the literature (37–38, 44, 57): Age (categorical and continuous), sex, race/ethnicity (categorized as “Non-Hispanic White,” “Non-Hispanic black,” “Mexican-American” and “other ethnicity”), education (categorized as “<High School,” “High School” and “>High School”), marital status (“Currently married” vs. “unmarried”) and poverty income ratio (PIR, categorized as “<100%,” “100%–<200%,” “≥200%”).
Lifestyle and health-related factors
An objective measure for physical activity was constructed based on individual leisure-time activities based on an intensity score assessed by metabolic equivalent (MET) multiplied by duration of the activity and frequency converted to per week unit. This MET×hr/week value was summed for each subject depending on the number of leisure-time physical activities elicited. Participants who did not elicit any activity were considered sedentary and given a score of zero. (58–60) This continuous score was categorized as “0–<5”; “5–10”; “>10”, in the main part of the analysis.
Questions on tobacco use history were elicited from NHANES adult participants aged 20 years or over during in-home interviews using computer-assisted personal interviewing system. Cigarette smoking status was defined as never, former or current smoker. This was a combination of two binary questions, one asking about lifetime history of smoking more than 100 cigarettes (i.e. ever smoked, yes vs. no) and a second about current smoking status (i.e. current smoker: yes vs. no). Those two questions were combined in a way as to form the three categories.
Dietary intake information was obtained from NHANES participants and amounts and types of foods or beverages consumed during the day before the interview. The dietary interview component of NHANES, called What We Eat in America (WWEIA), is a collaboration of the U.S. Department of Agriculture (USDA) with the U.S. Department of Health and Human Services (DHHS). All NHANES participants were eligible for two 24 hr recalls, the first one was administered during the MEC exam and second 3–10 days later by telephone interview. In this study, the average of the two 24 hr recalls was examined. Using estimated amounts of foods, nutrient intakes over two 24 hr recalls were computed at the individual-level using a revised nutrient database that converted amounts of specific food intakes into amounts of various nutrients. (61) In our present analyses, total energy intake and intakes of fiber, alcohol, caffeine, vitamin B-6, selected antioxidants (β-carotene, vitamin C and vitamin E), and n-3 highly unsaturated fatty acids were considered, given their potential confounding effects of the relationship between main exposures and depressive symptoms. (43–44, 46, 62–65) n-3 highly unsaturated fatty acids were defined as the sum of eicosapentaenoic acid, docosahexaenoic acid and n-3 docosapentaenoic acid. All dietary covariates were expressed as tertiles to account for non-linear relationships in the main parts of the analysis where outcome was depressive symptoms. In all other analyses, continuous values were used. Weight and height were measured during MEC examination, and body mass index (BMI) was computed as weight (kg) divided by height-squared in m2.
Statistical Analysis
All analyses were conducted using Stata release 10.0. (66) First, we described the NHANES 2005–06 study sample characteristics by sex and depressive symptoms status (i.e. PHQ≥10 vs. PHQ<10). Differences in means between groups were tested using t-tests. Associations between categorical variables were tested using design-based χ2 tests.
Second, multiple ordinary least square (OLS) linear regression models examined the associations of the three main exposures of interest (i.e. serum folate, vitamin B-12 and tHcy) with socio-demographic, lifestyle and health-related factors.
Third, multiple logistic regression models on high PHQ scores (i.e. PHQ≥10) were conducted to test associations between folate, vitamin B-12 and tHcy status (entered as tertiles) and depressive symptoms, controlling for confounders and stratifying by sex. Similarly, and because the distribution of the PHQ count measure was skewed and had a large proportion of zero scores, associations between exposures and continuous PHQ score were tested uusing multiple zero-inflated Poisson regression models (40, 67). Socio-demographic factors were retained in all models (Models 1 and 2), and other potential confounding variables were added in Model 2 (including lifestyle, health-related and dietary factors).
Fourth, interactions between the three exposures were tested by multiple logistic and linear regression models with PHQ as the outcome, exposures (folate, B-12 and tHcy levels, expressed as binary variables; 1=uppermost tertile; 0=lowest or middle tertile), and interactions among exposures (B-12×tHcy, FOL×tHcy, FOL×B-12, FOL×B-12×tHcy).
Finally, a sensitivity analysis was conducted in which the sample was stratified by age (<50, 50+) for both sexes combined and the main exposures were expressed as tertiles. Multiple logistic regression model findings controlling for the same covariates as above were presented graphically (i.e. adjusted odds ratios and 95% CI).
In all analyses, sampling design complexity was taken into account and adequate sampling weights, strata and primary sampling units were specified using Stata survey commands. Two-year MEC exam weights were used for all sample estimations and masked variance units were used to estimate variances using the Taylor series linearization method (68). All p values presented are 2-tailed; p < 0.05 was considered statistically significant.
RESULTS
Sample characteristics by sex and depressive symptoms status
Table 1 presents the distribution of sample characteristics by sex and depressive symptoms status based on PHQ score. The mean PHQ score was significantly higher among women than men. Participants with elevated depressive symptoms were generally less educated, more likely to belong to PIR<100% category, be unmarried, less physically active and have lower intake of vitamin B-6 than their less depressed counterparts. Women with depressive symptoms were additionally more likely to be current smokers, had a higher mean BMI, lower intakes of fiber, β-carotene, vitamin C, vitamin E, and n-3 highly unsaturated fatty acids, as compared to their less depressed counterparts. More importantly, serum folate level was significantly lower in women with elevated depressive symptoms than in less depressed women. In contrast, among men, tHcy level was directly related to elevated depressive symptoms. Additionally, there were notable differences between men and women in a number of those characteristics including age, race/ethnicity, smoking status, education, physical activity, BMI as well as levels of folate, vitamin B-12 and tHcy.
TABLE 1.
