Abstract
Calcium signaling and calcium transport play a key role during osteoblast differentiation and bone formation. Here, we demonstrate that DMP1 mediated calcium signaling, and its downstream effectors play an essential role in the differentiation of preosteoblasts to fully functional osteoblasts. DMP1, a key regulatory bone matrix protein, can be endocytosed by preosteoblasts, triggering a rise in cytosolic levels of calcium that initiates a series of downstream events leading to cellular stress. These events include release of store-operated calcium that facilitates the activation of stress-induced p38 MAPK leading to osteoblast differentiation. However, chelation of intracellular calcium and inhibition of the p38 signaling pathway by specific pharmacological inhibitors and dominant negative plasmid suppressed this activation. Interestingly, activated p38 MAPK can translocate to the nucleus to phosphorylate transcription factors that coordinate the expression of downstream target genes such as Runx 2, a key modulator of osteoblast differentiation. These studies suggest a novel paradigm by which DMP1-mediated release of intracellular calcium activates p38 MAPK signaling cascade to regulate gene expression and osteoblast differentiation.
Keywords: Bone, Gene Regulation, Heat Shock Protein, MAPKs, p38 MAPK
Introduction
Osteoblasts can react to a variety of biological signals. Among these, calcium signaling is essential for the proliferation and differentiation of osteoblasts. Earlier studies have shown that treating osteoblasts with parathyroid hormone or vitamin D3 induces an increase in intracellular calcium ([Ca2+]i) by increasing the release of Ca2+ from the intracellular stores (1–5). Store-operated Ca2+ channels, which are activated in response to Ca2+ store depletion, control homeostasis between the extracellular Ca2+ reservoir and intracellular Ca2+ storage and control a wide range of cellular functions.
Dentin matrix protein 1 (DMP1) initially identified and localized in the mineralized dentin and bone matrix (6) is thought to play a regulatory role only in the calcification of the extracellular matrix. Apart from its role in mineralization, one of the putative functions of DMP1 is its involvement during differentiation of osteoblasts and odontoblasts (7–9). DMP1-null mice displayed severe defects in bone formation (10). We had shown earlier that DMP1 is specifically localized in the nucleus of differentiating osteoblasts and odontoblasts, and this translocation from the extracellular matrix is facilitated by the endocytic receptor GRP78 (11). The 78-kDa glucose-regulated protein (GRP78) is a calcium-binding molecular chaperone expressed in the endoplasmic reticulum of eukaryotic cells. Identification of GRP78 as a cell surface receptor for DMP1 is particularly interesting as its induction is a protective response against several kinds of stress, including ER2 Ca2+ depletion and accumulation of unglycosylated proteins (12, 13). However, the specific signaling pathways activated following DMP1 stimulus and osteoblast differentiation are not delineated yet.
p38 MAPKs are widely expressed in many tissues and are strongly activated by cellular stress inducers, like inflammatory stress, cytokines, lipopolysaccharides, and osmotic stress (14). Activation of p38 involves dual phosphorylation on threonine (Thr-180) and tyrosine (Tyr-182) residues and is thought to play an essential role in signal transduction by modulating gene transcription in the nucleus in response to changes in the cellular environment. In general, the p38 MAPK pathway has been shown to mediate signals for the generation of important biological responses such as phosphorylation of transcription factors such as ATF-2 (activating transcription factor 2), MEF2C (myocyte enhancer factor 2C), C/EBP homologous protein, and heat shock proteins (15, 16). During bone and cartilage development, the stress-activated p38 kinase has been shown to be essential for osteoblast and chondrocyte differentiation (17–20).
The pathways that connect the rise in [Ca2+]i to osteoblast differentiation have not been elucidated yet. In this study, we have identified the molecular events that occur during DMP1-mediated osteoblast differentiation. Endocytosis of DMP1 mediates elevation of intracellular free calcium. This [Ca2+]i flux leads to activation of stress response and cellular mediators of the stress kinase pathway resulting in downstream induction of transcription factors, such as Runx-2, which is a key modulator of osteoblast differentiation. Additional investigations using pharmacological inhibitors supported a role for p38 MAPK during DMP1-mediated osteoblast differentiation. This study was performed in preosteoblast MC3T3-E1 cells, primary calvarial cells, and in pluripotent embryonic cell line C3H10T1/2 cells in which overt markers of osteogenesis are absent.
MATERIALS AND METHODS
Expression and Purification of DMP-1
The recombinant DMP1 protein was expressed in Escherichia coli as published earlier (21). Native protein was a kind gift by Dr. Chunlin Qin, Baylor College of Dentistry, Dallas, TX.
Cell Culture
Mouse calvarial preosteoblast MC3T3-E1 cells (a kind gift of Dr. R. T. Franceschi, University of Michigan) were cultured in α-minimum Eagle's medium, and C3H10T1/2 cells were grown in Eagle's Basal medium supplemented with 10% FBS and 1% penicillin/streptomycin. They were seeded in 6-well plates. Primary calvarial cells were isolated from day 3 mice, and grown as mentioned above. The cells were allowed to proliferate until it attained 70% confluence. Media were changed every 2 days. 12–16 h before the start of the experiment, the cells were cultured in α-minimum Eagle's medium or BME medium supplemented with 1% FBS (basal medium). These cells were then stimulated with 250 ng of rDMP1 (this was determined optimum from a pilot study using 100, 250, and 500 ng). The treated cells were then trypsinized, and RNA was extracted for RT-qPCR. Total proteins were extracted at 15, 30, and 45 min and 1, 1.5, and 2 h, respectively. MC3T3-E1 and C3H10T1/2 cells were also cultured in mineralization media to promote terminal differentiation. The mineralization microenvironment was induced by the addition of 10 mm β-glycerophosphate and 100 μg/ml ascorbic acid along with 10 nm dexamethasone in the basal media (9). Cells were stimulated with rDMP1 and total RNA, and total proteins were extracted at 7, 14, and 21 days.
Quantitative Real Time PCR
RNA was extracted according to the manufacturer's recommended protocol by using TRIzol (Invitrogen). RT-qPCR was performed with DNase I (Promega)-treated RNA. A total of 1 μg of total RNA was reverse-transcribed for 90 min at 50 °C with Superscript III (Invitrogen). Quantitative real time PCR (qPCR) analysis was then carried out using ABI StepOnePlus instrument. Expressions of osteocalcin, Runx2, and GAPDH transcripts were analyzed by qPCR during its linear phase. The relative gene expression level was estimated by using the 2−ΔΔCT method, where CT value = log linear plot of PCR signal versus the cycle number. ΔCT = CT value of target gene − CT value of GAPDH. Primers were obtained from Qiagen.
Mineralization Assay by von Kossa Staining
von Kossa staining was used to determine the presence of phosphate. MC3T3-E1 cells were grown to 60% confluency in growth media. Cells were then stimulated with either DMP1 or DMP1 and SB203580 inhibitor for 7, 14, and 21 days in mineralization media. The cells were fixed in ice-cold methanol for 10 min, washed twice with PBS, and then stained with 5% silver nitrate solution.
Detection of Protein Phosphorylation by Western Blot Analysis
Total proteins were extracted from rDMP1-stimulated MC3T3-E1 and C3H10T1/2 cells using M-per reagent (Pierce). Nuclear and cytoplasmic proteins were also extracted from MC3T3-E1 cells treated with rDMP1 using NE-Per reagent (Pierce). 30 μg of the total proteins were resolved on a 10% SDS-polyacrylamide gel under reducing conditions. After electrophoresis, the proteins were electrotransferred onto nitrocellulose membrane (Bio-Rad), blocked with 5% nonfat milk, probed with either anti-Gαq (1:500) (Santa Cruz Biotechnology), anti-p38 (1:500 (Cell Signaling), anti-phospho-p38 (1:500) (Santa Cruz Biotechnology), anti-phospho-HSP27 (1:500) (Cell Signaling), anti-MAPKAPK2 (1:500) (Santa Cruz Biotechnology), and anti-phospho-MAPKAPK2 (1:500) (Cell Signaling) for 16 h at 4 °C. Blots were then incubated with HRP-conjugated goat anti-rabbit IgG secondary antibody (Chemicon International). They were washed three times with PBS containing 0.05% Tween 20 and once with PBS. The bands were visualized by the ECL-Western blot reagent (PerkinElmer Life Sciences). Each membrane was then carefully washed, treated for 5 min with a stripping buffer (Pierce), and washed with PBS, and Western blot analysis was performed with anti-tubulin antibody (Sigma) and HRP-conjugated goat anti-mouse IgG secondary antibody.
