Abstract
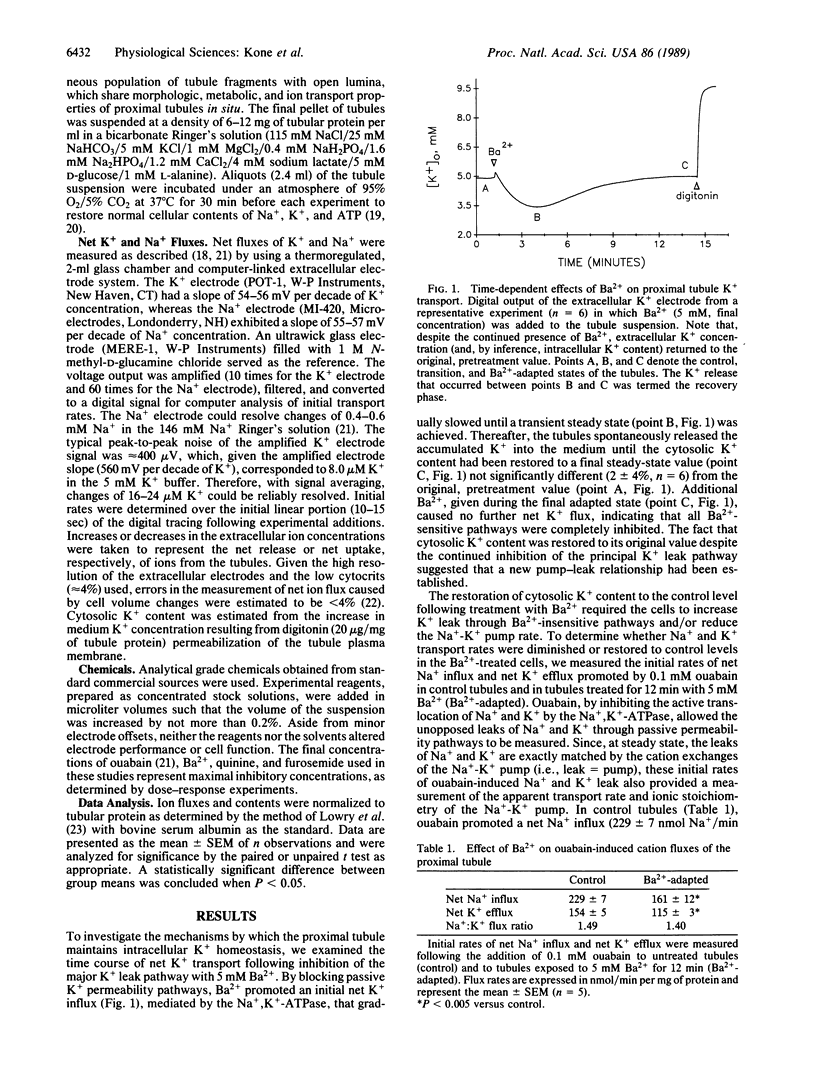
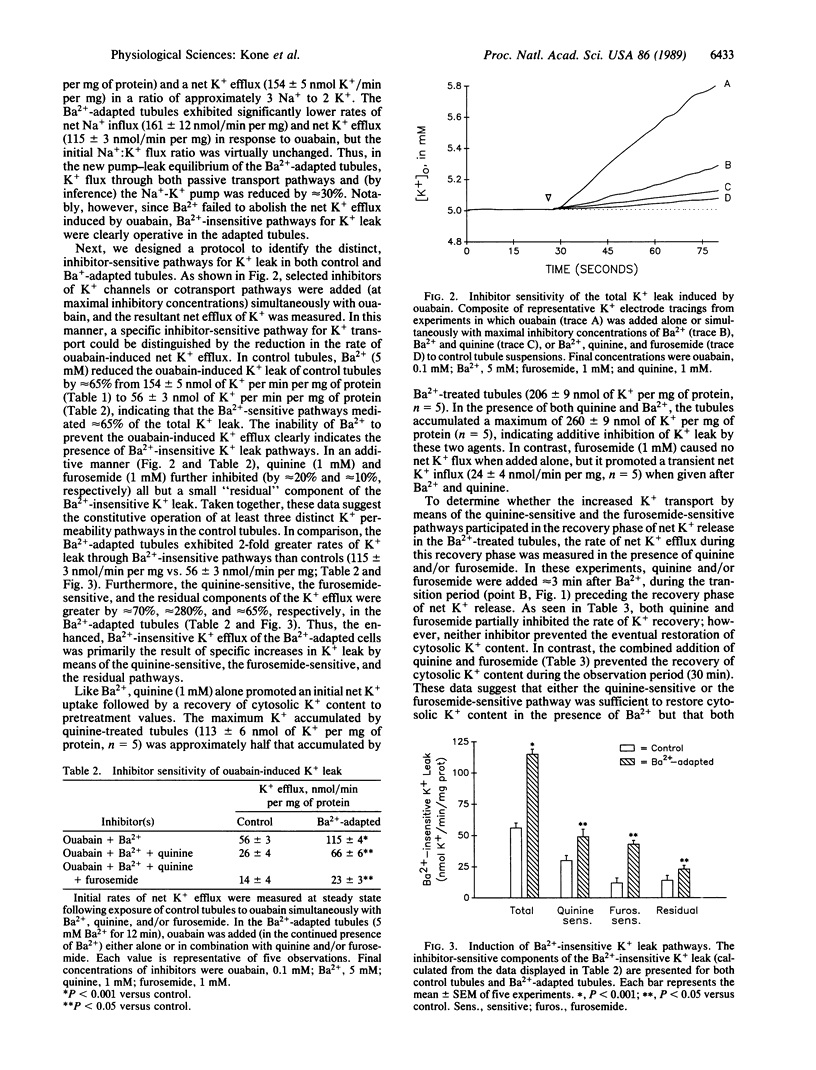
To avoid large changes in cell K+ content and volume during variations in Na+,K+-ATPase activity, Na+-transporting epithelia must adjust the rate of K+ exit through passive permeability pathways. Recent studies have shown that a variety of passive K+ transport mechanisms may coexist within a cell and may be functionally linked to the activity of the Na+,K+-ATPase. In this study, we have identified three distinct pathways for passive K+ transport that act in concert with the Na+,K+-ATPase to maintain intracellular K+ homeostasis in the proximal tubule. Under control conditions, the total K+ leak of the tubules consisted of discrete Ba2+-sensitive (approximately 65%), quinine-sensitive (approximately 20%), and furosemide-sensitive (approximately 10%) pathways. Following inhibition of the principal K+ leak pathway with Ba2+, the tubules adaptively restored cell K+ content to normal levels. This recovery of cell K+ content was inhibited, in an additive manner, by quinine and furosemide. Following adaptation to Ba2+, the tubules exhibited a 30% reduction in Na+-K+ pump rate coupled with an increase in K+ leak by means of the quinine-sensitive (approximately 70%) and furosemide-sensitive (approximately 280%) pathways. Thus, the proximal tubule maintains intracellular K+ homeostasis by the coordinated modulation of multiple K+ transport pathways. Furthermore, these results suggest that, like Ba2+, other inhibitors of K+ conductance will cause compensatory changes in both the Na+-K+ pump and alternative pathways for passive K+ transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avison M. J., Gullans S. R., Ogino T., Giebisch G. Na+ and K+ fluxes stimulated by Na+-coupled glucose transport: evidence for a Ba2+-insensitive K+ efflux pathway in rabbit proximal tubules. J Membr Biol. 1988 Nov;105(3):197–205. doi: 10.1007/BF01870997. [DOI] [PubMed] [Google Scholar]