Selected characteristics of NHANES 2005–06 participants by sex and Patient Health Questionnaire (PHQ) score (n=2,524)a
| Men |
Women |
Pb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| PHQ<10 (n=963) | PHQ≥10 (n =117) | All Men (n=1,080) | PHQ<10 (n=1,273) | PHQ≥10 (n =171) | All Women (n=1,444) | Men vs. women | Low vs. high PHQ score among men | Low vs. high PHQ score among women | |
| PHQ score | |||||||||
| PHQ continuous score mean±SEM | 3.0±0.1 | 13.8±0.4 | 4.0±0.1 | 3.3±0.1 | 14.5±0.4 | 4.5±0.2 | 0.005 | <0.001 | <0.001 |
| PHQ score≥10, %±SEP | 9.4±0.9 | __ | __ | 10.8±1.0 | __ | __ | 0.18 | __ | __ |
| Folate, B-12 and tHcy, mean±SEM | |||||||||
| Folate statusc, ng/mL | 12.2±0.3 | 11.9±0.8 | 12.1±0.3 | 14.7±0.4 | 11.2±0.5 | 14.3±0.4 | <0.001 | 0.99 | <0.001 |
| Vitamin B-12 statusc, pg/mL | 507.6±10.4 | 472.0±31.2 | 504.3±10.0 | 530.1±10.9 | 502.4±24.7 | 527.1±10.2 | 0.04 | 0.95 | 0.55 |
| Total homocysteinec level, μmol/L | 8.8±0.1 | 10.1±0.4 | 9.0±0.1 | 7.6±0.2 | 8.0±0.4 | 7.7±0.2 | <0.001 | 0.007 | 0.42 |
| Socio-demographic, lifestyle factors | |||||||||
| Age (y), %±SEP | 0.3231 | 0.5394 | 0.1743 | ||||||
| 20–29 | 22.5±2.0 | 17.5±4.8 | 22.0±1.7 | 18.4±1.3 | 21.4±4.0 | 18.7±1.4 | |||
| 30–39 | 22.2±1.8 | 17.9±4.1 | 21.9±1.7 | 20.6±1.7 | 18.7±3.0 | 20.4±1.7 | |||
| 40–49 | 20.9±1.9 | 20.0±6.1 | 20.8±1.8 | 19.3±2.0 | 28.8±6.1 | 20.3±2.0 | |||
| 50–59 | 15.5±1.6 | 23.3±4.0 | 16.2±1.5 | 18.6±1.7 | 16.2±3.4 | 18.3±1.5 | |||
| 60–69 | 9.2±1.0 | 12.2±5.5 | 9.5±1.2 | 10.6±1.0 | 7.6±3.0 | 10.3±0.8 | |||
| 70+ | 9.6±1.3 | 9.1±2.9 | 9.6±1.2 | 12.6±1.5 | 7.3±2.3 | 12.0±1.5 | |||
| mean±SEM | 44.6±0.8 | 47.4±2.3 | 44.9±0.8 | 47.3±0.9 | 44.9±1.7 | 47.1±0.9 | 0.008 | 0.21 | 0.26 |
| Race/ethnicity, %±SEP | |||||||||
| NH White | 75.0±2.8 | 70.0±4.9 | 74.6±2.8 | 73.3±2.9 | 64.9±5.0 | 72.4±3.0 | 0.047 | 0.44 | 0.09 |
| NH black | 9.1±1.5 | 13.7±3.1 | 9.6±1.2 | 11.1±2.1 | 16.4±3.8 | 11.7±2.2 | |||
| MA | 8.6±1.6 | 7.6±2.5 | 8.5±1.5 | 6.8±0.9 | 8.4±1.9 | 7.0±0.9 | |||
| Other ethnicity | 7.3±1.4 | 8.6±3.1 | 7.4±1.2 | 8.8±1.6 | 10.3±2.7 | 8.9±1.5 | |||
| Married, %±SEP | 58.8±1.8 | 47.0±5.0 | 57.7±1.9 | 54.9±2.0 | 45.2±4.5 | 53.9±2.0 | 0.10 | 0.02 | 0.04 |
| Education, %±SEP | |||||||||
| <High School | 6.4±0.7 | 8.5±2.5 | 6.6±0.7 | 4.7±0.7 | 7.9±2.2 | 5.1±0.7 | 0.02 | 0.005 | <0.001 |
| High School | 36.6±2.1 | 53.2±4.7 | 38.2±2.1 | 33.3±1.8 | 50.6±3.1 | 35.2±1.6 | |||
| >High School | 56.9±2.2 | 38.2±5.6 | 55.2±2.3 | 61.9±2.0 | 41.5±3.5 | 59.7±1.9 | |||
| Poverty Income Ratio, %±SEP | |||||||||
| <100% | 9.9±1.2 | 23.8±3.0 | 11.2±1.2 | 10.9±1.1 | 26.2±3.8 | 12.6±1.1 | 0.13 | <0.001 | <0.001 |
| 100%–<200% | 18.1±1.6 | 25.1±4.6 | 18.8±1.5 | 20.2±1.7 | 26.6±3.7 | 20.9±1.7 | |||
| ≥200% | 71.9±2.0 | 51.1±4.9 | 70.0±2.0 | 68.9±2.2 | 47.1±4.4 | 66.6±2.2 | |||
| Smoking status, %±SEP | |||||||||
| Never smoker | 41.3±2.0 | 26.2±7.3 | 39.9±2.1 | 56.1±1.6 | 47.0±4.3 | 55.1±1.6 | <0.001 | 0.09 | 0.002 |
| Former smoker | 28.0±1.5 | 31.7±5.2 | 28.3±1.6 | 23.4±1.5 | 17.0±4.4 | 22.7±1.6 | |||
| Current smoker | 30.7±1.9 | 42.1±6.8 | 31.8±1.9 | 20.4±1.7 | 36.0±3.6 | 22.1±1.7 | |||
| Physical activity, Mets.hr.wk−1, mean±SEM | 8.6±0.8 | 4.5±0.8 | 8.2±0.7 | 6.2±0.4 | 4.1±0.7 | 5.9±0.4 | <0.001 | 0.03 | 0.03 |
| Body mass index, kg.m−2 mean±SEM | 28.8±0.3 | 29.5±1.2 | 28.9±0.3 | 28.7±0.4 | 30.9±1.0 | 28.9±0.4 | 0.03 | 0.31 | 0.02 |
| Dietary Intake, mean±SEM | |||||||||
| Total Energy Intake, kcal/d | 2770.5±60.2 | 2595.7±146.1 | 2754.1±62.6 | 1830.0±25.5 | 1740.3±80.4 | 1820.3±29.6 | <0.001 | 0.39 | 0.61 |
| Alcohol intake, g/d | 15.0±1.4 | 20.1±5.9 | 15.5±1.3 | 5.6±0.8 | 3.5±1.0 | 5.4±0.7 | <0.001 | 0.84 | 0.42 |
| Caffeine intake, mg/d | 217.2±11.3 | 253.2±22.5 | 220.8±10.1 | 160.4±5.9 | 167.3±14.9 | 161.2±5.8 | <0.001 | 0.24 | 0.42 |
| Fiber, g/d | 17.8±0.4 | 16.6±1.1 | 17.7±0.4 | 14.6±0.3 | 11.8±0.6 | 14.3±0.3 | <0.001 | 0.22 | <0.001 |
| β-carotene, μg/d | 2198.9±137.7 | 1430.6±158.8 | 2124.4±121.3 | 2302.7±92.6 | 1241.8±200.7 | 2187.7±93.0 | 0.99 | 0.21 | <0.001 |
| vitamin C, mg/d | 94.38±3.47 | 90.7±10.0 | 94.0±2.8 | 81.6±2.3 | 70.3±6.2 | 80.4±2.1 | 0.03 | 0.87 | 0.04 |
| vitamin E, mg/d | 8.5±0.2 | 7.0±0.5 | 8.3±0.2 | 6.7±0.1 | 5.7±0.2 | 6.6±0.1 | <0.001 | 0.087 | 0.009 |
| vitamin B-6, mg/d | 2.4±0.0 | 2.2±0.1 | 2.4±0.0 | 1.7±0.0 | 1.5±0.1 | 1.7±0.0 | <0.001 | 0.042 | 0.004 |
| n-3 HUFA, g/d | 0.20±0.01 | 0.12±0.03 | 0.19±0.01 | 0.14±0.01 | 0.08±0.01 | 0.14±0.01 | <0.001 | 0.118 | 0.033 |
Abbreviations: HUFA=Highly Unsaturated fatty acids; MA=Mexican American; Met=Metabolic equivalents; NH=Non-Hispanic; NHANES=National Health and Nutrition Examination Survey; PHQ=Patient Health Questionnaire; SEM=Standard error of the mean; SEP=Standard Error of the Proportion; tHcy=total homocysteine.
Values are mean±SEM or percent±SEP. Sampling design complexity is taken into account in all analyses.
P-value was based on t-test when row variable is continuous and design-based χ2 test when row variable is categorical.
1 ng/mL of folate equivalent to 2.266 nmol/L. 1 pg/mL of vitamin B-12 is 1.355 pmol/L. Outliars were removed from this univariate analysis and defined by cutpoints of 50, 2000 and 50 for folate, B-12 and tHcy. Less than 1% of the eligible sample was dropped in each case.
Associations between serum folate, vitamin B-12 and tHcy
The three exposures of interest (serum folate, vitamin B-12 and tHcy) were weakly-to-moderately correlated with each other. In particular, a positive association was found between serum folate and vitamin B-12 levels (r=0.28, p<0.001); (data not shown).
Associations of serum folate, vitamin B-12 and tHcy with socio-demographic, lifestyle and health-related factors: OLS multiple regression analysis
Table 2 shows findings from OLS multiple regression analysis with outcome variables being folate, vitamin B-12 and tHcy serum levels as predicted by various socio-demographic, lifestyle, health-related and dietary factors. Higher folate status was related to older age, female gender, “married” status, a higher level of physical activity and higher intakes of fiber, vitamin-C and vitamin B-6. Lower folate status was observed among Non-Hispanic blacks and Mexican-Americans compared to Non-Hispanic whites, current smokers compared to non-smokers, and with higher BMI, energy, caffeine and n-3 highly unsaturated fatty acids intakes. Serum B-12 level was directly associated with age, significantly higher among Non-Hispanic blacks versus Non-Hispanic whites, inversely related to BMI but positively related to vitamin B-6 intake. Finally, tHcy was positively associated with age, lower among women, lower in Mexican-Americans and “other ethnicity” compared to Non-Hispanic whites, higher among current smokers, and inversely related to both fiber and vitamin B-6 intakes.