Blocking p38 MAPK Activation
Cells were cultured as described above and were treated with 10 μm of SB203580 (Biomol), an inhibitor specific for p38 MAPK. SB203580 was added to the DMP1 containing basal media and was preincubated for 30 min at 37 °C prior to the start of the experiment. Total RNA and cell lysates were harvested at various time points and were analyzed by qPCR and Western blotting performed as described above.
The dominant negative p38 plasmid was a generous gift from Dr. Philip Scherer (22), and transient transfections were performed on MC3T3-E1 cells using Superfect (Qiagen). These cells were treated with rDMP1 peptide for 15, 30, and 45 min and 1, 1.5, and 2 h, and Western blot analysis was performed.
MC3T3-E1 and C3H10T1/2 cells stimulated with rDMP1 were grown in mineralization media with or without SB203580 for 7, 14, and 21 days, and Western blot analysis was performed as mentioned above.
To study the role of intracellular calcium in activating the p38 MAPK pathway, cells were treated with BAPTA-AM (50 μm) for 30 min and then stimulated with rDMP1 for various time points. Total protein was isolated and analyzed by Western blotting for p38 activation.
Cytosolic Ca2+ Measurement
Changes in the [Ca2+]i was measured using the Ca2+-sensitive fluorescent dye Fura-2AM (23). MC3T3-E1 cells or primary calvarial cells were grown to confluence on tissue culture glass coverslips and then washed two times with serum-free medium and incubated for 2 h at 37 °C in culture medium containing 1% FBS. Cells were again washed and loaded with 3 μm Fura-2AM for 30 min. After loading, cells were washed with HBSS, and the coverslips were transferred on a perfusion chamber and imaged using an Axio Observer D1 semi-motorized microscope (Carl Zeiss, Sartrouville, France) equipped with a camera and a Fluar 40× oil immersion objective lens (Carl Zeiss). Light was provided by the DG-4 wavelength switcher (Princeton Instruments). A dual excitation at 340 and 380 nm was used, and emission was collected at 515 nm. The software AxioVision physiology module was used to acquire the images at a frequency of 1-s intervals, and the data were analyzed off line. Regions of interest in individual cells were marked and excited at 340 and 380 nm with emission at 520 nm at 5-s intervals. In each experiment, 30–40 cells were selected to measure change in [Ca2+]i.
For blocking experiments, cells were treated with various agents as follows. MC3T3-E1 cells were exponentially grown and were pretreated with anti-GRP78 antibody (1:100) (gift from Sylvie Blond, Dept. of Pharmaceutical Biotechnology, University of Illinois, Chicago), GRGDNP blocking peptide (2 mm), 2-APB (75 μm) (Sigma), U73122 (10 and 20 μm) (Sigma), or calphostin C (1 μm) (Sigma) for 30 min.
The cells were then loaded with Fura-2AM for 30 min. rDMP1 (250 ng/ml) was then added to the cells, and cytoplasmic Ca2+ concentration was measured. Cells triggered with BSA (250 ng/ml) served as negative control.
Blocking Integrin and GRP78 Receptors and Detection of Phospho-p38 Activation
MC3T3-E1 cells were grown to 70% confluency on tissue culture plates. GRP78 antibody (1:100) or GRGDNP blocking peptide (2 mm) was added to the cells and incubated for 60 min. The cells were then stimulated with DMP1 for 30 mi, and total proteins were extracted. Western blot was performed to determine phosphorylation of p38 kinase.
Activation of MAPKAPK2
Transient transfections were performed using MAPKAPK2 wild type (pcDNA3mycMK2WT), dominant negative kinase-inactive mutant (pcDNA3mycMK2K76R), and constitutively active mutant (pcDNA3mycMK2EE) plasmids (generous gift from Matthias Gaestel (Institute of Biochemistry, Medical School, Hannover, Germany)). These plasmids were transfected in MC3T3-E1 cells according to published protocols and then stimulated with DMP1 for 1 h. Total proteins were isolated and subjected to Western blot analysis to detect activated MAPKAPK2 and its downstream target HSP27.
Immunofluorescence
Activation of p38 signaling by DMP1 was analyzed by immunofluorescence. MC3T3-E1 cells or primary calvarial cells were seeded on glass slides and treated with 250 ng/ml rDMP1 for 30 min, 1 and 2 h. The cells were then washed with 1× PBS and fixed with 4% paraformaldehyde for 30 min. Fixed cells were then rinsed three times with 1× PBS and then permeabilized with 0.05% Triton X-100 in PBS for 20 min. The cells were then washed with PBS and blocked with 5% BSA in PBS for 1 h. After blocking, the cells were then incubated overnight with anti-phospho-p38 (1:100) (Santa Cruz Biotechnology) or with anti-phospho-HSP27 (1:100) (Cell Signaling) and followed by a 1-h incubation with a fluorescein-conjugated goat anti-rabbit IgG (Sigma). After washing with PBS, the cover glass was mounted using mounting media (Vector Shield), and labeling was detected with an Axio Observer D1 fluorescence microscope (Zeiss) equipped with Axiovision imaging software (Zeiss).
RESULTS
DMP1 Stimulation Results in Mobilization of Intracellular Calcium in Preosteoblasts
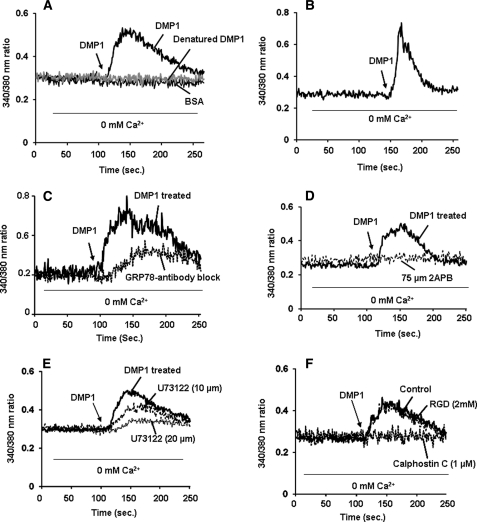
In the first set of experiments, we investigated the effect of DMP1 on the mobilization of intracellular calcium [Ca2+]i in MC3T3-E1 preosteoblast cells. Cells were loaded with the fluorescent calcium-sensitive dye Fura-2AM for 30 min followed by DMP1 stimulation and measured for [Ca2+]i. In the absence of external Ca2+, treatment of cells with DMP1 produced a 2–3-fold transient increase in [Ca2+]i when compared with unstimulated cells (Fig. 1A). Interestingly, cells stimulated with heat-denatured DMP1 or BSA (Fig. 1A) caused no observable changes in the [Ca2+]i levels. A 4-fold increase in the mobilization of [Ca2+]i was observed when primary calvarial cells were stimulated with DMP1 (Fig. 1B). To confirm that DMP1 was endocytosed by binding to GRP78 receptor as reported previously, MC3T3-E1 cells were treated with anti-GRP78 antibody and then loaded with Fura-2AM. Results (Fig. 1C) demonstrate that DMP1 binding to GRP78 was necessary for release of [Ca2+]i. Together, these data indicate that endocytosis of DMP1 by binding to GRP78 receptor mediates the transient rise in [Ca2+]i.
FIGURE 1.