- Avison M. J., Gullans S. R., Ogino T., Giebisch G., Shulman R. G. Measurement of Na+-K+ coupling ratio of Na+-K+-ATPase in rabbit proximal tubules. Am J Physiol. 1987 Jul;253(1 Pt 1):C126–C136. doi: 10.1152/ajpcell.1987.253.1.C126. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B., Sohtell M., Giebisch G. Intracellular potassium activity in the rabbit proximal straight tubule. Am J Physiol. 1981 Dec;241(6):F677–F686. doi: 10.1152/ajprenal.1981.241.6.F677. [DOI] [PubMed] [Google Scholar]
- Brady H. R., Kone B. C., Gullans S. R. Extracellular Na+ electrode for monitoring net Na+ flux in cell suspensions. Am J Physiol. 1989 May;256(5 Pt 1):C1105–C1110. doi: 10.1152/ajpcell.1989.256.5.C1105. [DOI] [PubMed] [Google Scholar]
- Cox T. C., Helman S. I. Na+ and K+ transport at basolateral membranes of epithelial cells. II. K+ efflux and stoichiometry of the Na,K-ATPase. J Gen Physiol. 1986 Mar;87(3):485–502. doi: 10.1085/jgp.87.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveloff J., Warnock D. G. K-Cl transport systems in rabbit renal basolateral membrane vesicles. Am J Physiol. 1987 May;252(5 Pt 2):F883–F889. doi: 10.1152/ajprenal.1987.252.5.F883. [DOI] [PubMed] [Google Scholar]
- Grasset E., Gunter-Smith P., Schultz S. G. Effects of Na-coupled alanine transport on intracellular K activities and the K conductance of the basolateral membranes of Necturus small intestine. J Membr Biol. 1983;71(1-2):89–94. doi: 10.1007/BF01870677. [DOI] [PubMed] [Google Scholar]
- Gullans S. R., Avison M. J., Ogino T., Giebisch G., Shulman R. G. NMR measurements of intracellular sodium in the rabbit proximal tubule. Am J Physiol. 1985 Jul;249(1 Pt 2):F160–F168. doi: 10.1152/ajprenal.1985.249.1.F160. [DOI] [PubMed] [Google Scholar]
- Gunter-Smith P. J., Grasset E., Schultz S. G. Sodium-coupled amino acid and sugar transport by Necturus small intestine. An equivalent electrical circuit analysis of a rheogenic co-transport system. J Membr Biol. 1982;66(1):25–39. doi: 10.1007/BF01868479. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. Properties of single K+ channels in the basolateral membrane of rabbit proximal straight tubules. Pflugers Arch. 1987 Oct;410(3):288–295. doi: 10.1007/BF00580279. [DOI] [PubMed] [Google Scholar]
