Abstract
The purpose of this study is to investigate the effects of Lang-du extract (LDE) from Traditional Chinese Medicine (TCM) Euphorbia fischeriana Steud on the in vitro and in vivo growth of melanoma cells and its molecular mechanisms of action. Our present results have shown that LDE significantly suppressed the in vitro melanoma cell growth in dose- and time-dependent manners. LDE also displayed the synergistic effect with γ-radiation on the reduction of the cell viability in melanoma cells. The animal experimental results further confirmed that compared with the control group without drug treatment, the tumor volume in mice was significantly and time-dependently less in LDE group. The absolute weight of solid tumor in the LDE group was 7-fold lower than that in the control group. Western blot analysis indicated that LDE markedly down-regulated the expression of anti-apoptotic protein Bcl-2 and up-regulated the level of pro-apoptotic protein Bax, eventually leading the reduction of Bcl-2/Bax protein ratios both in the cultured melanoma cells and in the tumors from melanoma-bearing mice. In addition, LDE significantly reduced the tumor progression-associated protein levels of vascular endothelial growth factor (VEGF), hepatocyte growth factor/scatter factor (HGF/SF), and osteopontin (OPN) in tumors from the LDE-treated mice. Our findings suggest that LDE may have a wide therapeutic and/or adjuvant therapeutic application in the treatment of melanoma and other cancer.
Keywords: Lang-du extract, Melanoma cells, Tumor growth, Bcl-2/Bax, VEGF, HGF, OPN
Introduction
Although melanoma accounts for only four percent of all dermatologic cancers, it is responsible for 80 percent of deaths from skin cancer (Miller and Mihm 2006); metastatic stage melanoma is an aggressive disease that few patients survive for >2 years. Compounding this, scores of clinical trials testing different adjuvant therapies have brought no significant improvement in the survival outlook for these patients (Sasse et al. 2007). To find novel natural compounds with low toxicity and high selectivity of killing various cancer cells including melanoma cells is an important area in cancer research. Due to their wide range of biological activities and low toxicity in animal models, some natural products have been used as alternative treatments for cancer. Lang-du (LD) is a naturally occurring product from Traditional Chinese Medicine (TCM) Euphorbia fischeriana Steud. In China, LD has been used to treat clinical patients with cancer, edema, and ascites for many years (Wang et al. 2006). Chemical investigation of this plant revealed that it mainly contained diterpenoids, triterpenoids, and steroids (Qin and Xu 1998). Some of these compounds in the LD have shown inhibitory effects on the in vitro growth of human leukemia K562 (Luo and Wang 2006), hepatoma HepG2 (Yan et al. 2008; Wang et al. 2009a, b) and human prostate LNCaP cancer cell line (Liu et al. 2002). However, whether or not the extract from LD has inhibitory activities against melanoma growth in animal is unclear. In this study, we investigated the in vitro and in vivo effects of LD extract (LDE) on the growth of melanoma cells and its molecular mechanisms of action. We show that the LDE significantly reduced the viability of melanoma cells by inducing apoptosis and regulating the expressions of Bcl-2 and Bax proteins in dose-dependent manners. More importantly, the LDE significantly suppressed the tumor growth in melanoma-bearing mice and reduced the ratio of Bcl-2/Bax and the levels of vascular endothelial growth factor (VEGF), hepatocyte growth factor/scatter factor (HGF/SF), and osteopontin (OPN) in tumors.
Materials and methods
Materials
RPMI 1640 medium, penicillin, streptomycin, fetal bovine serum (FBS), 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT), trypan blue dye, trypsin/EDTA, and all other chemicals employed in this study were purchased from Sigma Chemical Co. (St. Louis, MO). Antibodies against Bcl-2, Bax, and β-actin were purchased from Cell Signaling Technology, Inc. (Beverley, MA). Acrylamide and the protein assay kits were obtained from Bio-Rad (Hercules, CA).
Preparation for Lang-du extract
The roots of E. fischeriana Steud were purchased from Lunan Pharmaceuticals in Linyi, Shandong Province, China. The powdered roots of E. fischeriana Steud were extracted with 88% ethanol at 50 °C under mixing. After precipitation, the cooled solution was filtered and evaporated under reduced pressure to give a residue. Then, the extract was suspended in distilled water. After precipitation with water once more, the supernatant was condensed as a Lang-du extract (LDE) for the in vitro and in vivo experiments. There are about 0.53% of jolkinolide (A, B), 1.06% of fischeriana (A, B) and 1.75% of flavonoid in LDE.
