Abstract
Glucose homeostasis in mammals is mainly regulated by insulin signaling. It was previously shown that SIRT6 mutant mice die before 4 weeks of age, displaying profound abnormalities, including low insulin, hypoglycemia, and premature aging. To investigate mechanisms underlying the pleiotropic phenotypes associated with SIRT6 deficiency, we generated mice carrying targeted disruption of SIRT6. We found that 60% of SIRT6−/− animals had very low levels of blood glucose and died shortly after weaning. The remaining animals, which have relatively higher concentrations of glucose, survived the early post-weaning lethality, but most died within one year of age. Significantly, feeding the mice with glucose-containing water increased blood glucose and rescued 83% of mutant mice, suggesting that the hypoglycemia is a major cause for the lethality. We showed that SIRT6 deficiency results in more abundant membrane association of glucose transporters 1 and 4, which enhances glucose uptake. We further demonstrated that SIRT6 negatively regulates AKT phosphorylation at Ser-473 and Thr-308 through inhibition of multiple upstream molecules, including insulin receptor, IRS1, and IRS2. The absence of SIRT6, consequently, enhances insulin signaling and activation of AKT, leading to hypoglycemia. These data uncover an essential role of SIRT6 in modulating glucose metabolism through mediating insulin sensitivity.
Keywords: Akt PKB, Glucose, Glucose Transport, Insulin, Metabolism, GLUTs, Insulin Signaling
Introduction
Silent information regulator 2 (Sir2)3 in yeast, Caenorhabditis elegans, and Drosophila serves as a histone deacetylase that regulates DNA recombination, genomic stability, and lifespan (1–4). Mammalian homologs of Sir2 expand to a gene family of seven sirtuin proteins (SIRT1–7), which not only serve as type III histone deacetylases but also deacetylate many proteins that are involved in multiple biological processes, including cell fate determination, DNA damage repair, neuronal protection, adaptation to calorie restriction, organ metabolism and function, age-related diseases, and tumorigenesis (3–12).
Using gene targeting, six Sirtuins have been disrupted. Whereas these mutant mice displayed distinct phenotypes, SIRT1 and SIRT6 mutant mice showed the most severe phenotypes (8). SIRT1 mutant mice die during gestation and early postnatal life, displaying genetic instability and some developmental defects (13–15). SIRT1+/− mice were normal; however suffered spontaneous tumorigenesis when one wild-type allele of p53 is mutated (13). SIRT6 mutant mice died before 4 weeks of age, displaying profound lymphopenia, loss of subcutaneous fat, lordokyphosis, low insulin and hypoglycemia, and premature aging (16). Because SIRT6 is involved in base excision repair (BER), it was suspected that DNA damage could serve as a trigger for the death (16, 17). Recently, a study showed that SIRT6 deficiency activates NF-κB signaling through interaction with RelA/p65, which may be partially responsible for the lethality of SIRT6−/− mice (18). Consistent with this, deletion of one allele of RelA/p65 overcame lethality of ∼40% of SIRT6 mutant mice. However, the surviving mutant mice still have lower body weight and blood glucose for the first month after birth (18), suggesting these phenotypes are not a direct cause of activation of NF-κB signaling.
To understand the potential causes for the hypoglycemia and to study functions of SIRT6 further, we generated a SIRT6 mutant mouse strain independently and analyzed its phenotypes. Our data revealed that the absence of SIRT6 enhanced phosphorylation of AKT and glucose uptake in multiple tissues/organs, which accounts for the severe hypoglycemia in the mutant mice.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
TC1 ES cells derived from 129SvEv mice (19) were transfected with NotI-digested pLoxpneoSirt6 and selected with G418 and FIAU as described (20). Hepa1–6 cells were cultured in DMEM with 10% FBS. C2C12 cells were cultured in DMEM with 10% FBS. For differentiation into myotubes, 2% horse serum (Invitrogen) was added into DMEM instead of FBS. Cells were cultured for 4 days, serum starved (0.1% horse serum in DMEM) overnight, then incubated in KRH buffer (25 mm Hepes pH7.5, 140 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1.2 mm KH2PO4, 2.5 mm MgSO4, 5MM NaHCO3, and 0.1% BSA) for 3 h, then 5 mm glucose and insulin were added for 20 min at 37 °C. Cells were harvested for RNA and protein.