- Gögelein H., Greger R. Single channel recordings from basolateral and apical membranes of renal proximal tubules. Pflugers Arch. 1984 Aug;401(4):424–426. doi: 10.1007/BF00584348. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O., Lambert I. H. Volume-induced increase of K+ and Cl- permeabilities in Ehrlich ascites tumor cells. Role of internal Ca2+. J Membr Biol. 1984;78(3):211–222. doi: 10.1007/BF01925969. [DOI] [PubMed] [Google Scholar]
- Kirk K. L., DiBona D. R., Schafer J. A. Regulatory volume decrease in perfused proximal nephron: evidence for a dumping of cell K+. Am J Physiol. 1987 May;252(5 Pt 2):F933–F942. doi: 10.1152/ajprenal.1987.252.5.F933. [DOI] [PubMed] [Google Scholar]
- Kone B. C., Kaleta M., Gullans S. R. Silver ion (Ag+)-induced increases in cell membrane K+ and Na+ permeability in the renal proximal tubule: reversal by thiol reagents. J Membr Biol. 1988 Apr;102(1):11–19. doi: 10.1007/BF01875349. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang F., Messner G., Rehwald W. Electrophysiology of sodium-coupled transport in proximal renal tubules. Am J Physiol. 1986 Jun;250(6 Pt 2):F953–F962. doi: 10.1152/ajprenal.1986.250.6.F953. [DOI] [PubMed] [Google Scholar]
- Larson M., Spring K. R. Volume regulation by Necturus gallbladder: basolateral KCl exit. J Membr Biol. 1984;81(3):219–232. doi: 10.1007/BF01868715. [DOI] [PubMed] [Google Scholar]
- Matsumura Y., Cohen B., Guggino W. B., Giebisch G. Regulation of the basolateral potassium conductance of the Necturus proximal tubule. J Membr Biol. 1984;79(2):153–161. doi: 10.1007/BF01872119. [DOI] [PubMed] [Google Scholar]
- Messner G., Wang W., Paulmichl M., Oberleithner H., Lang F. Ouabain decreases apparent potassium-conductance in proximal tubules of the amphibian kidney. Pflugers Arch. 1985 May;404(2):131–137. doi: 10.1007/BF00585408. [DOI] [PubMed] [Google Scholar]
- Parent L., Cardinal J., Sauvé R. Single-channel analysis of a K channel at basolateral membrane of rabbit proximal convoluted tubule. Am J Physiol. 1988 Jan;254(1 Pt 2):F105–F113. doi: 10.1152/ajprenal.1988.254.1.F105. [DOI] [PubMed] [Google Scholar]