Cell culture and in vitro growth assays
The murine melanoma cell line B16 (B16M) was obtained from the American Type Culture Collection. The B16M cell line was incubated in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), glutamine (2 mM), penicillin (100 U/mL) and streptomycin (100 μg/mL) at 37 °C in a humidified incubator with 95% air/5% CO2 atmosphere. The in vitro assays were done according to our published methods (Liu et al. 2009; Huang et al. 2009; Zhang et al. 2000). The cells were cultured in RPMI 1640 medium supplemented with 10% FBS containing different concentrations of LDE. The cells in control group were treated with PBS vehicle. For detection of the effect of LDE on the in vitro growth of B16M cells, the relative cell viability was measured 24, 48, and 72 h after the treatments using MTT growth assay kit. For detection of the effect of LDE on the in vitro growth in γ-radiation-treated B16M cells, the cells were treated with γ-radiation at dose of 8 Gy in the absence or presence of LDE at 0.4 mg/mL. After the treated cells were cultured for 48 h, the relative cell viability was determined using a trypan blue dye exclusion assay as described previously (Zhang et al. 2000). Each experiment was repeated three times.
Morphological evaluation of apoptotic cells
This was done according to our published methods (Zhang et al. 2000). In brief, B16M cells at 70% confluence were, respectively treated for 48 h with LDE at concentrations of 0 (PBS vehicle as control), 0.4, and 1.2 mg/mL. The treated cells were fixed with 1% glutaraldehyde in PBS for 30 min at room temperature, washed in PBS, and stained with 1 mM Hoechst 33258 for 30 min at room temperature. The morphological changes in the nuclear chromatin were observed under a fluorescent microscope (Nikon, TE2000-U, Japan), using a 40× lens.
Animal experimentation
Female C57BL/6 J mice, 5 weeks old (from SLRC Laboratory Animal, Chinese Academy of Sciences, Shanghai, China) were given a standard laboratory diet (from the SLRC Laboratory Animal) and distilled water. The diet and water were available ad libitum. The mice were deprived of their diet at 9:00 a.m. on the day of killing, but allowed free access to water until killing, which was performed 4 h later. The mice were kept on a 12-h light/dark cycle at 22 ± 2 °C and allowed to adjust to their environment for 1 week before the initiation of experiments. The mice were injected s.c. in the left flank with 2 × 105 B16M cells in 100 μL serum-free medium. Two groups of mice (eight mice per group) were then treated i.p. with LDE (200 mg/mL/kg body weight) or with vehicle alone (PBS) every 2 days for 19 days. Animals were observed twice daily and the body weight as well as the food consumption of each mouse was weighed every 2 days. LDE treatment did not alter the weight of mice, and the body weight and consumption of food in the LDE group were not noticeably different from that in the control group without LDE treatment (vehicle alone). Tumor size was measured every 2 days with calipers, based on the formula L × W2/2 where L is the length and W is the width of the tumor (Villares et al. 2008). All mice were euthanized at day 19 after the injection of B16M cells following institutional regulation. After the mice had been killed, the solid tumor in each mouse was weighed.
Western blot analysis
This was performed according to the method published by Wang et al. (2009a, b), Zhang et al. (2009), and Yu et al. (2009). In brief, each tumor of the control group (n = 8) and LDE group (n = 8) were excised after the mice of both groups were killed. Equal amounts of protein lysates of each tumor were resolved by SDS–PAGE, transferred to nitrocellulose membranes, and probed with primary antibodies to mouse Bcl-2, Bax, and β-actin and then horseradish peroxidase-conjugated secondary antibodies, respectively. Anti-β-actin antibody was used as a loading control. Detection was done using an enhanced chemiluminescence system (GE Healthcare Life Sciences).
ELISAs for detection of protein levels of VEGF and HGF as well as OPN in tumors
This was performed according to the method published by Wang et al. (2009a, b) and Zhang et al. (2009). In brief, each tumor in the control group (n = 8) and LDE group (n = 8) were excised after the mice of both groups were killed. Equal amounts of protein lysates of each tumor were analyzed by ELISA using kits for VEGF, HGF and OPN from R&D Systems (Minneapolis, MN.). ELISAs were done according to the instructions of the manufacturer. Each experiment was repeated three times.
Statistical analysis
Statistical analysis was done using the one-way or two-way ANOVA followed by the appropriate post hoc test (Bonferroni) and Student’s t-test with the software SPPS 13.0. All statistical tests were two-sided. Values between different treatment groups were compared. For confirming the synergistic effect between LDE and γ-radiation treatment, comparison was made by two-way ANOVA followed by Bonferroni post hoc test. The relative cell viability (%), tumor volume, tumor weight, and the levels of VEGF, HGF and OPN are shown for each dose or each group; error bars indicate SD or SEM. Asterisk P < 0.05, double asterisk P < 0.01, and triple asterisk P < 0.001. For all tests, P values less than 0.05 were considered statistically significant.