A, effect of rDMP1 on [Ca2+]i in MC3T3-E1 cells. DMP1-induced [Ca2+]i was measured in MC3T3-E1 cells as described under “Materials and Methods.” Fura-2AM-loaded cells were washed three times, placed in Ca2+- and Mg2+-free HBSS, and then stimulated with rDMP1 (250 ng/ml). Heat-inactivated DMP1 protein was used to confirm the specificity, and BSA (250 ng/ml) was used as a control. The arrow indicates the time point when the cells were stimulated with DMP1 or heat-inactivated DMP1 or BSA. The experiment was repeated three times, and a representative plot is shown. B, effect of rDMP1 on [Ca2+]i in primary calvarial cells. Primary calvarial cells were loaded with Fura-2AM, washed, and stimulated with rDMP1. DMP1 stimulation results in the mobilization of intracellular calcium in primary calvarial cells. Triplicates of the experiments were performed. The arrow indicates the time point when the cells were stimulated with DMP1. C, GRP78 is necessary for release of [Ca2+]i. Cells were treated with anti-GRP78 antibody and then loaded with Fura-2AM and then stimulated with rDMP1 (250 ng/ml) as indicated. Blocking DMP1 internalization by anti-GRP78 suppressed release of [Ca2+]i. The arrow indicates the time point when the cells were stimulated with DMP1. D, 2-APB blocks DMP1-stimulated increase in [Ca2+]i in MC3T3-E1 cells. Cells pretreated with 2-APB (75 μm) for 2 h were loaded with Fura-2AM and placed in Ca2+- and Mg2+-free HBSS. The cells were then stimulated with rDMP1 (250 ng/ml) and then observed for Ca2+ store release. Cells with no 2-APB treatment served as control. Arrow indicates the time at which cells were stimulated with rDMP1. Experiments were repeated a minimum of four times. A representative plot is shown in this figure. E, PLC inhibitor, U-73122, inhibits DMP1-induced [Ca2+]i release in MC3T3-E1 cells. DMP1-induced [Ca2+]i was measured in confluent preosteoblast monolayers stimulated with 250 ng/ml rDMP1 after 30 min of pretreatment with U-73122 (PLC inhibitor). Cells were loaded with Fura-2AM and placed in Ca2+- and Mg2+-free HBSS and stimulated with rDMP1 (250 ng/ml) to measure store-Ca2+-release. Arrow indicates the time at which cells were stimulated with rDMP1. Experiments were repeated a minimum of four times. A representative plot is shown in this figure. F, calphostin C, a PKC inhibitor and an RGD-containing peptide, inhibits DMP1-induced [Ca2+]i. release in MC3T3-E1 cells. DMP1-induced [Ca2+]i was measured in confluent MC3T3-E1 cells loaded with 1 μm calphostin C or 2 mm RGD-containing peptide for 30 min prior to the addition of Fura-2AM. Fura-2AM-loaded cells were washed three times, placed in Ca2+- and Mg2+-free HBSS, and then stimulated with rDMP1 (250 ng/ml). The arrow indicates the time point when the cells were stimulated with DMP1. The experiment was repeated four times, and a representative plot is shown.
Intracellular Ca2+ Is Released from Endoplasmic Reticulum Stores through IP3Rs
We next investigated if release of Ca2+ from ER stores occurs through the calcium release channel, namely IP3Rs. Therefore, cells were treated with 2-APB, an IP3R antagonist prior to the administration of DMP1. Results in Fig. 1D demonstrate that 2-APB completely inhibited the store-Ca2+-release in preosteoblasts. These data demonstrate that 2-APB, an IP3 receptor blocker, could suppress the intracellular Ca2+ changes elicited by DMP1 and strongly suggest that Ca2+ release is mediated through IP3R-gated stores in DMP1-stimulated preosteoblasts.
Studies have conclusively reported that IP3R-mediated Ca2+ release from the endoplasmic reticulum involves activation of phospholipase C (PLC). PLC activation produces IP3 and diacylglycerol from phosphoinositol 2-phosphate. IP3 binds to its receptors that gate Ca2+ release from intracellular stores. Therefore, we tested the effect of PLC inhibitor U73122 on DMP1-induced Ca2+ store release. As shown in Fig. 1E, treatment of cells with 20 μm U73122 significantly prevented DMP1-induced store-Ca2+-release. Together, these results suggest that DMP1-mediated release of intracellular Ca2+ through the calcium-permeable IP3R channel is regulated by the soluble second messenger IP3, which is produced in response to activation of PLC. This release of Ca2+ from ER stores could generate cytosolic Ca2+ signals that could control downstream signaling events.
To demonstrate the role of PKC in DMP1-induced Ca2+ release, calphostin C, a highly specific pharmacological inhibitor for PKC, was used. Store Ca2+ release was completely inhibited when cells were pretreated with calphostin C (1 μm) for 30 min followed by stimulation with DMP1 (Fig. 1F). We next examined if integrins played a role in activating the store-Ca2+-release. Results demonstrate that MC3T3-E1 cells treated with GRGDNP blocking peptide (2 mm) for 30 min followed by stimulation with DMP1 (Fig. 1F) did not block intracellular Ca2+ release.
Osteoblast Differentiation Can Be Mediated by DMP1 Stimulation
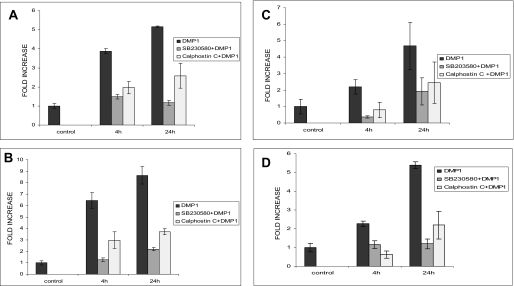
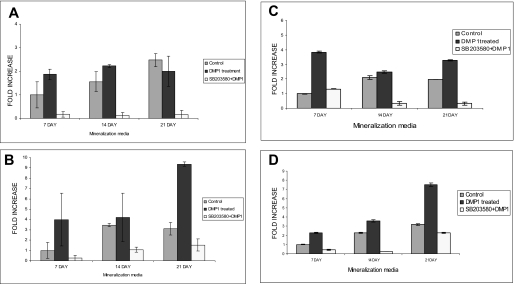
To gain insight into the downstream effect of DMP1-mediated calcium signaling on gene expression and osteoblast differentiation, MC3T3-E1 and C3H10T1/2 cells were treated with rDMP1 (250 ng/ml) for 4 and 24 h in basal medium. Quantitative real time RT-PCR on RNA samples showed that DMP1 stimulation caused a 4- and 5-fold increase in Runx2 mRNA expression at 4 and 24 h, respectively (Fig. 2A). Interestingly, osteocalcin, a differentiation marker for osteoblast differentiation, had a 6- and 8-fold mRNA expression at 4 and 24 h, respectively (Fig. 2B). These results were also confirmed by using an embryonic mesenchymal cell line C3H10T1/2. Upon stimulation with recombinant DMP1, we observed a 4–5-fold increase in the two osteogenic differentiation markers at the 24-h time point (Fig. 2, C and D). To gain insight into the role of DMP1 in terminal differentiation of osteoblasts, MC3T3-E1 cells (Fig. 3, A and B) and C3H10T1/2 (Fig. 3, C and D) were grown in differentiation media for 7, 14, and 21 days in the presence of DMP1. Significant increase in Runx2 (Fig. 3, A and C) and osteocalcin gene (Fig. 3, B and D) expression were seen in both cell types with cellular differentiation.
FIGURE 2.
Effect of DMP1 on osteogenic gene expression and their abrogation in the presence of p38 inhibitor SB203580 and PKC inhibitor calphostin. MC3T3-E1 cells were either left untreated (control) or starved for 24 h prior to stimulation with 250 ng/ml rDMP1 peptide or with SB203580 and rDMP1 or with calphostin C and rDMP in basal medium for 4 and 24 h. Total RNA was isolated and subjected to real time PCR and analyzed for the expression of Runx2 (A) and osteocalcin (B). Similar experiments were performed with C3H10T1/2 cells (Runx2 (C) and osteocalcin (D)). Stimulation with DMP1 significantly up-regulated the expression levels of Runx2 and osteocalcin. Treatment with SB203580, a specific inhibitor for p38 MAPK activation or calphostin, down-regulated gene expression. Note basal levels of gene expression were not affected by the addition of SB203580 alone. These results were normalized with the loading control GAPDH. Experiments were done in triplicate.