- Richards N. W., Dawson D. C. Single potassium channels blocked by lidocaine and quinidine in isolated turtle colon epithelial cells. Am J Physiol. 1986 Jul;251(1 Pt 1):C85–C89. doi: 10.1152/ajpcell.1986.251.1.C85. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Ishibashi K., Yoshiyama N., Shiigai T. KCl co-transport across the basolateral membrane of rabbit renal proximal straight tubules. J Clin Invest. 1988 Jan;81(1):194–199. doi: 10.1172/JCI113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G. Homocellular regulatory mechanisms in sodium-transporting epithelia: avoidance of extinction by "flush-through". Am J Physiol. 1981 Dec;241(6):F579–F590. doi: 10.1152/ajprenal.1981.241.6.F579. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. I. Transport and metabolic studies. J Gen Physiol. 1984 Oct;84(4):601–622. doi: 10.1085/jgp.84.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Potassium transport in the rabbit renal proximal tubule: effects of barium, ouabain, valinomycin, and other ionophores. J Membr Biol. 1986;94(2):153–161. doi: 10.1007/BF01871195. [DOI] [PubMed] [Google Scholar]
- TOSTESON D. C., HOFFMAN J. F. Regulation of cell volume by active cation transport in high and low potassium sheep red cells. J Gen Physiol. 1960 Sep;44:169–194. doi: 10.1085/jgp.44.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völkl H., Greger R., Lang F. Potassium conductance in straight proximal tubule cells of the mouse. Effect of barium, verapamil and quinidine. Biochim Biophys Acta. 1987 Jun 30;900(2):275–281. doi: 10.1016/0005-2736(87)90342-7. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A. Importance of anion in hypotonic volume regulation of rabbit proximal straight tubule. Am J Physiol. 1988 Nov;255(5 Pt 2):F853–F860. doi: 10.1152/ajprenal.1988.255.5.F853. [DOI] [PubMed] [Google Scholar]
- Welling P. A., Linshaw M. A., Sullivan L. P. Effect of barium on cell volume regulation in rabbit proximal straight tubules. Am J Physiol. 1985 Jul;249(1 Pt 2):F20–F27. doi: 10.1152/ajprenal.1985.249.1.F20. [DOI] [PubMed] [Google Scholar]
- White J. F. Intracellular potassium activities in Amphiuma small intestine. Am J Physiol. 1976 Oct;231(4):1214–1219. doi: 10.1152/ajplegacy.1976.231.4.1214. [DOI] [PubMed] [Google Scholar]