TABLE 2.
Socio-demographic, lifestyle and dietary predictors of serum folate, vitamin B-12 and total homocysteine among NHANES 2005–06 participants: OLS multiple regression modelsa
|
MODEL 1: Serum folate, ng/mL |
MODEL 2: Serum vitamin B-12, pg/mL |
MODEL 3: Serum total homoycysteine, μmol/L |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=2,197) | Men (n=920) | Women (n=1,277) | All (n=2,200) | Men (n=924) | Women (n=1,276) | All (n=2,214) | Men (n=925) | Women (n=1,289) | |
| Age (y) | +0.12±0.01b | +0.08±0.02b | +0.14±0.10 | +1.39±0.29b | −0.21±0.60 | +2.68±0.49 b | +0.08±0.01b | +0.08±0.01b | +0.08±0.01b |
| Sex, Women vs. Men | +1.96±0.36b | __ | __ | +25.7±17.3 | __ | __ | −1.56±0.17b | __ | __ |
| Race/ethnicity | |||||||||
| NH White | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| NH black | −2.13±0.35b | −2.22±0.50b | −2.11±0.46b | +90.3±20.6b | +28.8±28.6 | +135.5±22.6 | +0.21±0.16 | +0.11±0.25 | +0.31±0.20 |
| MA | −1.90±0.47b | −2.12±0.52b | −2.00±0.57b | +17.7±21.2 | −9.9±28.6 | +37.6±22.7 | −0.40±0.16b | −0.05±0.20 | −0.62±0.25b |
| Other ethnicity | −0.60±0.70 | −0.69±0.89 | −0.47±0.66 | +26.7±24.6 | −13.8±36.4 | +68.8±38.2 | −0.87±0.14b | −0.48±0.29 | −1.23±0.21b |
| Married | +0.90±0.40b | +0.75±0.35 | 1.27±0.53b | +34.3±9.6 | +9.6±14.2 | +67.3±13.8 | −0.39±0.12b | −0.41±0.17b | −0.44±0.18b |
| Education | |||||||||
| <High School | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| High School | +0.66±0.60 | +0.47±0.88 | +1.04±0.73 | +11.2±24.6 | +13.2±25.4 | +23.2±32.1 | +0.19±0.21 | +0.02±0.28 | 0.02±0.31 |
| >High School | +1.07±0.64 | +0.82±1.00 | +1.54±0.61b | +13.9±32.8 | +1.25±27.8 | +36.7±40.7 | +0.30±0.28 | +0.44±0.35 | −0.05±0.36 |
| Poverty Income Ratio | |||||||||
| <100% | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 100%–<200% | +0.21±0.40 | +0.26±0.60 | +0.10±0.65 | −3.94±14.8 | 26.8±17.6 | −26.7±22.9 | −0.35±0.26 | −0.61±0.43 | −0.11±0.29 |
| ≥200% | +0.47±0.44 | +0.52±0.37 | +0.30±0.80 | −4.69±14.3 | 20.2±12.3 | −34.2±27.1 | −0.39±0.23 | −0.63±0.35 | −0.12±0.35 |
| Smoking status | |||||||||
| Never smoker | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Former smoker | +0.06±0.47 | +0.42±0.49 | +0.05±0.70 | +18.6±18.7 | −8.31±24.9 | 61.3±23.7b | −0.16±0.10 | +0.02±0.25 | −0.34±0.12b |
| Current smoker | −1.31±0.52b | −0.77±0.57 | −1.49±0.73 | −38.8±22.0 | −30.6±24.1 | −33.6±30.8 | +0.65±0.24b | +0.02±0.22 | +1.20±0.45b |
| Physical activity, Mets.hr−1.wk−1 | +0.02±0.01b | 0.01±0.01 | +0.03±0.02 | −0.06±0.34 | −0.35±0.33 | 0.35±0.82 | −0.01±0.01 | −0.00±0.01 | −0.01±0.01 |
| Body mass index, kg.m−2 | −0.08±0.02b | −0.05±0.04 | −0.10±0.02b | −3.24±1.12b | −2.50±2.02 | −3.81±1.07b | −0.01±0.01 | −0.00±0.01 | −0.02±0.01 |
| Total Energy Intake, per 100 kcal/d | −0.08±0.01b | −0.09±0.01b | −0.06±0.02b | +0.86±0.87 | 1.00±0.78 | −22.4±13.2 | +0.01±0.12 | −0.03±0.09 | 0.05±0.24 |
| Alcohol intake, per 10 g/d | −0.04±0.08 | −0.01±0.08 | −0.11±0.17 | −3.84±2.55 | −3.57±2.71 | −0.85±0.43b | +0.11±0.06 | +0.02±0.01b | 0.01±0.10 |
| Caffeine intake, per 10 mg/d | −0.03±0.00b | −0.02±0.01b | −0.04±0.01b | −0.29±0.43 | −0.28±0.37 | −0.58±0.88 | +0.00±0.00 | +0.00±0.00 | 0.01±0.01 |
| Fiber, g/d | +0.07±0.02b | 0.09±0.04b | +0.04±0.02b | +0.43±1.00 | −1.62±1.24 | +3.28±1.67 | −0.05±0.01b | −0.06±0.02b | −0.05±0.02 |
| β-carotene, per 1000 μg/d | −0.07±0.07 | −0.11±0.07 | −0.04±0.12 | −0.03±2.45 | 0.70±4.68 | −0.86±3.90 | 0.00±0.00 | 0.01±0.03 | +0.00±0.00 |
| vitamin C, per 10 mg/d | +0.04±0.01b | +0.02±0.02 | +0.07±0.03b | +1.03±1.14 | +1.33±1.71 | +6.30±1.75 | +0.00±0.00 | +0.01±0.01 | −0.01±0.01 |
| vitamin E, mg/d | −0.02±0.04 | 0.01±0.06 | −0.06±0.07 | +0.86±1.92 | +5.59±3.45 | −2.03±3.84 | +0.03±0.03 | +0.05±0.04 | +0.02±0.03 |
| vitamin B-6, mg/d | 0.93±0.19b | +0.43±0.26 | +1.46±0.48b | +22.0±8.29b | +11.4±10.8 | +29.0±9.43b | −0.20±0.07b | −0.09±0.14 | −0.28±0.07b |
| n-3 HUFA, g/d | −0.72±0.30b | −0.47±0.29 | −1.14±0.61 | +10.3±24.9 | −6.83±26.36 | +26.6±28.0 | −0.02±0.17 | −0.09±0.20 | +0.29±0.39 |
Abbreviations: HUFA=Highly Unsaturated Fatty acids; Kcal=kilocalories; MA=Mexican-American; Met=Metabolic equivalents; NH=Non-Hispanic; NHANES=National Health and Nutrition Examination Survey; OLS=Ordinary Least Square; Ref=Referent category; SEE=Standard error of the estimate.
Values are β±SEE. Sampling design complexity is taken into account in all analyses. All models were multivariate-adjusted for all variables included in this table.
P<0.05 for null hypothesis that β=0 based on Wald test.