Results and discussion
In vitro suppression of growth in B16M cells by LDE and its effect on γ-radiation treatment
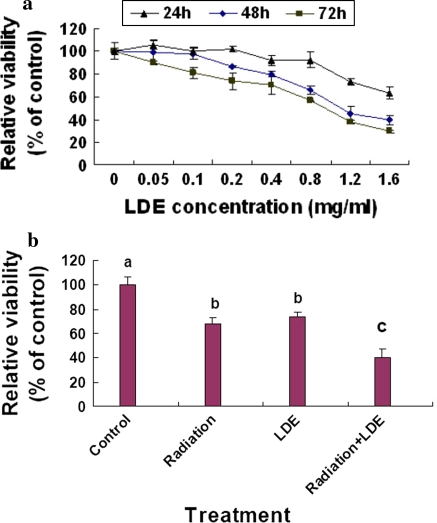
The in vitro growth assay showed that LDE suppressed the growth of B16M cells in dose- and time-dependent manners after the cells were treated with LDE at concentrations of 0.05–1.6 mg/mL for 24, 48 and 72 h, respectively. The IC50 values at 48 and 72 h were 1.0 and 0.9 mg/mL, respectively (Fig. 1a). In addition, LDE at 0.4 mg/mL enhanced the effect on the reduction of the cell viability in B16M cells treated with γ-radiation at the dose of 8 Gy (Fig. 1b). The results suggest that LDE may not only have the potential to inhibit the B16M cell growth in vivo but also have a therapeutic and/or adjuvant therapeutic application in the treatment of human melanoma and other cancer.
Fig. 1.
In vitro effects of Long-du extract (LDE) on the growth in B16M cells (a) and γ-radiation-treated B16M cells (b). a B16M cells were cultured for 24, 48, and 72 h in DMEM medium containing LDE at the concentrations indicated in figure. The effects of LDE on the cell growth were determined by MTT assay as described in the “Materials and methods” section. b B16M cells were treated with γ-radiation at dose of 8 Gy in the absence or presence of LDE at 0.4 mg/mL. After the treated cells were cultured for 48 h, the relative cell viability was determined using the trypan blue dye exclusion assay as described in the “Materials and methods” section. Each point and vertical bar represents the mean and SD for 6 wells. The figures (a, b) are the representative of three similar experiments performed. Statistical analysis was carried out using the ANOVA and Bonferroni test. All statistical tests were two-sided. Values with different letters (a–c) differ significantly (P < 0.05)
LDE induced apoptosis by reducing the Bcl-2/Bax protein ratio in B16M cells
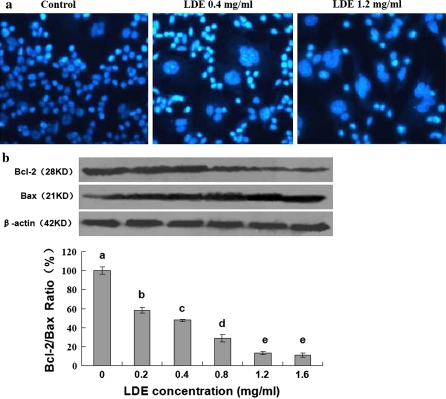
To understand the mechanisms of action of LDE against the growth in the B16M cells, we investigated the effects of LDE on apoptosis and expressions of Bcl-2/Bax proteins in the cells. Hoechst 33258 staining indicated that the typical morphological changes, such as formation of apoptotic bodies appeared in the B16M cells after the cells were treated for 48 h with LDE at 0.4 and 1.2 mg/mL, whereas the control cells without LDE treatment did not show the evident apoptotic morphological changes (Fig. 2a). Furthermore, Western blot analysis confirmed that LDE dose-dependently down-regulated the expression of anti-apoptotic protein Bcl-2 and up-regulated the level of pro-apoptotic protein Bax, eventually leading to the reduction of the Bcl-2/Bax protein ratio in the B16M cells (Fig. 2b). There have been studies showing that Bcl-2 and its dominant inhibitor Bax are key regulators of cell proliferation and apoptosis. Overexpression of Bcl-2 enhances cell survival by suppressing apoptosis, but overexpression of Bax accelerates cell death (Oltvai et al. 1993). Induction of apoptosis and decrease in the ratio of Bcl-2/Bax proteins by LDE may be one of the important mechanisms of action of LDE against the B16M cell growth in vitro.
Fig. 2.