FIGURE 3.
Effect of p38 kinase stimulated by DMP1 on terminal differentiation of osteoblast. DMP1 enhances terminal differentiation of osteoblast in MC3T3-E1 (B) and C3H10T1/2 cells (C and D). Cells were treated with DMP1 for 7, 14, and 21 days in the presence of mineralization media and with or without SB203580. Relative amounts of mRNA for Runx2 (A and C) and osteocalcin (B and D) were determined using real time PCR. Values are normalized to the levels of GAPDH mRNA.
p38 MAPK Signaling Pathway Is Required for Osteoblast Differentiation
Next, we investigated the signaling pathway mediating the increase in gene expression. As p38 MAPK can be activated by cellular stresses, MC3T3-E1 and C3H10T1/2 cells were treated with SB203580, a specific inhibitor for the p38 MAPK. RT-qPCR results demonstrate that the mRNA expression of Runx2 and osteocalcin in the two cell types were markedly down-regulated in the presence of the p38 inhibitor (Fig. 2). To confirm that the down-regulation was not mediated through DMSO, a vehicle used for dissolving the SB203580 inhibitor, RT-qPCR was performed on mRNA extracted at 4 and 24 h from MC3T3-E1 cells stimulated with DMSO. Results demonstrate that MC3T3-E1 cells treated with DMSO had little or no effect on gene expression (supplemental Fig. S1). Interestingly, SB203580 inhibited gene expression during the terminal differentiation of osteoblast in both cell types when subjected to in vitro differentiation (Fig. 3). Moreover, we analyzed the expression levels of Runx2 and osteocalcin in the presence of calphostin C, a protein kinase C inhibitor. Results in Fig. 2 demonstrate a significant decrease in the expression levels in both the cell types. Together, the data suggest that the augmented gene expression levels in DMP1 stimulated cells are mediated through PKC/p38 signaling pathways.
DMP1 Induces Nuclear Localization of RUNX2
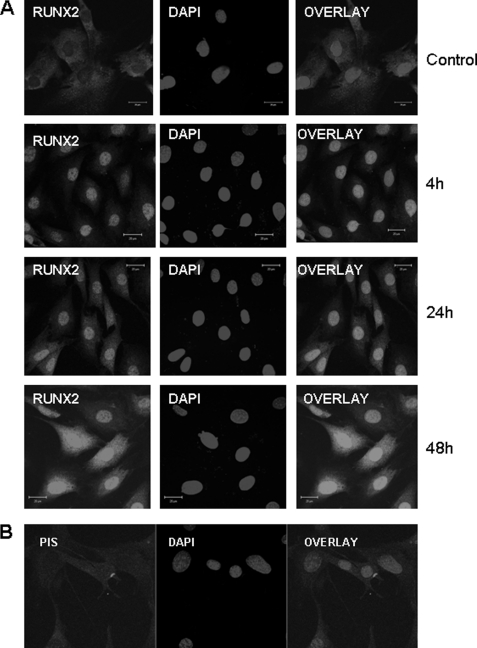
Increase in nuclear localization and cytoplasmic staining of Runx2 was observed when MC3T3-E1 cells were stimulated with rDMP1 for 4, 24, and 48 h (Fig. 4A). No nuclear localization was observed with preimmune serum (Fig. 4B). These results confirm that DMP1 mediates the nuclear translocation of Runx2 protein.
FIGURE 4.
DMP1 stimulates the nuclear translocation of RUNX2. Confocal image showing nuclear and cytoplasmic staining of Runx2 when stimulated with rDMP1 for 4, 24, and 48 h, although predominant cytoplasmic localization was present in unstimulated cells (A). MC3T3-E1 cells stained with control preimmune serum did not show nuclear translocation of RUNX2 (B). The scale bar indicates 20 μm.
Activation of p38 MAPK DMP1 during “Early” Differentiation of Osteoblasts
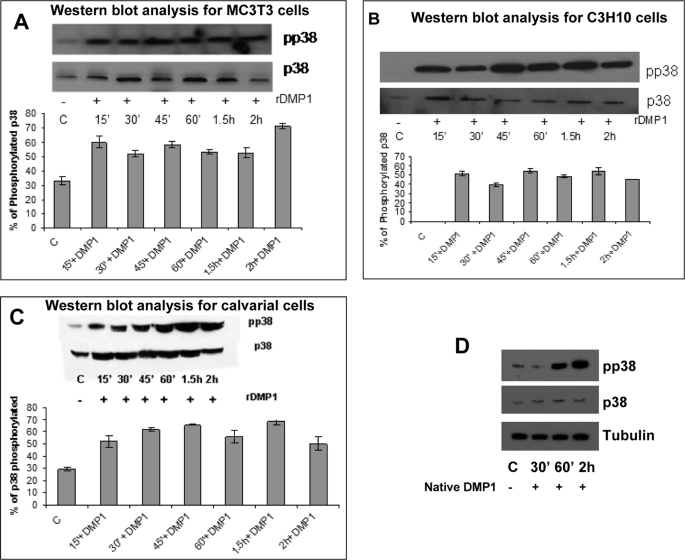
We next determined if the stress-induced MAPK, p38, is activated early on during osteoblast differentiation. Western blot results presented in Fig. 5 demonstrate that rDMP1 and native DMP1 stimulation can activate p38 kinase (phosphorylated) in both MC3T3-E1 and C3H10T1/2 cells. This activation is observed as early as 15 min and can be sustained for 2 h after DMP1 stimulation. Maximum levels of phosphorylation were observed at 2 h in MC3T3-E1 cells (Fig. 5A), whereas C3H10T1/2 cells showed maximum phosphorylation at 45 min after DMP1 stimulation (Fig. 5B). Similar p38 activation was also observed in primary calvarial cells (Fig. 5C). Unstimulated control cells showed no significant increase in p38 kinase activity. MC3T3-E1 cells stimulated with native DMP1 showed activation of p38 MAPK (Fig. 5D). Together, these data suggest the involvement of p38 MAPK in early osteoblast differentiation.
FIGURE 5.
DMP1 stimulates phosphorylation of p38 MAPK in MC3T3-E1 and C3H10T1/2 cells and primary calvarial cells. Cells in basal medium were untreated (control) or treated with rDMP1 (250 ng/ml) for 15, 30, 45, and 60 min and 1.5 and 2 h. Cell lysates were harvested and subjected to SDS-PAGE, and Western blot analysis was performed with phospho-p38 antibody. The blots were stripped and probed for total p38. Stimulation by DMP1 phosphorylates p38 MAPK in MC3T3-E1 cells (A), C3H10T1/2 cells (B), and primary calvarial cells (C). The graphs show the quantification of the percentage levels of phosphorylated p38. MC3T3-E1 cells stimulated with native DMP1 showed activation of p38 MAPK (D).
Activation of p38 Kinase by DMP1 Can Be Abrogated by Treatment with 2-APB, BAPTA-AM, SB203580, anti-GRP78 Antibody and in the Presence of Dominant Negative p38 Plasmid
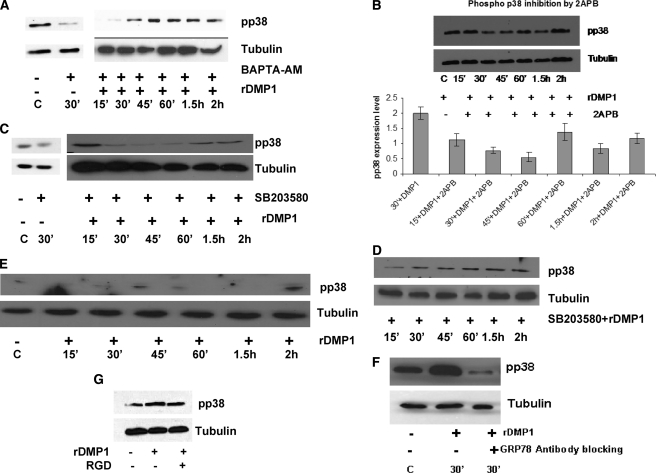
To examine whether an increase in [Ca2+]i was required for p38 activation, cells were treated with the Ca2+ chelator BAPTA-AM first and then stimulated with DMP1 and subjected to Western blot analysis. BAPTA-AM is inactive in the extracellular environment; however, once this membrane-permeable chelator enters cells, the AM group is cleaved by intracellular esterases to form active BAPTA. Results in Fig. 6A show that BAPTA-AM caused a considerable decrease in p38 activation, implicating that [Ca2+]i was involved in downstream signaling.