Associations of PHQ score (binary and continuous) with serum folate, vitamin B-12 and tHcy tertiles: logistic and zero-inflated Poisson multiple regression analysis
Table 3 shows estimated associations between serum folate, vitamin B-12, total homocysteine levels and PHQ score (both binary and continuous), using a series of multiple logistic and zero-inflated Poisson regression models, in the total population and stratified by sex. In both socio-demographic (Model 1) and fully-adjusted (Model 2) models, there was an inverse association between serum folate and depressive symptoms in the total population and among women. In the total population, [Model 2 adjusted odds ratio (T3 vs. T1) = 0.52 (95% CI = 0.35–0.76)] indicated that the odds of elevated depressive symptoms in the upper tertile of serum folate was close to half that in the lowest tertile. Among women, being in the upper tertile of folate status was associated with a reduced odds of “elevated depressive symptoms” by almost one-third of those in the lowest tertile [Model 2 adjusted odds ratio (T3 vs. T1) = 0.37 (95% CI = 0.17–0.86)]. Similarly, in the fully-adjusted zero-inflated poisson regression model (Model 2), the upper tertile of serum folate was associated with a significantly lower PHQ continuous score (β=−0.17±0.07) among women compared to the lowest tertile. In separate unstratified models where folate×sex interaction was added in addition to the main effects of folate and sex as well as other covariates, there was no indication for statistically significant effect modification by sex (p>0.10 for folate×sex interaction term in both logistic and zero-inflated Poisson regression models). This was verified by observing an overlap between men and women in 95% CI of Loge(OR) and β coefficients obtained from Model 2 logistic and zero-inflated Poisson regression models, respectively. Neither of the other exposures was significantly associated with the PHQ score or with odds of having elevated depressive symptoms. Examining interactions between exposures (Appendix table 1) indicated that serum folate’s association with PHQ score (both binary and continuous) was homogeneous across levels of the other two exposures (p>0.05 for all interaction terms).
TABLE 3.
Associations between serum folate, vitamin B-12, total homocysteine levels and PHQ score: Multiple logistic and zero-inflated poisson regression modelsa
| PHQ score≥10 |
PHQ continuous score |
|||||
|---|---|---|---|---|---|---|
| All | Men | Women | All | Men | Women | |
| OR 95% CI | OR 95% CI | OR 95% CI | β±SEE | β±SEE | β±SEE | |
| MODEL 1c | (n=2,388) | (n=1,030) | (n=1,358) | (n=2,388) | (n=1,030) | (n=1,358) |
| Serum folate, ng/mL | ||||||
| T1 : 7.02±0.08 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 12.0±0.06 | 0.74(0.52; 1.06) | 0.62(0.27; 1.40) | 0.82(0.48; 1.38) | −0.03±0.04 | −0.10±0.07 | 0.01±0.07 |
| T3 : 21.1±0.29 | 0.43(0.27; 0.69) b | 0.76(0.42; 1.39) | 0.31(0.13; 0.72) b | −0.20±0.06b | −0.14±0.07b | −0.22±0.09b |
| Serum vitamin B-12, pg/mL | ||||||
| T1 : 289.2±3.0 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 467.1±2.5 | 0.88(0.54; 1.41) | 1.03(0.47; 2.25) | 0.80(0.38; 1.66) | −0.07±0.07 | 0.02±0.10 | −0.13±0.11 |
| T3 : 807.2±10.1 | 0.97(0.61; 1.57) | 0.95(0.36; 2.53) | 1.03(0.52; 2.02) | +0.00±0.07 | 0.08±0.12 | −0.05±0.11 |
| Serum total homocysteine, μmol/L | ||||||
| T1 : 5.5±0.04 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 7.6±0.03 | 0.92(0.57; 1.48) | 1.12(0.52; 2.43) | 0.93(0.52; 1.69) | −0.06±0.07 | 0.04±0.08 | −0.10±0.08 |
| T3 : 11.5±0.2 | 1.11(0.64; 1.90) | 1.59(0.55; 4.60) | 0.95(0.49; 1.81) | +0.01±0.09 | 0.07±0.13 | −0.00±0.10 |
| MODEL 2d | (n=2,178) | (n=918) | (n=1,260) | (n=2,178) | (n=918) | (n=1,260) |
| Serum folate, ng/mL | ||||||
| T1 : 7.02±0.08 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 12.0±0.06 | 0.79(0.55; 1.12) | 0.55(0.24; 1.25) | 0.94(0.52; 1.69) | −0.00±0.04 | −0.08±0.10 | 0.05±0.08 |
| T3 : 21.1±0.29 | 0.52(0.35; 0.76) b | 0.85(0.41; 1.74) | 0.37(0.17; 0.80) b | −0.13±0.05b | −0.06±0.09 | −0.17±0.07b |
| Serum vitamin B-12, pg/mL | ||||||
| T1 : 289.2±3.0 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 467.1±2.5 | 0.92(0.56; 1.53) | 0.95(0.46; 1.96) | 0.86(0.38; 1.93) | −0.07±0.08 | 0.00±0.10 | −0.13±0.10 |
| T3 : 807.2±10.1 | 1.09(0.71; 1.69) | 1.04(0.37; 2.87) | 1.15(0.63; 2.10) | +0.03±0.06 | 0.08±0.12 | −0.02±0.10 |
| Serum total homocysteine, μmol/L | ||||||
| T1 : 5.5±0.04 | 1.00 | 1.00 | 1.00 | Ref | Ref | Ref |
| T2 : 7.6±0.03 | 1.00(0.56; 1.70) | 1.33(0.64; 2.77) | 0.97(0.46; 2.06) | −0.06±0.07 | 0.08±0.08 | −0.12±0.07 |
| T3 : 11.5±0.2 | 1.15(0.65; 2.03) | 1.74(0.51; 5.89) | 1.02(0.51; 1.02) | +0.01±0.08 | 0.10±0.16 | −0.00±0.09 |
Abbreviations: CI=confidence interval; Met=Metabolic Equivalent; NHANES=National Health and Nutrition Examination Survey; OR=odds ratio; Ref=Referent category; SEE=Standard error of the estimate; T=tertile.
Values are odds ratios with 95% confidence intervals or β±SEE. Sampling design complexity is taken into account in all analyses.
P<0.05 for null hypothesis that β=0 or Loge(OR)=0 based on Wald test.
Model 1 included all three exposures simultaneously and adjusted only for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and poverty income ratio.
Model 2 is model 1 but additionally adjusted for other potential confounders: Lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”) and dietary intakes (total energy intake (as is), alcohol, caffeine, vitamin B-6, β-carotene, vitamin C, vitamin E and n-3 HUFA intakes, expressed as tertiles).
Appendix Table 1.
Associations between serum folate, vitamin B-12, total homocysteine levels and PHQ score: Multiple logistic and zero-inflated poisson regression models (exposures expressed as 1=upper tertile; 0=lower or middle tertile)a
| PHQ score ≥ 10 |
PHQ continuous score |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n=2,178) | Men (n=918) | Women (n=1,260) | All (n=2,178) | Men (n=918) | Women (n=1,260) | ||||
| Loge (OR) | 95% CI | Loge (OR) | 95% CI | Loge (OR) | 95% CI | β±SEE | β±SEE | β±SEE | |
| Serum folate (FOL) | −0.84b | (−1.47; −0.20) | 0.27 | (−0.81; 1.36) | −1.33b | (−2.28; −0.40) | −0.16±0.06b | 0.04±0.13 | −0.23±0.07b |
| Serum vitamin B-12 (B-12) | 0.08 | (−0.34; 0.50) | 0.27 | (−1.07; 1.62) | 0.02 | (−0.56; 0.61) | 0.06±0.06 | 0.04±0.14 | 0.06±0.11 |
| Serum total homocysteine (tHcy) | 0.10 | (−0.51; 0.70) | 0.58 | (−0.60; 1.76) | −0.22 | (−0.84; 0.41) | 0.03±0.10 | 0.03±0.15 | 0.03±0.08 |
| FOL×B-12 | 0.27 | (−1.03; 1.56) | −0.34 | (−1.70; 1.01) | 0.44 | (−1.20; 2.09) | 0.02±0.14 | −0.07±0.16 | 0.02±0.15 |
| FOL× tHcy | 0.56 | (−0.34; 1.47) | −0.45 | (−1.67; 0.77) | 1.03 | (−0.38; 2.45) | 0.11±0.08 | −0.14±0.13 | 0.21±0.15 |
| B-12×tHcy | −0.09 | (−0.59; 0.42) | −0.74 | (−2.14; 0.66) | 0.90 | (−0.18; 1.99) | 0.08±0.10 | 0.04±0.15 | 0.22±0.16 |
| FOL×B-12× tHcy | −0.08 | (−1.57; 1.41) | 1.32 | (−1.30; 3.94) | −1.42 | (−3.30; 0.45) | −0.16±0.20 | 0.27±0.36 | −0.49±0.28 |
Abbreviations: B-12=Serum vitamin B-12; CI=confidence interval; FOL=Serum folate; NHANES=National Health and Nutrition Examination Survey;
OR=odds ratio; SEE=Standard error of the estimate; T=tertile; tHcy=Total homocysteine level.