In vitro effects of LDE on induction of apoptosis and reduction of Bcl-2/Bax protein ratio in B16M cells. a Induction of apoptosis in B16M cells by treatment for 48 h with LDE at the concentrations indicated in figure. The treated cells were fixed with 1% glutaraldehyde and then stained with Hoechst 33258. Apoptotic morphological changes in the nuclear chromatin were observed under a fluorescent microscope as described in the “Materials and methods” section. b Reduction of Bcl-2/Bax protein ratio in B16M cells by treatment for 48 h with LDE at the concentrations indicated in figure. The expressions of Bcl-2 and Bax proteins were analyzed by Western blotting as described in “Materials and methods” section. The cells were treated for 48 h with LDE at the indicated concentrations; Anti-β-actin was used in Western blot analysis as a sample loading control. The ratio of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of 0 (vehicle) as the control (designated as 100%). For one experiment, three assays were carried out and only one set of gels is shown. Statistical analysis was carried out using the ANOVA and Bonferroni test. * P < 0.05. All statistical tests were two-sided. Values with different letters (a–e) differ significantly (P < 0.05)
LDE suppressed tumor growth in melanoma-bearing mice and reduced the Bcl-2/Bax protein ratios in the tumors
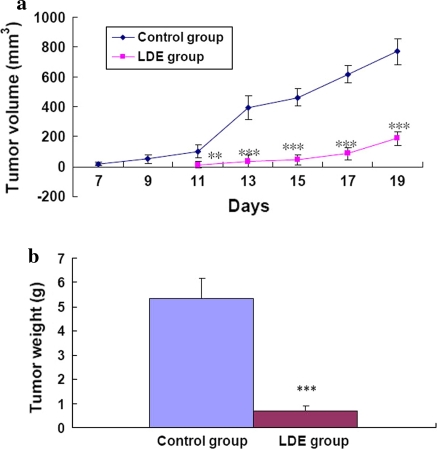
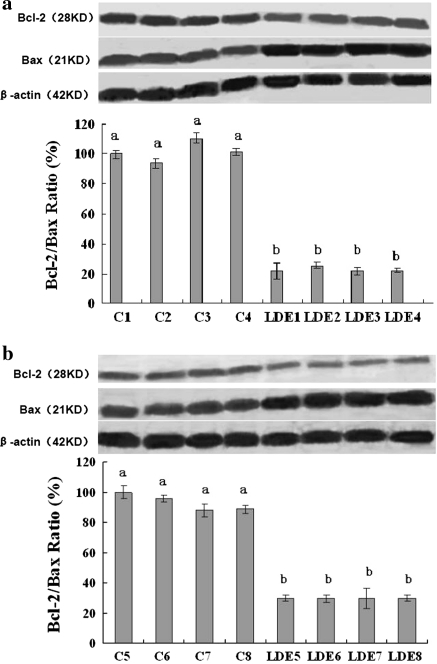
To determine whether or not LDE could suppress melanoma growth in vivo and reduce Bcl-2/Bax protein ratios in tumors, we conducted an animal experiment. The result of the animal experiment showed that the incidence of tumors in mice was on the 7th day in the control group and on the 11th day in the LDE group. Compared with the control group, the tumor volume in mice was significantly and time-dependently lower in LDE group (Fig. 3a). The absolute weight of solid tumors in the LDE group was 7-fold lower than that in the control group (Fig. 3b). Western blot analysis confirmed that compared with the control group, the Bcl-2 protein level in the tumors was lower and the Bax protein level in the tumors was high and thus the ratios of Bcl-2/Bax protein level in the tumors were lower in the LDE group (Fig. 4a, b). These in vivo results further demonstrate that the reduction of Bcl-2/Bax protein ratios is one of molecular mechanisms of action of LDE suppressing the B16M cell growth in mice. These results partially explain why LDE has its therapeutic and/or adjuvant therapeutic effects on treatment for some cancer patients.
Fig. 3.
In vivo effects of LDE on the growth (a Volume; b weight) of tumors in melanoma-bearing mice. a Tumor volume was determined in live animals by measuring the largest and the smallest diameters of each tumor with calipers, based on the formula L x W2/2 where L is the length and W is the width of the tumor. b After the mice had been killed, each tumor in mice was weighed as described in the “Materials and methods” section. Each value and bar represents the mean and SEM of eight mice in each group. **/*** Values are significantly different at P < 0.01/P < 0.001 by using the ANOVA and Student’s t-test when compared to the control group
Fig. 4.
In vivo effects of LDE on the expressions of tumor Bcl-2/Bax proteins and reduction of Bcl-2/Bax ratio in melanoma-bearing mice. After the mice in the control group (n = 8) and LDE group (n = 8) were killed, each tumor in both groups was excised. The expressions of Bcl-2 and Bax proteins in each tumor were analyzed by Western blotting as described in “Materials and methods” section. Anti-β-actin was used in Western blot analyses as a sample loading control. In (a) and (b), C1, C2, C3, C4, C5, C6, C7 and C8 are the analyzed samples, respectively from the tumors of each mouse in the PBS-treated control group; LDE1, LDE2, LDE3, LDE4, LDE5, LDE6, LDE7 and LDE8 are the analyzed samples, respectively from the tumors of each mouse in the LDE-treated group. Compared with the PBS-treated control group, each sample in the LDE-treated group shows significantly lower ratio of Bcl-2 and Bax protein levels. The ratio (%) of Bcl-2 and Bax, (the ratio of relative density of each band normalized to β-actin), shown as mean ± SD (Bar) is relative to that of C1 in (a) or C5 in (b) as the control (designated as 100%). For one experiment, three assays were carried out and only one set of gels is shown. Statistical analysis was carried out using the ANOVA and Student’s t-test. Values with different letters (a–b) differ significantly (P < 0.05)
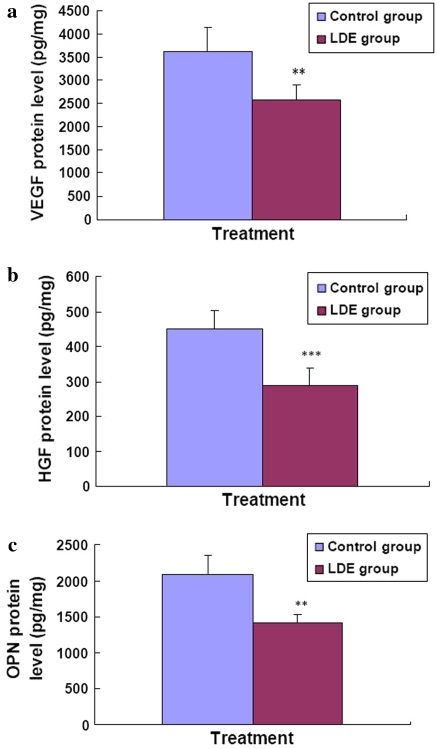
LDE reduced tumor progression-associated protein levels of VEGF, HGF, and OPN in melanoma-bearing mice
To further understand the molecular mechanisms of action of LDE against the B16M cell growth in mice, we investigated the effects of LDE on tumor protein levels of VEGF, HGF, and OPN in melanoma-bearing mice. The ELISA analysis indicated that compared with the control group, the tumor protein levels of VEGF, HGF, and OPN in melanoma-bearing mice were significantly lower in LDE group (Fig. 5a, b, c). The tumor VEGF level decreased by an average of 1,052 ± 318 pg/mg, which represents a mean inhibition of 29.0% (Fig. 5a). The tumor HGF level decreased by an average of 165 ± 52 pg/mg, which represents a mean inhibition of 36.6% (Fig. 5b). The tumor OPN level decreased by an average of 672 ± 104 pg/mg, which represents a mean inhibition of 32.1% (Fig. 5c).