FIGURE 6.
Activation of p38 kinase by DMP1 can be abrogated by treatment with 2-APB, BAPTA-AM, SB203580, and anti-GRP78 antibody and in the presence of dominant negative p38 plasmid. MC3T3-E1 cells were treated with BAPTA-AM (50 μm) followed by stimulation with rDMP1 (A). Abrogation of p38 activation was observed in the presence of IP3 receptor inhibitor 2-APB in MC3T3-E1 cells (B). MC3T3-E1 cells (C) and C3H10T1/2 cells (D) were treated with p38 inhibitor 10 μm SB203580 and stimulated with or without DMP1 for the indicated time points, and Western blots were developed with anti-phospho-p38 antibody. E, MC3T3-E1 cells were transiently transfected with dominant negative p38 plasmid and then stimulated with rDMP1 and Western blots developed with anti-phospho-p38 antibody. F, MC3T3-E1 cells were treated with GRP78 antibody (1:100) for 60 min followed by stimulation with rDMP1. Total proteins were isolated, and Western blots were developed with anti-phospho-p38 antibody. G, cells were blocked with RGD blocking peptide (2 mm) for 30 min followed by stimulation with rDMP1. Total proteins were isolated and probed for anti-phospho-p38 antibody. Equal loading of the proteins was confirmed by stripping the blot followed by probing it with tubulin.
To further demonstrate the involvement of cytosolic Ca2+ in the activation of p38, cells were treated with 2-APB and then stimulated with DMP1 and observed for p38 activation. Results in Fig. 6B demonstrate that p38 activation was reduced in the presence of IP3 receptor inhibitor 2-APB.
To confirm the role of DMP1 in mediating p38 kinase activation, MC3T3-E1 cells and C3H10T1/2 cells were pretreated for specific time points with SB203580, a potent chemical inhibitor specific for the p38 MAPK. Interestingly, immunoblot results using phospho-p38 antibody confirmed the ability of the DMP1-induced increase in p38 phosphorylation was effectively blocked by SB203580 (Fig. 6, C and D). MC3T3-E1 cells treated with DMSO (supplemental Fig. S2A) or SB203580 (Fig. 6C) had little or no effect on suppressing p38 activation. To further confirm the specificity of the results obtained with SB203580, MC3T3-E1 cells were transfected with dominant negative p38 plasmid. Fig. 6E shows that the p38 dominant negative plasmid blocked p38 phosphorylation, validating the chemical inhibitor data. Furthermore, blocking endocytosis of DMP1 by anti-GRP78 antibody resulted in decreased p38 phosphorylation (Fig. 6F) indicative of the necessity for DMP1 binding to GRP78 to mediate downstream p38 activation. Together, these findings suggest that DMP1 internalization increases cytosolic Ca2+ influx resulting in p38 MAPK activation.
Role of Integrins in the Activation of p38 Kinase by DMP1 Stimulation
To investigate if the RGD domain in DMP1 was involved in p38 activation through integrins, we blocked the integrin receptor by an RGD-containing peptide before DMP1 stimulation. Results in Fig. 6G show that blocking integrin receptor interactions had much less effect on p38 activation relative to GRP78 interactions.
Stimulation by DMP1 Facilitates Translocation of Phospho-p38 from the Cytoplasm to the Nucleus in MC3T3-E1 Cells
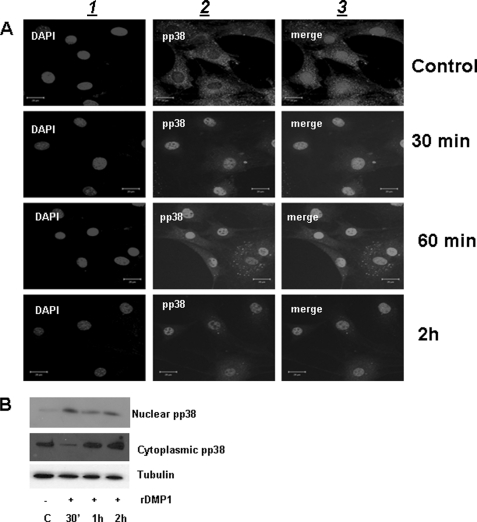
We next examined whether activated p38 kinase was translocated to the nuclear compartment of preosteoblasts stimulated with DMP1. Confocal microscopy images in Fig. 7A indicated that in unstimulated cells phospho-p38 was uniformly distributed throughout the cytoplasm and sparsely in the nucleus. However, in DMP1-stimulated cells, phospho-p38 was localized in the nucleus as early as 30 min. Predominant nuclear localization was observed at 2 h. These results were further confirmed by immunoblotting experiments, demonstrating greater amounts of phospho-p38 in the nuclear fraction at 30 min than in the cytoplasmic fraction (Fig. 7B). This suggests that preosteoblasts stimulated with DMP1 can activate p38 kinase, which then translocates to the nucleus within 30 min.
FIGURE 7.
DMP1 stimulates the nuclear translocation of phospho-p38. A, immunofluorescence analysis showing localization of phospho-p38 (panel 2) in MC3T3-E1 cells stimulated with rDMP1 at the indicated time points. DNA was stained with DAPI (panel 1). Note nuclear localization of phospho-p38 in the nucleus as early as 30 min and overlap between the two images is depicted in panel 3. The scale bar indicates 20 μm. B, total proteins were isolated from the nuclear and cytoplasmic compartment of MC3T3-E1 cells stimulated with DMP1 at the indicated time point. Western blot analysis was performed with anti-phospho-p38 antibody.
Effect of DMP1 on Activation of pMAPKAPK2 and HSP27, Downstream Targets of p38 MAPK Signaling Pathway
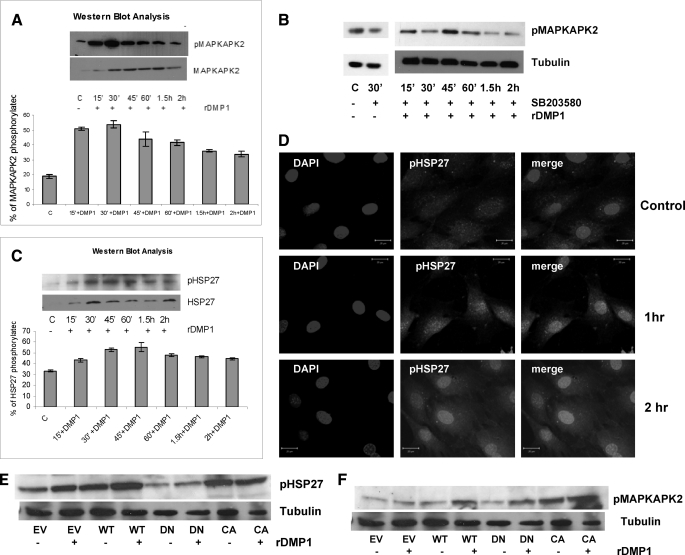
Having established the importance of p38 MAPK in regulating DMP1-mediated osteoblast differentiation, we then began to identify downstream effectors of p38 MAPK. MAPKAPK2 is a known substrate of p38 MAPK; therefore, we analyzed its involvement in DMP1-mediated osteoblast differentiation. Western blot analysis revealed that stimulation by DMP1 increased the levels of phosphorylated MAPKAPK2 from 15 min until 2 h in MC3T3-E1 cells (Fig. 8A). However, unstimulated cells had little-to-no effect. Importantly, inhibition of MAPKAPK2 activation was observed with SB203580, which blocked MAPKAPK2 phosphorylation (Fig. 8B), or with DMSO (supplemental Fig. S2B). These results demonstrate that DMP1 stimulation leads to activation of p38 kinase, which in turn leads to increased levels of phosphorylated MAPKAPK2 in preosteoblasts.