Mating and Genotyping Mice
Chimeric mice were mated with NIH Black Swiss females (Taconic) to screen for germline transmission. Male mice bearing germline transmission were mated with female FVB EIIa-Cre mice (21) to generate whole body deletion of exons 2 and 3 of the Sirt6 gene according to a procedure described (22). Mice carrying the Sirt6Δ2–3 allele were genotyped by PCR using primers F1 (GCTAATGGGAACGAGACCAA) and R3 (GCGTCCACTTCTCTTTCCTG), which detect a band of 524bp. The wild-type allele was detected by PCR using the primers F1 and R1 (ACCCACCTCTCTCCCCTAAA), which detect a band of 390 bp. All experiments were approved by the Animal Care and Use Committee of the National Institute of Diabetes, Digestive and Kidney Diseases (ACUC, NIDDK).
2-Deoxy-glucose Uptake
For mice: mice (25–28-days old) were injected with 2-deoxy-d-[1-14C]glucose (Perkin Elmer, 10μCi per mouse) with or without insulin (0.75U per kg body weight) intraperitoneally. Animals were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine 40 min after injection. Under deep anesthesia multiple tissues were dissected and frozen immediately in liquid nitrogen. Tissue samples (50 mg) were homogenized in 500 μl of dH2O, incubated at room temperature for 30 min, then in hot water bath (100 °C) for 10 min, and allowed to cool down to room temperature. After centrifugation (4000 rev/min at 20 °C for 20 min), the supernatant was transferred to ion-exchange columns (Bio-Rad Poly-Prep Prefilled Chromatography Columns, catalogue 731-6211). The columns were washed with 2 ml of dH2O three times (6 ml of dH2O total), the samples were eluted with 2 ml of 0.2 m formic acid/0.5 m ammonium acetate three times, 1 ml of eluate was mixed with 10 ml of scintillation mixture and counted on scintillation counter. 2-Deoxy-glucose is expressed in dmp/mg tissue. For cells: differentiated C2C12 cells transduced with lentiviral shRNA specific to SIRT6 or to luciferase, or MEF cells with wild type or mutated Sirt6 were serum-starved overnight, and incubated with KRH buffer. The cells were then incubated with 0 nm or 100 nm insulin for 20 min in 450 μl of KRH buffer. Glucose uptake was initiated by the addition of 50 μl of KRH buffer containing 0.5 mm 2-deoxy-d-[1,2-3H]glucose (0.25 μCi) (Perkin Elmer) to each well; after 5 min, uptake was terminated by washing the cells three times with ice-cold KRH buffer. The cells were solubilized, and the incorporated radioactivity was measured by liquid scintillation counting.
Plasma Insulin Assay
Plasma insulin concentrations were measured using rat insulin RIA (Millipore).
Plasmids and Lentiviral Vectors
The sh-SIRT6 lentiviral construct was purchased from Open Biosystems. For lentiviral expression, mouse SIRT6 Flag was cloned into pCDH-CMV-MCS-EF1-GFP from System Biosciences (Mountain View, CA) to make pCDH-SIRT6. The packaging and envelope vectors psPAX2 and VSV-G were obtained from Addgene. 293T cells were transfected with sh-SIRT6 or pCDH-SIRT6, psPAX2, and VSV-G using Fugene 6 for 24 h. The medium was changed and collected after 24 and 48 h, respectively.
RT-PCR and Real-time PCR
Total RNA from cells or tissues were extracted with RNA STAT-60TM following the manufacturer's protocol (TEL-TEST, INC), and cDNA was generated by Cells-to-cDNATMII (Ambion, Inc). Quantitative RT-PCR was performed using a SYBR green PCR Master Mix (Applied Biosystems) and the 7500 Real Time PCR system (Applied Biosystems).
Western Blotting
Western blot was carried out with antibodies against GLUT1 (Abcam), GLUT4 (a gift from Dr. Samuel Cushman), IR, p-IR, IRS2 (Upstate), IRS1, pan-AKT, p-AKT (S473 and T308) (Cell Signaling Technology), α-tubulin, and β-actin (Sigma). Signals were detected by chemiluminescent substrate (Thermo Scientific and Millipore).
Immunohistochemistry and Immunofluorescence Staining
Paraffin sections of 5 μm thickness were deparaffinized, hydrated through a graded alcohol series, and stained with monoclonal antibody against Glut1 (Abcam) using regular procedure. Mouse muscle fibers were teased from soleus and gastrocnemius muscle and blocked with animal-free blocker (Vector)/1.5% BSA for 1 h at room temperature in a well of a 24-well plate, then stained overnight with antibody against GLUT4 at 4 °C with shaking. After washing with PBS three times, muscle fibers were stained with Alexa Fluor 488 goat anti-rabbit IgG(H+L) (Invitrogen) for 1 h at room temperature, washed, and mounted on slides with Prolong Gold antifade reagent (Invitrogen) and photographed.