Values are Loge of odds ratios with 95% confidence intervals or β±SEE. Means for upper tertile of serum folate, vitamin B-12 and tHcy were 21.1, 807.2, and 11.5, respectively. Sampling design complexity is taken into account in all analyses.
P<0.05 for null hypothesis that β=0 or Loge(OR)=0 based on Wald test.
Models adjusted for socio-demographic factors: age, sex, race/ethnicity, marital status, educational level and poverty income ratio, lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”) and dietary intakes (total energy intake, alcohol, caffeine, vitamin B-6, β-carotene, vitamin C, vitamin E and n-3 HUFA intakes, expressed as tertiles).
Associations of PHQ score (binary) with serum folate, vitamin B-12 and tHcy tertiles: logistic multiple regression analysis stratified by age group
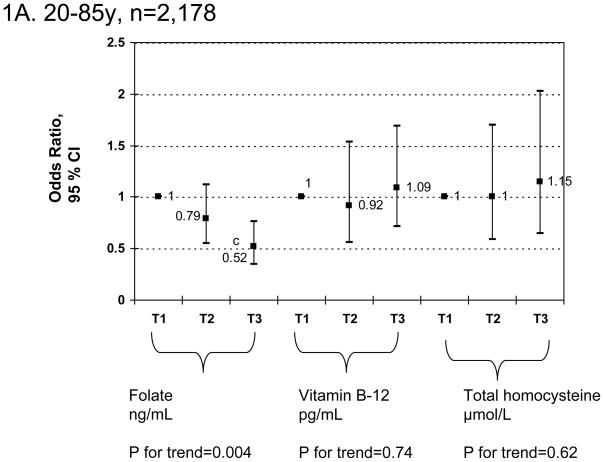
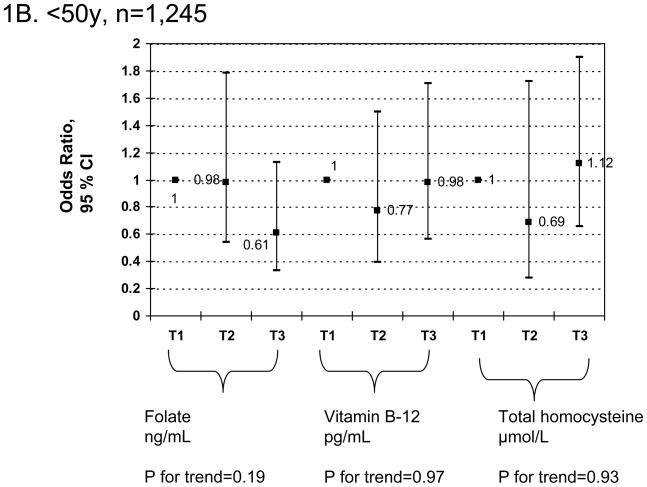
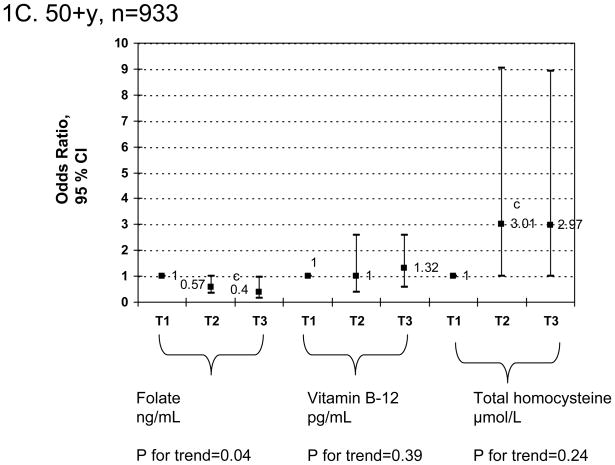
Finally, a sensitivity analysis using similar multiple logistic regression models as in Table 3 (Figures 1A–1C) but stratifying by age group (<50 vs. 50+) indicated that for both sexes combined and controlling for socio-demographic, lifestyle and dietary factors, there was only a significant linear dose-response relationship between serum folate tertiles and elevated depressive symptoms (p-value for trend=0.04) among the older adults age group (50+) and was highly significant overall (p-value for trend=0.004). Examining individual odds ratios, there was a significant positive association between tHcy tertiles [T2 vs. T1 : OR=3.01; 95%CI: 1.01–9.03] and elevated depressive symptoms also among older adults only, without a significant dose-response relationship based on p-value for trend.
Figure 1.
FIGURE 1A–C Adjusted odds ratios (95% CI) and p-values for trend of elevated depressive symptoms and folate, vitamin B-12 and total homocysteine tertiles, stratified by age group (1A: 20–85y; 1B: <50; 1C: 50+y): Multiple logistic regression modelsa,b
a Values are odds ratios with 95% confidence intervals or β±SEE. Sampling design complexity is taken into account in all analyses. See Table 3 for values of exposure tertiles.
b Models adjusted for socio-demographic factors (age, sex, race/ethnicity, marital status, educational level and poverty income ratio), lifestyle and health-related factors (smoking status, BMI, physical activity: Mets.hr.wk−1, recoded as “0–<5”; “5–10”; “>10”) and dietary intakes (total energy intake (as is), alcohol, caffeine, vitamin B-6, β-carotene, vitamin C, vitamin E and n-3 HUFA intakes, expressed as tertiles).
c P<0.05 for null hypothesis that β=0 or Loge(OR)=0 based on Wald test.
DISCUSSION
We assessed whether serum concentrations of folate, vitamin B-12 and tHcy predicted depressive symptoms among US adults (aged 20–85 years) using data from NHANES (2005–06). Overall, mean PHQ scores were significantly higher among women compared to men. Second, in multivariate models adjusting for socio-demographic, lifestyle and dietary factors, elevated depressive symptoms were inversely associated with folate status but not tHcy or vitamin B-12, and significant associations were restricted to women. Among women, odds of elevated depressive symptoms in the upper tertile of folate status was almost one-third of those in the lowest tertile. tHcy and vitamin B-12 levels did not interact with folate status to affect its inverse association with depressive symptoms among women.
Several studies previously examined associations of folate status (or folate intake) with depressive symptoms (or diagnosis of depression). Similar to our main finding in the total population, case-control studies showed that psychiatric patients with major depression had lower serum and erythrocyte levels of folate compared to controls (23, 25, 28, 32–36) and large population-based cross-sectional studies corroborated this evidence for both serum and dietary folate (27, 31, 37–38, 40–41, 46–47, 57, 69), with inconsistent effect modification by sex found in some. (27, 37–38, 46) In two US studies, however, this inverse association between folate status and depressive symptoms was mostly found among women, which was also the case in our present study. (37–38) This can either be due to sample size differences between men and women in our present study and previous ones or a real difference in the association and in serum folate’s ability to affect mental health to a greater extent among women compared to men.