FIGURE 8.
DMP1 stimulation activates MAPKAPK2 and HSP27 that are downstream targets of the p38 MAPK signaling pathway. Confluent adherent MC3T3-E1 cells were treated without (control, C) or with DMP1, and Western blot analysis was performed with anti-phospho-MAPKAPK2 and total MAPKAPK2. A, phosphorylation of MAPKAPK2 was assessed following DMP1 stimulation. Densitometric quantification of the blots was performed by assessing tubulin and total MAPKAPK2. B, phosphorylation of MAPKAPK2 was assessed following treatment with SB203580 and then stimulated by DMP1. C, Western blot was also performed with anti-phospho-HSP27 antibody after stimulating cells without (control) or with DMP1. Equal amounts of proteins were loaded as assessed by tubulin. D, confocal microscopy images showing nuclear localization of HSP27 in DMP1-stimulated cells. The scale bar indicates 20 μm. MC3T3-E1 cells were transfected with MAPKAPK2 wild type (WT), dominant negative kinase-inactive mutant (DN), or constitutively active mutant (CA) or with empty vector (EV). 48 h after transfection, cells were changed to serum-free media, and 24 h later cells were treated with or without DMP1 for 1 h, and phospho-HSP27 (E), phospho-MAPKAPK2 (F), and tubulin were detected by Western blot analysis.
Heat shock protein 27 (HSP27) is constitutively expressed in a variety of mammalian cell types, and its expression increases in response to sublethal stresses, including heat shock, heavy metal, toxins, and oxidative stress. HSP27 has previously been reported to be phosphorylated by MAPKAPK2 downstream of p38 MAPK in the stress response pathway (24). Activation of HSP27 was therefore evaluated as a potential regulator of osteoblast differentiation. We therefore determined if DMP1 stimulation can phosphorylate HSP27 in a time-dependent manner. In Fig. 8C expression of phospho-HSP27 increased after 15 min of DMP1 treatment. This was further confirmed by the nuclear translocation of the phosphorylated form of HSP27 in DMP1-treated cells (Fig. 8D).
To further confirm that MAPKAPK2 and HSP27 are downstream signaling effectors of p38 MAPK with respect to DMP1-mediated osteoblast differentiation, cells were transiently transfected with either wild type (WT) MAPKAPK2, a kinase-inactive dominant negative mutant, a constitutively active mutant, or with empty vector control, and the effects upon phosphorylation of HSP27 were measured by Western blot (Fig. 8E). Treatment with DMP1 increased phosphorylation of MAPKAPK2 in wild type and dominant negative (which is kinase-inactive but can still be phosphorylated) (Fig. 8F). Furthermore, DMP1 stimulation led to an increase in phospho-HSP27 levels in cells transfected with wild type MAPKAPK2 (Fig. 8E). Interestingly, in cells transfected with dominant negative MAPKAPK2, stimulatory effects were abrogated, although in cells transfected with constitutively active MAPKAPK2, the expression of phospho-HSP27 was enhanced. These results definitely prove that DMP1-mediated signaling is responsible for phosphorylation of p38 kinase, MAPKAPK2, and HSP27 in preosteoblasts.
p38 MAPK Signaling Pathway Is Required for Terminal Differentiation of Osteoblasts
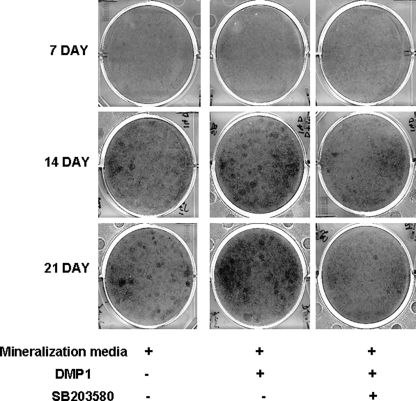
Mineralized nodule formation is the hallmark of in vitro osteogenic differentiation. MC3T3-E1 cells were grown for 7, 14, and 21 days in mineralization media containing DMP1 or DMP1 and SB203580. The cells were then stained by von Kossa. As expected, the cells grown in the presence of DMP1 and in mineralization media for 14 and 21 days stained positive for a mineralized matrix. However, mineralized nodule formation was suppressed in the presence of SB203580 (Fig. 9). We have also determined that p38 is activated during the terminal differentiation process in MC3T3-E1 cells (supplemental Fig. S2C).
FIGURE 9.
Effect of p38 kinase stimulated by DMP1 on mineralized nodule formation. MC3T3-E1 cells were grown in mineralization media in the presence of DMP1 or SB203580 and DMP1 for 7, 14, and 21 days. Mineralized nodules were assayed with von Kossa staining. DMP1 stimulated the formation of mineralized nodules at 14 and 21 days, although SB203580 inhibitor suppressed nodule formation.
Role of G Protein Signaling in Mediating Downstream p38 Activation
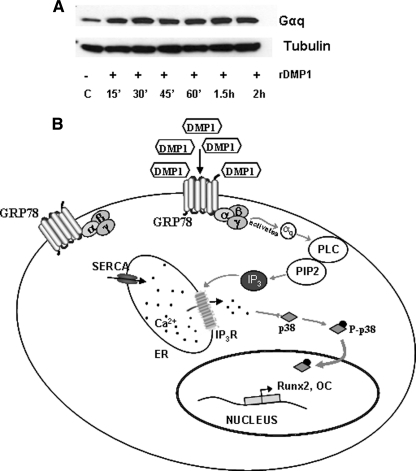
We next sought to identify if the signals resulting from DMP1 endocytosis are transmitted intracellularly from the cell surface through the activation of G protein subunits. Specifically, we explored the role of Gαq-mediated p38 signaling on osteoblast differentiation as Gαq signaling played a crucial role in bone formation. Results in Fig. 10A show that stimulation by DMP1 results in increased Gαq expression.
FIGURE 10.
Effect of DMP1 on the expression of Gαq11 and a hypothetical model. A, total proteins were extracted from DMP1-stimulated MC3T3-E1 at the indicated time points. Western blot analysis was performed with anti-Gαq11 antibody. Tubulin was used as the loading control. B, hypothetical model depicting the depletion of ER Ca2+ stores upon DMP1 endocytosis and the subsequent activation of p38 MAPK and downstream gene transcription.
DISCUSSION
Bone is a complex mineralized tissue that undergoes a continuous process of renewal and repair. Remodeling of adult skeleton is carried about by osteoblasts (bone-forming cells) and osteoclasts (bone resorption cells) (25, 26). During bone formation, the osteoblasts undergo a maturation process resulting in the synthesis and secretion of several matrix molecules (27). The differentiated cells first produce an unmineralized matrix called osteoid, which eventually calcifies. Finally, maturation of osteoblasts results in the formation of osteocytes, which are embedded within the mineralized matrix.
The osteoblast differentiation cascade is mainly orchestrated by matrix cues, transcription factors, cytokines, morphogens, and secreted growth factors. Studies have shown that the differentiation of primary osteoblasts can be stimulated by local factors such as bone morphogenetic proteins (28, 29) and members of the TGF-β family. BMPs control osteoblast differentiation mainly through the Smad signaling pathway (17, 30–33). Furthermore, BMP2 has been reported to require the transcription factor Runx2 for induction of osteoblast differentiation (34, 35). Recently, tissue-specific ER stress transducers that convert ER stress to the transcription of target genes for development, differentiation, and maturation events have been identified (36). In bone, the endoplasmic reticulum stress transducer OASIS has been recently identified and determined critical for bone formation through the transcription of type I collagen and the secretion of bone matrix proteins (37).