Fig. 5.
In vivo effects of LDE on the expressions of tumor VEGF, HGF, and OPN proteins in melanoma-bearing mice. After the mice in the control group (n = 8) and LDE group (n = 8) were killed, each tumor in both the groups were excised. The expressions of VEGF, HGF, and OPN proteins was in each tumor were analyzed by ELISA as described in “Materials and methods” section. Each point and vertical bar represents the mean and SD for six wells. The figures (a, b, and c) are the representatives of three similar experiments performed. Statistical analysis was carried out using the ANOVA and Bonferroni test. All statistical tests were two-sided. ** P < 0.01, *** P < 0.001
Increased protein levels of VEGF, HGF and OPN are associated with cancer cell growth, angiogenesis and metastasis (Kienast et al. 2010; Eder et al. 2009; McGill et al. 2006; Rangaswami and Kundu 2007; Rangel et al. 2008; Johnston et al. 2008; Philip et al. 2001). VEGF is a key regulator of angiogenesis and VEGF expression correlates with tumor progression (Goh et al. 2007; Chen et al. 2009; Kienast et al. 2010). Vascular endothelial growth factor-A (VEGF-A) inhibition induced long-term dormancy of lung cancer micrometastases by preventing angiogenic growth to macrometastases. VEGF becomes the important target of therapies (Kienast et al. 2010; Chen et al. 2009; Kerr 2004). Inappropriate c-Met signaling through autocrine, paracrine, amplification, and mutational activation occurs in virtually all types of solid tumors, contributing to one or a combination of proliferative, invasive, survival, or angiogenic cancer phenotypes. The c-Met ligand, HGF/SF participates in all stages of malignant progression and represents a promising drug targets in a variety of cancer types, including melanoma (Eder et al. 2009; McGill et al. 2006). OPN is a glycophosphoprotein cytokine associated with the growth and metastasis of various cancer cells including melanoma cells (Johnston et al. 2008; Rangaswami and Kundu 2007; Rangel et al. 2008). In the present study, the suppression of VEGF, HGF and OPN in B16M tumor by LDE may be related to inhibition of phosphatidylinositol 3′-kinase (PI3 K)/Akt (protein kinase B) and nuclear factor-κB (NF-κB) signaling pathway. There have been studies showing that OPN stimulated tumor growth and activated NF-κB signaling pathway in melanoma cells (Rangaswami et al. 2004); OPN induced PI3K dependent Akt phosphorylation and NF-κB activity (Das et al. 2003, 2005) in cancer cells; overexpression of Akt led to upregulation of VEGF and rapidly growing melanomas in vivo (Govindarajan et al. 2007); the activation of PI3K/Akt pathway induced by the HGF/c-Met axis was involved in promoting the enhanced motility and migration of uveal melanoma cells (Ye et al. 2008); HGF/c-Met-activated PI3K/Akt signaling pathway was important for melanoma cell survival (Carlson et al. 2007); activation of NF-κB signaling pathway led to upregulation of VEGF and HGF in cancer cells (Xie et al. 2010; Fan et al. 2005; Maroni et al. 2007). Inhibition of NF-κB activity decreased the VEGF mRNA expression in cancer cells (Shibata et al. 2002). Our previous results also confirmed that the inhibition of PI3K/Akt and NF-κB signaling pathway and reduction of expressions of VEGF and OPN were associated with decrease in cell viability in cancer cells (Wang et al. 2009a, b; Yu et al. 2009).