A power analysis of our multiple logistic regression model was done, taking into account the following: (A) The average correlations between predictor variables (assuming R2=0.2), (B) The proportion with elevated depressive symptoms among men and women in the uppermost tertile of serum folate (6.33% among women and 10.67% among men) and (C) The observed odds ratio point estimates [Model 2, Table 3 (0.37 among women and 0.85 among men)], (D) An alpha level of 0.05 and (E) an equal balance between referent group and index group (i.e. lowest vs. uppermost tertile). A power curve was obtained based on those parameters and for a power of 0.80, the sample size needed to detect the odds ratio observed among women was n=1,084, when the actual one was n=1,358 and thus was adequate. However, among men, the minimum sample size needed for a power of 0.80 to detect the odds ratio that was observed was n=16,196 whereas the actual sample size available was only n=1,030. When R2 between predictors was reduced to 0.10, the minimal sample size was still inadequate among men. Thus, in fact, the study was under-powered among men, given the small difference in proportion “with elevated depressive symptoms” between lowest and uppermost tertiles.
Moreover, at least two cross-sectional studies were unable to detect a significant association (70–71). Among large population-based cohort studies, (26, 30, 43, 45, 47, 62, 72) an inverse association between folate status (or folate intake) and depressive symptoms (or depression) was observed in most, (26, 43, 45, 47) with inconsistent effect modification by sex found in some. (43, 47) Null findings with respect of folate-depressive symptoms associations in some of the observational studies (30, 62, 70–72) may be due to the selection of older age groups and differences in measurements of depressive symptoms, including the use of specific instruments (e.g. Geriatric Depression Scale instead of CES-D or PHQ). In particular, the proportion of somatic items relative to items addressing strictly depressed affect differs in various instruments. In this instrument (PHQ), five out of the ten items were somatic items (i.e. sleep, appetite, trouble concentrating, trouble moving/speaking and poor energy). Depressed affect was elicited using the five other items (i.e. loss of interest, feeling down, feeling bad about self, feeling better off dead, difficulty these problems have caused). In fact, when a sensitivity analysis was further conducted using binary outcomes of elevated somatic complaints sub-scale (1: score≥6; 0: score<6, cut-point corresponding to the 90th percentile) and a similar binary outcome for the depressed affect sub-scale, only somatic complaints were found to be inversely related to folate tertiles after controlling for vitamin B-12, tHcy, socio-demographic, lifestyle and dietary factors as was done in Model 2 of Table 3 in the total population [T3 vs. T1: OR=0.56; 95% CI=0.36–0.88; p=0.02] and among women only [T3 vs. T1: OR=0.52; 95% CI=0.28–0.96; p=0.04], without a significant effect modification by sex for that sub-scale.
Similar to our study, previous research did not find any association when considering tHcy (62, 73) and vitamin B-12 (40, 46, 62, 70, 72–73) levels as exposures in relation to depressive symptoms. However, a number of observational studies found that depressed individuals had higher tHcy levels (21–27) and/or lower vitamin B-12 levels. (25–26, 31), among which many were conducted in older adults. (21–23, 25–26, 31) Thus, their study population differed markedly from ours which had a wider age range (20–85 years, mean in the mid-40s) and from those who found null associations between those two exposures and depressive symptoms. Our sensitivity analysis on those aged 50 years or older demonstrated that in fact there was a statistically significant and positive association between tHcy status and elevated depressive symptoms.
Our findings suggest that serum folate level may reflect brain tissue folate, which in turn may be playing a primary role in neuroprotection with the BH4 pathway as a possible mechanism (See introduction). In fact, levels of tHcy and vitamin B-12 did not seem to play a direct or interactive role with the levels of folate in protecting against elevated depressive symptoms.
Our study has several strengths. First, to our knowledge, it is the only large nationally representative observational study to assess the associations among serum folate, vitamin B-12, tHcy and elevated depressive symptoms in U.S. adults after mandatory folic acid fortification of foods was implemented in the U.S. Second, the study examined associations stratifying by sex and assessed interactions between exposures in the main association of interest. However, our study is limited by its cross-sectional design which precludes temporality ascertainment and reverse causality is a possibility whereby folate status is indicative poor dietary quality among depressed individuals compared to their non-depressed counterparts. Nevertheless, it was observed in at least one previous cohort study that serum folate was inversely related to incident depression (26). Another limitation is the lack of control for several potential confounders such as participant’s family history of depression, social isolation or personality dimensions commonly associated with depression, in addition to the presence of chronic illness. Adjusting for those potential confounders would attenuate the observed association given their positive relationship with the outcome (elevated depressive symptoms), only if their association with serum folate was an inverse one. The use of depressive symptoms as outcome and not a clinical diagnosis of depression is also an important limitation to be considered when interpreting our findings, although the CES-D has been used for similar purposes in a number of previous studies. (37–38) Finally, residual confounding caused by measurement error in dietary and other factors including in regression models cannot be ruled out.
To ascertain temporality of those main associations, future longitudinal studies should further examine whether trajectories in folate status are associated with trajectories in depressive symptoms and whether those associations differ between men and women and across various age groups. Future interventions and randomized controlled trials for improving mental health outcomes should take into account dietary and other factors that would increase levels of serum folate, and stratification by age and sex is important, and possibly race/ethnicity, to uncover possible differences in the effects of folate status-enhancing lifestyles (including folate dietary supplementation) on various socio-demographic groups.
In conclusion, our results suggest that depressive symptoms may be associated with low serum folate status, despite folate fortification efforts, specifically for women, but not by increasing vitamin B-12 or reducing tHcy serum level. Thus even with a lower prevalence of folate deficiency in the US, serum folate level may still be an important factor to consider for the prevention of elevated depressive symptoms.
Acknowledgments
This research was supported entirely by the Intramural Research Program of the NIH, National Institute on Aging. The authors would like to thank Dr. Alyssa Gamaldo and Dr. Lori L. Beason-Held for their thoughtful comments on the manuscript.
ABBREVIATIONS
- BH4
Tetrahydro biopterins
- CES-D
Center for Epidemiological Studies-Depression
- CNS
Central Nervous System
- CSF
Cerebrospinal fluid
- DSM
Diagnostic and Statistical Manual
- MEC
Mobile Examination Center
- MR
methylation reactions
- NHANES
National Health and Nutrition Examination Surveys
- OLS
Ordinary Least Square
- PHQ
Patient Health Questionnaire
- PIR
Poverty Income Ratio
- tHcy
total homocysteine
- T
tertile
- US
United States
Footnotes
Contributions of each co-author: MAB: Conceptualization, literature search, plan of analysis, data management, statistical analysis, write-up of the manuscript, revision of the manuscript.
MRS: Literature review, write-up of parts of the manuscript, plan of analysis, revision of the manuscript.
HAB: Literature review, write-up of parts of the manuscript, plan of analysis, revision of the manuscript.
ABZ: Plan of analysis, revision of the manuscript.
Disclosure: (a) Sources of funding: This study was entirely supported by the National Institute on Aging, Intramural Research Program (NIA/NIH/IRP). (b) May A. Beydoun: “No conflict of interest”; Monal R. Shroff: “No conflict of interest”; Hind A. Beydoun: “No conflict of interest”; Alan B. Zonderman: “No conflict of interest”.