Dentin matrix protein 1, an acidic protein present in the mineral phase of both vertebrates and invertebrates, is a key regulatory protein during biogenic formation of mineral deposits. We have previously shown that binding of DMP1 with GRP78 receptor might be an important mechanism by which DMP1 is internalized (11). In this study, we confirm that binding of DMP1 with GRP78 is necessary for intracellular calcium release. As DMP1 contains an RGD domain, we therefore explored the possibility that integrins might facilitate the endocytosis of DMP1 and store-Ca2+-release. Blocking integrin receptors with an RGD peptide did not have any significant effect on calcium release and other downstream signaling events.
The pathways that connect the rise in [Ca2+]i to osteoblast differentiation have not been elucidated yet. In this study, we investigated whether DMP1-mediated ER Ca2+ depletion, resulting in an increase in the intracellular free calcium ion concentration, could activate the stress-induced MAPK, p38, and mediate osteoblast differentiation. Activation of p38 MAPK has been reported to be necessary in the differentiation of a variety of cell types, including adipocytes, chondrocytes, osteoclasts, and neurons (31).
Osteoblasts handle large amounts of calcium during bone formation, and how the osteoblasts deposit mineral is a mystery. In a recent study, the expression level of ER stress markers, BiP, CHOP, ATF4, and EDEM, were significantly up-regulated by BMP2 stimulation. This finding suggests that the BMP2 signaling pathway can induce mild ER stress in osteoblasts. Thus, ER stress response has important roles not only in protection from a state of emergency in cells exposed to ER stress but also in physiological processes such as normal bone formation (22, 38). In this study, we demonstrate that release of calcium from intracellular stores by DMP1 stimulation in preosteoblasts is perceived as a cellular stress.
The stress-induced MAPK, p38, can be activated in preosteoblasts by DMP1 stimulation. This up-regulation was seen as early as 15 min suggesting the activation of p38 at early stages of osteoblast differentiation. The chemical inhibitor SB230580, known to play an important role in inhibiting the phosphorylated form of p38, confirmed our findings. Published studies demonstrate the role of p38 isoforms in osteoblastic differentiation (31). Three different isoforms of p38 MAPK exist, namely p38α, p38β, and p38γ (31). The results from this study have been found to be in accordance with other groups that have shown that SB203580 can inhibit the phosphorylated form of p38α and p38β, although the dominant negative form of p38 inhibits p38α (31). Thus, DMP1 can specifically activate p38α isoform and facilitate osteoblast differentiation.
Propagation of p38-mediated phosphorylation and activation of several downstream protein kinases are necessary to elicit cellular response. MAPKAPK2 is known to be a p38 MAPK downstream signaling protein (40). Interestingly, MAPKAPK2 is observed to be activated with DMP1 stimulation and inhibited in the presence of the inhibitor SB203580. This is the first study demonstrating that DMP1 activates MAPKAPK2 through p38 MAPK. Miguel et al. (41) have demonstrated that the osteogenic growth peptide could activate MAPKAPK2 in osteoblasts by phosphorylation and the transcriptional activity of the cAMP-response element-binding protein. Indirect activation of MAPKAPK2 by vascular growth factor was inhibited by SB203580 in endothelial cells (42). Activation of a multilayered protein kinase cascade downstream of p38 is known to control a broad range of cellular functions.
Hsp27, a small heat shock protein, functions in protein folding. However, Hsp27 is also known to intervene in the modulation of differentiation, which in turn is dependent on the phosphorylation state of Hsp27. Hsp27 has previously been reported to be phosphorylated by MAPKAPK2 downstream of p38 MAPK in the stress response pathway (24). Results from our study demonstrate that DMP1 can up-regulate HSP27 protein expression in preosteoblasts. Activation of Hsp27 was therefore an indication of its function as a potential regulator of osteoblast differentiation. Published reports have shown a transient increase in the expression of Hsp27 during the transition of the cellular states between proliferation and differentiation in several in vitro cellular systems (43, 44). This result corroborates well with published reports on Hsp27 expression during development of the craniofacial bones, suggesting that this protein could be involved in the balance between differentiation and apoptosis, by modulating the viability of osteoblasts and chondrocytes (45).
An interesting observation in this study is that stimulation of preosteoblasts with DMP1 induced Runx2 expression by activating the p38 MAPK pathway. Runx2 is a transcription factor essential for osteoblast differentiation, maturation, and bone formation during embryonic development. Induction of Runx2 gene transcription is a requirement for osteoblast differentiation in vivo. Furthermore, Runx2 regulates the expression of several osteoblast proteins and extracellular matrix protein genes (46).
Overexpression of Runx2 in nonosteogenic cells such as C3H10T1/2 cells and skin fibroblasts induced them to express osteoblast-related genes (47, 48). Antisense oligonucleotides for Runx2 down regulated the expression of osteoblast-related mRNAs in ROS17.2/8 osteoblastic cells (47). Reports also demonstrate that antisense oligonucleotides for Runx2 inhibited osteoblast differentiation, including formation of bone nodules in vitro (49).
Ablation of the Runx2 gene in mice resulted in no calcification in the skull, mandible, humerus, or femur at E18.5 when compared with the wild type (39, 48, 51). Histological examinations on the Runx2-deficient mice confirmed that it is an important transcription factor for bone formation. Furthermore, Ducy et al. (50) confirmed that Runx2 plays a crucial role in not only osteoblast differentiation but also in osteoblast function. Thus, Runx2 regulates the expression of major bone matrix proteins during the early stage of osteoblast differentiation, but it is not essential to maintain these gene expressions in mature osteoblasts.
In conclusion, we describe a new function for DMP1 besides its classical role as a hydroxyapatite nucleator. We demonstrate that stimulation of preosteoblasts with DMP1 results in depletion of ER Ca2+ stores and a rise in cytosolic Ca2+. This perturbation of Ca2+ homeostasis elicits a stress-like response, resulting in the activation of the p38 MAPK signal transduction cascade. Upon activation, p38 kinase can coordinate the expression of downstream target genes that regulate osteoblast differentiation. The net result is the osteoblast maturation process illustrated in the hypothetical molecular pathway shown in Fig 10B. Thus, calcium fluxes mediated by DMP1 in osteoblasts might exert potent anabolic effects during bone remodeling.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant DE 11657.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
- ER
- endoplasmic reticulum
- qPCR
- quantitative PCR
- BAPTA
- 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester)
- IP3R
- inositol 1,4,5-triphosphate receptor
- HBSS
- Hanks' balanced salt solution
- PLC
- phospholipase C
- IP3
- inositol 1,4,5-triphosphate
- 2-APB
- 2-aminoethoxydiphenyl borate.