NF-κB is a nuclear transcription regulator with a specific motif for Bcl-2 transcription (Viatour et al. 2003; Wei et al. 2001; Marsden et al. 2002). The PI3K/Akt pathway acts as a survival (anti-apoptotic) signal and plays a key role in the regulation of apoptotic change in cancer cells. Akt can exert its anti-apoptotic effects in several different ways, such as negatively regulating pro-apoptotic factors, stimulating the NF-κB survival pathway (Sizemore et al. 1999; Ozes et al. 1999; Mayo and Donner 2002; Nicholson and Anderson 2002). Activation of p-Akt and the NF-κB/Bcl-2 pathway leads to inhibition of chemotherapy-induced apoptosis, which results in treatment resistance (Wang et al. 1996). The Bax, Bcl-2, p-Akt and NF-κB have become the important targets of action by anticancer agents (Wang et al. 1996; Marsden et al. 2002; Patel et al. 2007; Gupta et al. 2002; Emi et al. 2005; Aggarwal 2004). There has been a report that 17-acetoxyjolkinolide B (17-AJB), one of the LDE constituents induced apoptosis of hepatoma cells, which was associated with the inhibition of NF-κB pathway and NF-κB-regulated genes such as Bcl-2, BAX-α, etc. (Yan et al. 2008). Our present results have shown that LDE inhibited the B16M cell growth, induced apoptosis and reduced the Bcl-2/Bax protein ratio in cancer cells. We also found that LY294002, the inhibitor of PI3K/Akt, and Bay 11-7082, the inhibitor of NF-κB induced cell viability and the Bcl-2/Bax protein ratio in B16M cells (data not shown). These results suggest that the inhibitory effects of LDE on cell growth and Bcl-2/Bax protein ratio may be relevant to the inhibition of PI3K/Akt and NF-κB pathway, and its upstream and/or downstream targets such as OPN, HGF and VEGF although LDE may not directly target these factors. It should be noted that LDE may have no or low toxic effect on normal cells because the results from both the present animal experiment and our ongoing clinical experiment have not shown evident toxic effects on LDE-treated mice and patients who received LDE treatment.
In the present study, we have demonstrated that LDE inhibits the B16M cell growth and reduces the Bcl-2/Bax protein ratios not only in vitro but also in vivo in mice. Induction of apoptosis and down-regulation of Bcl-2/Bax protein ratios as well as reduction of the protein levels of VEGF, HGF and OPN may be the important mechanisms of action of LDE against the B16M cell growth. There has been no report to show the inhibitory effects of LDE or a compound in LDE on tumor growth in melanoma-bearing mice as yet. This is the first time for us to report here that LDE suppresses melanoma growth in vivo, reduces tumor Bcl-2/Bax protein ratios as well as decreases the tumor protein levels of VEGF, HGF, and OPN in the melanoma-bearing mice. There have been studies showing that the compounds such as jolkinolide A, 17-AJB, jolkinolide B, fischeriana A, and fischeriana B in LDE inhibited the in vitro cell growth of some cancer lines such as human leukemia K562, hepatoma HepG2 (Luo and Wang 2006; Wang et al. 2009a, b; Yan et al. 2008) and/or human prostate LNCaP cancer cells (Liu et al. 2002). Our present results suggest that the synergistic effects of jolkinolide (A, B) and fischeriana (A, B) as well as some unidentified compounds in LDE may be responsible for the suppression of both in vitro and in vivo growth of the B16M cells as well as the tumor protein expressions of Bcl-2/Bax, VEGF, HGF, and OPN in melanoma-bearing mice. Our present results also suggest that LDE may have the therapeutic and/or adjuvant therapeutic application in the treatment of human melanoma and other cancer. The related clinic investigation of the LDE therapeutic and adjuvant therapeutic effects on cancer patients is ongoing in the PLA 107 hospital in China.
Acknowledgments
Grant support This work is supported in part by grants from the Ministry of Education of the People’s Republic of China to G.Z, from the Ministry of Human Resources and Social Security of the People’s Republic of China to G.Z, Projects of Yantai University to GZ, Project from the National Natural Science Foundation of China to GZ (No. 30973553), and grants from the Department of Science and Technology of Shandong Province to GZ (Y2008C71; 2009GG10002087), and the grant from the PLA research fund of medicine and health to Y.W (06G034). The authors declare no conflict of interest.
Abbreviations
- LDE
Lang-du extract
- VEGF
Vascular endothelial growth factor
- HGF/SF
Hepatocyte growth factor/scatter factor
- OPN
Osteopontin
- PI3K
Phosphatidylinositol 3′-kinase
- 17-AJB
17-acetoxyjolkinolide B
Footnotes
Liping Wang and Huiying Duan contributed equally to this work.
Contributor Information
Yishan Wang, Phone: +86-535-6884952, FAX: +86-535-6884952, Email: wys@107zlzx.com.
Guoying Zhang, Phone: +86-535-6884952, FAX: +86-535-6884952, Email: zhang_zhang6173@yahoo.com.cn.