References
- 1.Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 2.Bottiglieri T. Folate, vitamin B12, and neuropsychiatric disorders. Nutr Rev. 1996;54:382–90. doi: 10.1111/j.1753-4887.1996.tb03851.x. [DOI] [PubMed] [Google Scholar]
- 3.Bottiglieri T. Homocysteine and folate metabolism in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1103–12. doi: 10.1016/j.pnpbp.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Community Health. 2007;61:631–7. doi: 10.1136/jech.2006.050385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppen A, Bailey J. Enhancement of the antidepressant action of fluoxetine by folic acid: a randomised, placebo controlled trial. J Affect Disord. 2000;60:121–30. doi: 10.1016/s0165-0327(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 6.Alpert M, Silva RR, Pouget ER. Prediction of treatment response in geriatric depression from baseline folate level: interaction with an SSRI or a tricyclic antidepressant. J Clin Psychopharmacol. 2003;23:309–13. doi: 10.1097/01.jcp.0000084024.22282.cd. [DOI] [PubMed] [Google Scholar]
- 7.Chanarin I, editor. The Megaloblastic Anaemias. 2. Oxford: Blackwell Scientific; 1979. [Google Scholar]
- 8.Paul RT, McDonnell AP, Kelly CB. Folic acid: neurochemistry, metabolism and relationship to depression. Hum Psychopharmacol. 2004;19:477–88. doi: 10.1002/hup.614. [DOI] [PubMed] [Google Scholar]
- 9.Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the Rate-Limiting Step in Norepinephrine Biosynthesis in the Perfused Guinea-Pig Heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- 10.Coppen A, Swade C, Jones SA, Armstrong RA, Blair JA, Leeming RJ. Depression and tetrahydrobiopterin: the folate connection. J Affect Disord. 1989;16:103–7. doi: 10.1016/0165-0327(89)90062-1. [DOI] [PubMed] [Google Scholar]
- 11.Anderson DN, Abou-Saleh MT, Collins J, Hughes K, Cattell RJ, Hamon CG, Blair JA, Dewey ME. Pterin metabolism in depression: an extension of the amine hypothesis and possible marker of response to ECT. Psychol Med. 1992;22:863–9. doi: 10.1017/s0033291700038435. [DOI] [PubMed] [Google Scholar]
- 12.Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. J Neurosci Res. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg G, Colla M, Endres M. Folic acid, neurodegenerative and neuropsychiatric disease. Curr Mol Med. 2009;9:315–23. doi: 10.2174/156652409787847146. [DOI] [PubMed] [Google Scholar]
- 14.Scott TM, Tucker KL, Bhadelia A, Benjamin B, Patz S, Bhadelia R, Liebson E, Price LL, Griffith J, Rosenberg I, Folstein MF. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12:631–8. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- 15.Dufouil C, Alperovitch A, Ducros V, Tzourio C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol. 2003;53:214–21. doi: 10.1002/ana.10440. [DOI] [PubMed] [Google Scholar]
- 16.Sachdev PS, Valenzuela M, Wang XL, Looi JC, Brodaty H. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology. 2002;58:1539–41. doi: 10.1212/wnl.58.10.1539. [DOI] [PubMed] [Google Scholar]
- 17.Bleich S, Kornhuber J. Relationship between plasma homocysteine levels and brain atrophy in healthy elderly individuals. Neurology. 2003;60:1220. doi: 10.1212/wnl.60.7.1220. author reply. [DOI] [PubMed] [Google Scholar]
- 18.den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain. 2003;126:170–5. doi: 10.1093/brain/awg006. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann W, Obeid R. Biomarkers of folate and vitamin B(12) status in cerebrospinal fluid. Clin Chem Lab Med. 2007;45:1614–20. doi: 10.1515/CCLM.2007.310. [DOI] [PubMed] [Google Scholar]
- 20.Mischoulon D, Fava M. Role of S-adenosyl-L-methionine in the treatment of depression: a review of the evidence. Am J Clin Nutr. 2002;76:1158S–61S. doi: 10.1093/ajcn/76/5.1158S. [DOI] [PubMed] [Google Scholar]
- 21.Forti P, Rietti E, Pisacane N, Olivelli V, Dalmonte E, Mecocci P, Ravaglia G. Blood homocysteine and risk of depression in the elderly. Arch Gerontol Geriatr. 2009 doi: 10.1016/j.archger.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Almeida OP, McCaul K, Hankey GJ, Norman P, Jamrozik K, Flicker L. Homocysteine and depression in later life. Arch Gen Psychiatry. 2008;65:1286–94. doi: 10.1001/archpsyc.65.11.1286. [DOI] [PubMed] [Google Scholar]
- 23.Tiemeier H, van Tuijl HR, Hofman A, Meijer J, Kiliaan AJ, Breteler MM. Vitamin B12, folate, and homocysteine in depression: the Rotterdam Study. Am J Psychiatry. 2002;159:2099–101. doi: 10.1176/appi.ajp.159.12.2099. [DOI] [PubMed] [Google Scholar]
- 24.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69:228–32. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimopoulos N, Piperi C, Salonicioti A, Psarra V, Gazi F, Papadimitriou A, Lea RW, Kalofoutis A. Correlation of folate, vitamin B12 and homocysteine plasma levels with depression in an elderly Greek population. Clin Biochem. 2007;40:604–8. doi: 10.1016/j.clinbiochem.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS. Predictive value of folate, vitamin B12 and homocysteine levels in late-life depression. Br J Psychiatry. 2008;192:268–74. doi: 10.1192/bjp.bp.107.039511. [DOI] [PubMed] [Google Scholar]
- 27.Nanri A, Mizoue T, Matsushita Y, Sasaki S, Ohta M, Sato M, Mishima N. Serum folate and homocysteine and depressive symptoms among Japanese men and women. Eur J Clin Nutr. 2010 doi: 10.1038/ejcn.2009.143. [DOI] [PubMed] [Google Scholar]
- 28.Abou-Saleh MT, Coppen A. Serum and red blood cell folate in depression. Acta Psychiatr Scand. 1989;80:78–82. doi: 10.1111/j.1600-0447.1989.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 29.Fava M, Borus JS, Alpert JE, Nierenberg AA, Rosenbaum JF, Bottiglieri T. Folate, vitamin B12, and homocysteine in major depressive disorder. Am J Psychiatry. 1997;154:426–8. doi: 10.1176/ajp.154.3.426. [DOI] [PubMed] [Google Scholar]
- 30.Kendrick T, Dunn N, Robinson S, Oestmann A, Godfrey K, Cooper C, Inskip H. A longitudinal study of blood folate levels and depressive symptoms among young women in the Southampton Women’s Survey. J Epidemiol Community Health. 2008;62:966–72. doi: 10.1136/jech.2007.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ng TP, Feng L, Niti M, Kua EH, Yap KB. Folate, vitamin B12, homocysteine, and depressive symptoms in a population sample of older Chinese adults. J Am Geriatr Soc. 2009;57:871–6. doi: 10.1111/j.1532-5415.2009.02229.x. [DOI] [PubMed] [Google Scholar]
- 32.Carney MW, Chary TK, Laundy M, Bottiglieri T, Chanarin I, Reynolds EH, Toone B. Red cell folate concentrations in psychiatric patients. J Affect Disord. 1990;19:207–13. doi: 10.1016/0165-0327(90)90093-n. [DOI] [PubMed] [Google Scholar]
- 33.Carney MW, Sheffield BF. Serum folic acid and B12 in 272 psychiatric in-patients. Psychol Med. 1978;8:139–44. doi: 10.1017/s0033291700006711. [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Wing YK, Fong S. A controlled study of folate levels in Chinese inpatients with major depression in Hong Kong. J Affect Disord. 1998;49:73–7. doi: 10.1016/s0165-0327(97)00200-0. [DOI] [PubMed] [Google Scholar]
- 35.Lerner V, Kanevsky M, Dwolatzky T, Rouach T, Kamin R, Miodownik C. Vitamin B12 and folate serum levels in newly admitted psychiatric patients. Clin Nutr. 2006;25:60–7. doi: 10.1016/j.clnu.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Tiemeier H, Fekkes D, Hofman A, van Tuijl HR, Kiliaan AJ, Breteler MM. Plasma pterins and folate in late life depression: the Rotterdam Study. Psychiatry Res. 2006;145:199–206. doi: 10.1016/j.psychres.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Ramos MI, Allen LH, Haan MN, Green R, Miller JW. Plasma folate concentrations are associated with depressive symptoms in elderly Latina women despite folic acid fortification. Am J Clin Nutr. 2004;80:1024–8. doi: 10.1093/ajcn/80.4.1024. [DOI] [PubMed] [Google Scholar]
- 38.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, Shroff MR, Mason MA, Evans MK, Zonderman AB. The sex-specific role of plasma folate in mediating the association of dietary quality with depressive symptoms. J Nutr. 2010;140:338–47. doi: 10.3945/jn.109.113878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris DW, Trivedi MH, Rush AJ. Folate and unipolar depression. J Altern Complement Med. 2008;14:277–85. doi: 10.1089/acm.2007.0663. [DOI] [PubMed] [Google Scholar]
- 40.Sachdev PS, Parslow RA, Lux O, Salonikas C, Wen W, Naidoo D, Christensen H, Jorm AF. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–38. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- 41.Bjelland I, Tell GS, Vollset SE, Refsum H, Ueland PM. Folate, vitamin B12, homocysteine, and the MTHFR 677C->T polymorphism in anxiety and depression: the Hordaland Homocysteine Study. Arch Gen Psychiatry. 2003;60:618–26. doi: 10.1001/archpsyc.60.6.618. [DOI] [PubMed] [Google Scholar]
- 42.Kim JM, Kim SW, Shin IS, Yang SJ, Park WY, Kim SJ, Shin HY, Yoon JS. Folate, vitamin b(12), and homocysteine as risk factors for cognitive decline in the elderly. Psychiatry Investig. 2008;5:36–40. doi: 10.4306/pi.2008.5.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astorg P, Couthouis A, de Courcy GP, Bertrais S, Arnault N, Meneton P, Galan P, Hercberg S. Association of folate intake with the occurrence of depressive episodes in middle-aged French men and women. Br J Nutr. 2008;100:183–7. doi: 10.1017/S0007114507873612. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B(6) and vitamin B(12) intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009;22:122–33. doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 45.Tolmunen T, Hintikka J, Ruusunen A, Voutilainen S, Tanskanen A, Valkonen VP, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and the risk of depression in Finnish middle-aged men. A prospective follow-up study. Psychother Psychosom. 2004;73:334–9. doi: 10.1159/000080385. [DOI] [PubMed] [Google Scholar]
- 46.Murakami K, Mizoue T, Sasaki S, Ohta M, Sato M, Matsushita Y, Mishima N. Dietary intake of folate, other B vitamins, and omega-3 polyunsaturated fatty acids in relation to depressive symptoms in Japanese adults. Nutrition. 2008;24:140–7. doi: 10.1016/j.nut.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Villegas A, Doreste J, Schlatter J, Pla J, Bes-Rastrollo M, Martinez-Gonzalez MA. Association between folate, vitamin B and vitamin B intake and depression in the SUN cohort study. J Hum Nutr Diet. 2009 doi: 10.1111/j.1365-277X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 48.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. Jama. 2006;295:1549–55. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 49.Center for Disease Control and Prevention (CDC) [Access date: September 25th, 2006];National Health and Nutrition Examination Survey. 2006 2006 http://www.cdc.gov/nchs/nhanes.htm.