REFERENCES
- 1.Green J., Kleeman C. R., Schotland S., Ye L. H. (1992) Am. J. Physiol. 263, E1070–E1076 [DOI] [PubMed] [Google Scholar]
- 2.Boden S. D., Kaplan F. S. (1990) Orthop. Clin. North Am. 21, 31–42 [PubMed] [Google Scholar]
- 3.Dvorak M. M., Riccardi D. (2004) Cell Calcium 35, 249–255 [DOI] [PubMed] [Google Scholar]
- 4.Liao J., McCauley L. K. (2006) Cancer Metastasis Rev. 25, 559–571 [DOI] [PubMed] [Google Scholar]
- 5.Sharan K., Siddiqui J. A., Swarnkar G., Chattopadhyay N. (2008) Indian J. Med. Res. 127, 274–286 [PubMed] [Google Scholar]
- 6.George A., Sabsay B., Simonian P. A., Veis A. (1993) J. Biol. Chem. 268, 12624–12630 [PubMed] [Google Scholar]
- 7.Narayanan K., Srinivas R., Peterson M. C., Ramachandran A., Hao J., Thimmapaya B., Scherer P. E., George A. (2004) J. Biol. Chem. 279, 44294–44302 [DOI] [PubMed] [Google Scholar]
- 8.Narayanan K., Ramachandran A., Hao J., George A. (2002) Connect. Tissue Res. 43, 365–371 [DOI] [PubMed] [Google Scholar]
- 9.Narayanan K., Srinivas R., Ramachandran A., Hao J., Quinn B., George A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 4516–4521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye L., Mishina Y., Chen D., Huang H., Dallas S. L., Dallas M. R., Sivakumar P., Kunieda T., Tsutsui T. W., Boskey A., Bonewald L. F., Feng J. Q. (2005) J. Biol. Chem. 280, 6197–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravindran S., Narayanan K., Eapen A. S., Hao J., Ramachandran A., Blond S., George A. (2008) J. Biol. Chem. 283, 29658–29670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lien Y. C., Kung H. N., Lu K. S., Jeng C. J., Chau Y. P. (2008) Histol. Histopathol. 23, 1299–1308 [DOI] [PubMed] [Google Scholar]
- 13.Yeung B. H., Kwan B. W., He Q. Y., Lee A. S., Liu J., Wong A. S. (2008) Oncogene 27, 6782–6789 [DOI] [PubMed] [Google Scholar]
- 14.Cano E., Mahadevan L. C. (1995) Trends Biochem. Sci. 20, 117–122 [DOI] [PubMed] [Google Scholar]
- 15.Tindberg N., Porsmyr-Palmertz M., Simi A. (2000) Neurochem. Res. 25, 527–531 [DOI] [PubMed] [Google Scholar]
- 16.Zayzafoon M., Botolin S., McCabe L. R. (2002) J. Biol. Chem. 277, 37212–37218 [DOI] [PubMed] [Google Scholar]
- 17.Ulsamer A., Ortuño M. J., Ruiz S., Susperregui A. R., Osses N., Rosa J. L., Ventura F. (2008) J. Biol. Chem. 283, 3816–3826 [DOI] [PubMed] [Google Scholar]
- 18.Goodman S. B., Ma T., Spanogle J., Chiu R., Miyanishi K., Oh K., Plouhar P., Wadsworth S., Smith R. L. (2007) J. Biomed. Mater. Res. A 81, 310–316 [DOI] [PubMed] [Google Scholar]
- 19.Tuli R., Seghatoleslami M. R., Tuli S., Howard M. S., Danielson K. G., Tuan R. S. (2002) Ann. N.Y. Acad. Sci. 961, 172–177 [DOI] [PubMed] [Google Scholar]
- 20.Nishihara A., Fujii M., Sampath T. K., Miyazono K., Reddi A. H. (2003) Biochem. Biophys. Res. Commun. 301, 617–622 [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan R., Chen B., Gorski J. P., George A. (1999) Connect. Tissue Res. 40, 251–258 [DOI] [PubMed] [Google Scholar]
- 22.Engelman J. A., Lisanti M. P., Scherer P. E. (1998) J. Biol. Chem. 273, 32111–32120 [DOI] [PubMed] [Google Scholar]
- 23.Sundivakkam P. C., Kwiatek A. M., Sharma T. T., Minshall R. D., Malik A. B., Tiruppathi C. (2009) Am. J. Physiol. Cell Physiol. 296, C403–C413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stokoe D., Engel K., Campbell D. G., Cohen P., Gaestel M. (1992) FEBS Lett. 313, 307–313 [DOI] [PubMed] [Google Scholar]
- 25.Seeman E. (2008) J. Bone Miner. Metab. 26, 1–8 [DOI] [PubMed] [Google Scholar]
- 26.Seeman E. (2002) J. Bone Miner. Res. 17, 373–380 [DOI] [PubMed] [Google Scholar]
- 27.Mundy G. R., Boyce B., Hughes D., Wright K., Bonewald L., Dallas S., Harris S., Ghosh-Choudhury N., Chen D., Dunstan C., et al. (1995) Bone 17, 71S–75S [DOI] [PubMed] [Google Scholar]
- 28.Aubin J. E., Liu F., Malaval L., Gupta A. K. (1995) Bone 17, 77S–83S [DOI] [PubMed] [Google Scholar]
- 29.Kim I. S., Song Y. M., Cho T. H., Park Y. D., Lee K. B., Noh I., Weber F., Hwang S. J. (2008) Dev. Growth Differ. 7, 553–564 [DOI] [PubMed] [Google Scholar]
- 30.Matsubara T., Kida K., Yamaguchi A., Hata K., Ichida F., Meguro H., Aburatani H., Nishimura R., Yoneda T. (2008) J. Biol. Chem. 283, 29119–29125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y., Chan E., Wang S. X., Li B. (2003) Endocrinology 144, 2068–2074 [DOI] [PubMed] [Google Scholar]
- 32.Nöth U., Tuli R., Seghatoleslami R., Howard M., Shah A., Hall D. J., Hickok N. J., Tuan R. S. (2003) Exp. Cell Res. 291, 201–211 [DOI] [PubMed] [Google Scholar]
- 33.Reilly G. C., Golden E. B., Grasso-Knight G., Leboy P. S. (2005) Cell Commun. Signal. 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Goh C. H., Li B. (2007) Endocrinology 148, 1629–1637 [DOI] [PubMed] [Google Scholar]
- 35.Javed A., Bae J. S., Afzal F., Gutierrez S., Pratap J., Zaidi S. K., Lou Y., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2008) J. Biol. Chem. 283, 8412–8422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murakami T., Saito A., Hino S., Kondo S., Kanemoto S., Chihara K., Sekiya H., Tsumagari K., Ochiai K., Yoshinaga K., Saitoh M., Nishimura R., Yoneda T., Kou I., Furuichi T., Ikegawa S., Ikawa M., Okabe M., Wanaka A., Imaizumi K. (2009) Nat. Cell Biol. 11, 1205–1211 [DOI] [PubMed] [Google Scholar]
- 37.Hino S. I., Kondo S., Yoshinaga K., Saito A., Murakami T., Kanemoto S., Sekiya H., Chihara K., Aikawa Y., Hara H., Kudo T., Sekimoto T., Funamoto T., Chosa E., Imaizumi K. (2009) J. Bone Miner. Metab. 2, 131–138 [DOI] [PubMed] [Google Scholar]
- 38.Hamamura K., Yokota H. (2007) FEBS Lett. 581, 1769–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto F., Thornell A. P., Crompton T., Denzel A., Gilmour K. C., Rosewell I. R., Stamp G. W., Beddington R. S., Mundlos S., Olsen B. R., Selby P. B., Owen M. J. (1997) Cell 89, 765–771 [DOI] [PubMed] [Google Scholar]
- 40.Stokoe D., Caudwell B., Cohen P. T., Cohen P. (1993) Biochem. J. 296, 843–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miguel S. M., Namdar-Attar M., Noh T., Frenkel B., Bab I. (2005) J. Biol. Chem. 280, 37495–37502 [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi M., Nishita M., Mishima T., Ohashi K., Mizuno K. (2006) EMBO J. 25, 713–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davidson S. M., Morange M. (2000) Dev. Biol. 218, 146–160 [DOI] [PubMed] [Google Scholar]
- 44.Walsh D., Grantham J., Zhu X. O., Wei Lin J., van Oosterum M., Taylor R., Edwards M. (1999) Environ. Med. 43, 79–87 [PubMed] [Google Scholar]
- 45.Leonardi R., Barbato E., Paganelli C., Lo Muzio L. (2004) Calcif. Tissue Int. 75, 509–516 [DOI] [PubMed] [Google Scholar]
- 46.Komori T. (2010) Cell Tissue Res. 339, 189–195 [DOI] [PubMed] [Google Scholar]
- 47.Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997) Cell 89, 747–754 [DOI] [PubMed] [Google Scholar]
- 48.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. (1997) Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
- 49.Banerjee C., McCabe L. R., Choi J. Y., Hiebert S. W., Stein J. L., Stein G. S., Lian J. B. (1997) J. Cell. Biochem. 66, 1–8 [DOI] [PubMed] [Google Scholar]
- 50.Ducy P., Starbuck M., Priemel M., Shen J., Pinero G., Geoffroy V., Amling M., Karsenty G. (1999) Genes Dev. 13, 1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aberg T., Cavender A., Gaikwad J. S., Bronckers A. L., Wang X., Waltimo-Sirén J., Thesleff I., D'Souza R. N. (2004) J. Histochem. Cytochem. 52, 131–139 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.