References
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Carlson JA, Linette GP, Aplin A, Ng B, Slominski A. Melanocyte receptors: clinical implications and therapeutic relevance. Dermatol Clin. 2007;25:541–557. doi: 10.1016/j.det.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Lu Y, Wu JM, Xu B, Zhang LJ, Gao M, Zheng SZ, Wang AY, Zhang CB, Zhang WW, Lei N. Ligustrazine inhibits B16F10 melanoma metastasis and suppresses angiogenesis induced by vascular endothelial growth factor. Biochem Biophys Res Commun. 2009;386:374–379. doi: 10.1016/j.bbrc.2009.06.042. [DOI] [PubMed] [Google Scholar]
- Das R, Mahabeleshwar GH, Kundu GC. Osteopontin stimulates cell motility and nuclear factor kappaB-mediated secretion of urokinase type plasminogen activator through phosphatidylinositol 3-kinase/Akt signaling pathways in breast cancer cells. J Biol Chem. 2003;278:28593–28606. doi: 10.1074/jbc.M303445200. [DOI] [PubMed] [Google Scholar]
- Das R, Philip S, Mahabeleshwar GH, Bulbule A, Kundu GC. Osteopontin: it’s role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel therapeutic inhibitors of the c-Met signaling pathway in cancer. Clin Cancer Res. 2009;15:2207–2214. doi: 10.1158/1078-0432.CCR-08-1306. [DOI] [PubMed] [Google Scholar]
- Emi M, Kim R, Tanabe K, Uchida Y, Toge T. Targeted therapy against Bcl-2-related proteins in breast cancer cells. Breast Cancer Res. 2005;7:R940–R952. doi: 10.1186/bcr1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S, Pestell RG, Goldberg ID, Rosen EM. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–1766. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–758. doi: 10.2174/156800907783220462. [DOI] [PubMed] [Google Scholar]
- Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martínez-Diez M, Cuezva JM, Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Afaq F, Mukhtar H. Involvement of nuclear factor-kappa B, Bax and Bcl-2 in induction of cell cycle arrest and apoptosis by apigenin in human prostate carcinoma cells. Oncogene. 2002;21:3727–3738. doi: 10.1038/sj.onc.1205474. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Q, Liu K, Yagasaki K, Zhang G. Suppression of growth of highly-metastatic human breast cancer cells by norcantharidin and its mechanisms of action. Cytotechnology. 2009;59:201–208. doi: 10.1007/s10616-009-9210-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston NI, Gunasekharan VK, Ravindranath A, O’Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361–4372. doi: 10.2741/3009. [DOI] [PubMed] [Google Scholar]
- Kerr DJ. Targeting angiogenesis in cancer: clinical development of bevacizumab. Nat Clin Pract Oncol. 2004;1:39–43. doi: 10.1038/ncponc0026. [DOI] [PubMed] [Google Scholar]
- Kienast Y, Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–122. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- Liu WK, Ho JC, Qin G, Che CT. Jolkinolide B induces neuroendocrine differentiation of human prostate LNCaP cancer cell line. Biochem Pharmacol. 2002;63:951–957. doi: 10.1016/S0006-2952(01)00938-8. [DOI] [PubMed] [Google Scholar]
- Liu Q, Duan H, Luan J, Yagasaki K, Zhang G. Effects of theanine on growth of human lung cancer and leukemia cells as well as migration and invasion of human lung cancer cells. Cytotechnology. 2009;59:211–217. doi: 10.1007/s10616-009-9223-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Wang A. Induction of apoptosis in K562 cells by jolkinolide B. Can J Physiol Pharmacol. 2006;84:959–965. doi: 10.1139/Y06-045. [DOI] [PubMed] [Google Scholar]
- Maroni P, Bendinelli P, Matteucci E, Desiderio MA. HGF induces CXCR4 and CXCL12-mediated tumor invasion through Ets1 and NF-kappaB. Carcinogenesis. 2007;28:267–279. doi: 10.1093/carcin/bgl129. [DOI] [PubMed] [Google Scholar]
- Marsden VS, O’Connor L, O’Reilly LA, Silke J, Metcalf D, Ekert PG, Huang DC, Cecconi F, Kuida K, Tomaselli KJ, Roy S, Nicholson DW, Vaux DL, Bouillet P, Adams JM, Strasser A. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochromec/Apaf-1/caspase-9 apoptosome. Nature. 2002;419:634–637. doi: 10.1038/nature01101. [DOI] [PubMed] [Google Scholar]
- Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–467. doi: 10.1016/S0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- McGill GG, Haq R, Nishimura EK, Fisher DE. c-Met expression is regulated by Mitf in the melanocyte lineage. J Biol Chem. 2006;281:10365–10373. doi: 10.1074/jbc.M513094200. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355:51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-O. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Patel JB, Mehta J, Belosay A, Sabnis G, Khandelwal A, Brodie AM, Soprano DR, Njar VC. Novel retinoic acid metabolism blocking agents have potent inhibitory activities on human breast cancer cells and tumour growth. Br J Cancer. 2007;96:1204–1215. doi: 10.1038/sj.bjc.6603705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip S, Bulbule A, Kundu GC. Osteopontin stimulates tumor growth and activation of promatrix metalloproteinase-2 through nuclear factor-kappa B-mediated induction of membrane type 1 matrix metalloproteinase in murine melanoma cells. J Biol Chem. 2001;276:44926–44935. doi: 10.1074/jbc.M103334200. [DOI] [PubMed] [Google Scholar]