- 50.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282:1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 52.Abbott Homocysteine (HCY) assay package insert fo IMX Analyzer.
- 53.Pernet P, Lasnier E, Vaubourdolle M. Evaluation of the AxSYM homocysteine assay and comparison with the IMx homocysteine assay. Clin Chem. 2000;46:1440–1. [PubMed] [Google Scholar]
- 54.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 55.Ueland PM, Refsum H, Stabler SP, Malinow MR, Andersson A, Allen RH. Total homocysteine in plasma or serum: methods and clinical applications. Clin Chem. 1993;39:1764–79. [PubMed] [Google Scholar]
- 56.Pfeiffer CM, Huff DL, Smith SJ, Miller DT, Gunter EW. Comparison of plasma total homocysteine measurements in 14 laboratories: an international study. Clin Chem. 1999;45:1261–8. [PubMed] [Google Scholar]
- 57.Morris MS, Fava M, Jacques PF, Selhub J, Rosenberg IH. Depression and folate status in the US Population. Psychother Psychosom. 2003;72:80–7. doi: 10.1159/000068692. [DOI] [PubMed] [Google Scholar]
- 58.Cheng YJ, Gregg EW, De Rekeneire N, Williams DE, Imperatore G, Caspersen CJ, Kahn HS. Muscle-strengthening activity and its association with insulin sensitivity. Diabetes Care. 2007;30:2264–70. doi: 10.2337/dc07-0372. [DOI] [PubMed] [Google Scholar]
- 59.Lagerros YT, Lagiou P. Assessment of physical activity and energy expenditure in epidemiological research of chronic diseases. Eur J Epidemiol. 2007;22:353–62. doi: 10.1007/s10654-007-9154-x. [DOI] [PubMed] [Google Scholar]
- 60.McCullough ML, Feskanich D, Rimm EB, Giovannucci EL, Ascherio A, Variyam JN, Spiegelman D, Stampfer MJ, Willett WC. Adherence to the Dietary Guidelines for Americans and risk of major chronic disease in men. Am J Clin Nutr. 2000;72:1223–31. doi: 10.1093/ajcn/72.5.1223. [DOI] [PubMed] [Google Scholar]
- 61.United States Department of Agriculture (USDA), Agriculture Research Service FSRG. Food and Nutrient Database for Dietary Studies, 3.0. Beltsville, MD: USDA; [Accessed: March, 2008]. 2008. http://www.ars.usda.gov/Services/docs.htm?docid=17031. [Google Scholar]
- 62.Kamphuis MH, Geerlings MI, Grobbee DE, Kromhout D. Dietary intake of B(6-9-12) vitamins, serum homocysteine levels and their association with depressive symptoms: the Zutphen Elderly Study. Eur J Clin Nutr. 2008;62:939–45. doi: 10.1038/sj.ejcn.1602804. [DOI] [PubMed] [Google Scholar]
- 63.Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009;63:9–17. doi: 10.18926/AMO/31854. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Villegas A, Henriquez P, Figueiras A, Ortuno F, Lahortiga F, Martinez-Gonzalez MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. Eur J Nutr. 2007;46:337–46. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 65.Sublette ME, Hibbeln JR, Galfalvy H, Oquendo MA, Mann JJ. Omega-3 polyunsaturated essential fatty acid status as a predictor of future suicide risk. Am J Psychiatry. 2006;163:1100–2. doi: 10.1176/ajp.2006.163.6.1100. [DOI] [PubMed] [Google Scholar]
- 66.STATA. Statistics/Data Analysis: Release 10.0. Texas: Stata Corporation; 2007. [Google Scholar]
- 67.Bandiera FC, Arheart KL, Caban-Martinez AJ, Fleming LE, McCollister K, Dietz NA, Leblanc WG, Davila EP, Lewis JE, Serdar B, Lee DJ. Secondhand Smoke Exposure and Depressive Symptoms. Psychosom Med. 2009 doi: 10.1097/PSY.0b013e3181c6c8b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lohr SL. Sampling: Design and Analysis. Duxbury-Press; 1999. [Google Scholar]
- 69.Tolmunen T, Voutilainen S, Hintikka J, Rissanen T, Tanskanen A, Viinamaki H, Kaplan GA, Salonen JT. Dietary folate and depressive symptoms are associated in middle-aged Finnish men. J Nutr. 2003;133:3233–6. doi: 10.1093/jn/133.10.3233. [DOI] [PubMed] [Google Scholar]
- 70.Lindeman RD, Romero LJ, Koehler KM, Liang HC, LaRue A, Baumgartner RN, Garry PJ. Serum vitamin B12, C and folate concentrations in the New Mexico elder health survey: correlations with cognitive and affective functions. J Am Coll Nutr. 2000;19:68–76. doi: 10.1080/07315724.2000.10718916. [DOI] [PubMed] [Google Scholar]
- 71.Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B(12) deficiency and depression in physically disabled older women: epidemiologic evidence from the Women’s Health and Aging Study. Am J Psychiatry. 2000;157:715–21. doi: 10.1176/appi.ajp.157.5.715. [DOI] [PubMed] [Google Scholar]
- 72.Eussen SJ, Ferry M, Hininger I, Haller J, Matthys C, Dirren H. Five year changes in mental health and associations with vitamin B12/folate status of elderly Europeans. J Nutr Health Aging. 2002;6:43–50. [PubMed] [Google Scholar]
- 73.Nguyen PH, Grajeda R, Melgar P, Marcinkevage J, DiGirolamo AM, Flores R, Martorell R. Micronutrient supplementation may reduce symptoms of depression in Guatemalan women. Arch Latinoam Nutr. 2009;59:278–86. [PubMed] [Google Scholar]