- Qin GW, Xu RS. Recent advances on bioactive natural products from Chinese medicinal plants. Med Res Rev. 1998;18:375–382. doi: 10.1002/(SICI)1098-1128(199811)18:6<375::AID-MED2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Rangaswami H, Kundu GC. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep. 2007;18:909–915. [PubMed] [Google Scholar]
- Rangaswami H, Bulbule A, Kundu GC. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem. 2004;279:38921–38935. doi: 10.1074/jbc.M404674200. [DOI] [PubMed] [Google Scholar]
- Rangel J, Nosrati M, Torabian S, Shaikh L, Leong SP, Haqq C, Miller JR, 3rd, Sagebiel RW, Kashani-Sabet M. Osteopontin as a molecular prognostic marker for melanoma. Cancer. 2008;112:144–150. doi: 10.1002/cncr.23147. [DOI] [PubMed] [Google Scholar]
- Sasse AD, Sasse EC, Clark LG, Ulloa L, Clark OA (2007) Chemoimmunotherapy versus chemotherapy for metastatic malignant melanoma. Cochrane Database Syst Rev 1:CD005413 [DOI] [PubMed]
- Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002;73:237–243. doi: 10.1023/A:1015872531675. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19:4798–4805. doi: 10.1128/mcb.19.7.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatour P, Bentires-Alj M, Chariot A, Deregowski V, Leval L, Merville MP, Bours V. NF- kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349–1356. doi: 10.1038/sj.leu.2402982. [DOI] [PubMed] [Google Scholar]
- Villares GJ, Zigler M, Wang H, Melnikova VO, Wu H, Friedman R, Leslie MC, Vivas-Mejia PE, Lopez-Berestein G, Sood AK, Bar-Eli M. Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA. Cancer Res. 2008;68:9078–9086. doi: 10.1158/0008-5472.CAN-08-2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wang YB, Huang R, Wang HB, Jin HZ, Lou LG, Qin GW. Diterpenoids from the roots of Euphorbia fischeriana. J Nat Prod. 2006;69:967–970. doi: 10.1021/np0600088. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ma X, Yan S, Shen S, Zhu H, Gu Y, Wang H, Qin G, Yu Q. 17-hydroxy-jolkinolide B inhibits signal transducers and activators of transcription 3 signaling by covalently cross-linking Janus kinases and induces apoptosis of human cancer cells. Cancer Res. 2009;69:7302–7310. doi: 10.1158/0008-5472.CAN-09-0462. [DOI] [PubMed] [Google Scholar]
- Wang S, Liu Q, Zhang Y, Liu K, Yu P, Liu K, Luan J, Duan H, Lu Z, Wang F, Wu E, Yagasaki K, Zhang G. Suppression of growth, migration and invasion of highly-metastatic human breast cancer cells by berbamine and its molecular mechanisms of action. Mol Cancer. 2009;8:81. doi: 10.1186/1476-4598-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie TX, Xia Z, Zhang N, Gong W, Huang S. Constitutive NF-kappaB activity regulates the expression of VEGF and IL-8 and tumor angiogenesis of human glioblastoma. Oncol Rep. 2010;23:725–732. doi: 10.3892/or_00000813. [DOI] [PubMed] [Google Scholar]
- Yan SS, Li Y, Wang Y, Shen SS, Gu Y, Wang HB, Qin GW, Yu Q. 17-Acetoxyjolkinolide B irreversibly inhibits IkappaB kinase and induces apoptosis of tumor cells. Mol Cancer Ther. 2008;7:1523–1532. doi: 10.1158/1535-7163.MCT-08-0263. [DOI] [PubMed] [Google Scholar]
- Ye M, Hu D, Tu L, Zhou X, Lu F, Wen B, Wu W, Lin Y, Zhou Z, Qu J. Involvement of PI3K/Akt signaling pathway in hepatocyte growth factor-induced migration of uveal melanoma cells. Invest Ophthalmol Vis Sci. 2008;49:497–504. doi: 10.1167/iovs.07-0975. [DOI] [PubMed] [Google Scholar]
- Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-kappaB signaling. Cytotechnology. 2009;59:219–229. doi: 10.1007/s10616-009-9225-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Miura Y, Yagasaki K. Induction of apoptosis and cell cycle arrest in cancer cells by in vivo metabolites of teas. Nutr Cancer. 2000;38:265–273. doi: 10.1207/S15327914NC382_16. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang H, Yu P, Liu Q, Liu K, Duan H, Luan G, Yagasaki K, Zhang G. Effects of matrine against the growth of human lung cancer and hepatoma cells as well as lung cancer cell migration. Cytotechnology. 2009;59:191–200. doi: 10.1007/s10616-009-9211-2. [DOI] [PMC free article] [PubMed] [Google Scholar]