RESULTS
SIRT6 Deficiency Results in Hypoglycemia and Early Post-weaning Lethality of Most Mutant Mice
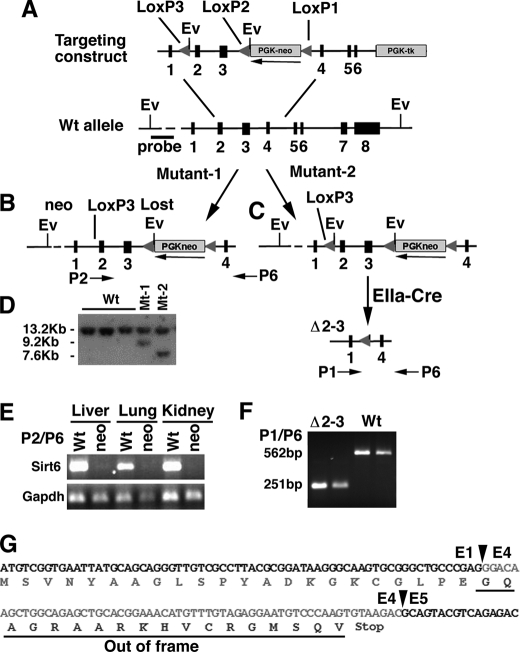
The Sirt6 gene was mutated by either inserting a neomycin gene at the opposite orientation of the Sirt6 gene into intron 3 or deleting exons 2 and 3 of Sirt6 gene (Fig. 1, A–D). RT-PCR analysis did not detect Sirt6 transcripts in mice homozygous for neo insertion (Sirt6neo/neo), indicating that the presence of the neo gene in intron 3 of the Sirt6 gene blocked normal splicing of Sirt6 (Fig. 1E). We detected a smaller transcript generated by directly splicing from exon 1 to exon 4 in mice carrying a homozygous deletion of exons 2 and 3 (Sirt6Δ2–3/Δ2–3) (Fig. 1F). This aberrant transcript cannot generate a normal SIRT6 protein, as the joining of exons 1 and 4 makes a frameshift and a stop codon before the end of exon 4 (Fig. 1G). Both Sirt6neo/neo and Sirt6Δ2–3/Δ2–3 mice did not contain SIRT6 protein as revealed by Western blot analysis (Fig. 2A) and showed identical phenotypes.
FIGURE 1.
Generation of Sirt6 mutant mice. A–C, targeted disruption of SIRT6 in mice. The Sirt6 targeting construct contains three loxP sites, one in intron 1 (LoxP3) and two flanked a neo gene (LoxP1 and P2) (A). Homologous recombination in embryonic stem (ES) cells can generate two types of mutant ES cells, i.e. mutant-1 that loses the third loxP site (B) and mutant-2 that maintains the third loxP site (C). In the mutant-2 animals, exons 2 and 3 can be deleted by crossing them with an EIIa-Cre mouse. D, Southern blot analysis of genomic DNA of ES cells that are cut by EcoRV and hybridized with a 5′ external probe. The wild-type allele is about 13.2 kb, whereas the targeted alleles are about 7.6 kb (mutant-2) and 9.2 kb (Mutant-1), respectively. E, RT-PCR analysis of Sirt6 mRNA in the liver, lung, and kidney of a mutant-1 animal using primer pair located in exon 2 (P2) and exon 6 (P6). F, RT-PCR analysis using primer pair located in exons 1 (P1) and P6 detects a smaller transcript (251 bp) in the mutant-2 animal. G, sequence of the 251-bp fragment showing the junctions (arrowheads) between exons 1 and 4, and exons 4 and 5. The joining of exons 1 and 4 would generate a frameshift product (underlined) if it could be produced.
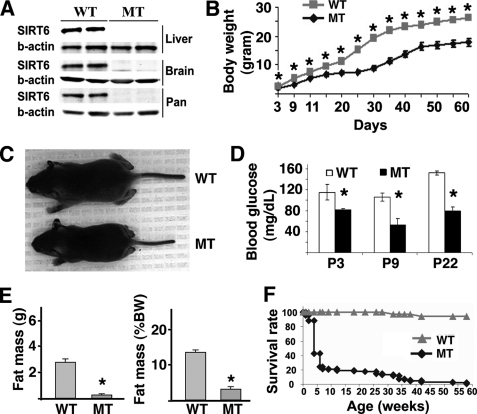
FIGURE 2.
Characterization of the SIRT6 mutant mice. A, Western blot analysis showing SIRT6 protein in the brain, liver, and pancreas of WT mice but not in SIRT6 mutant (MT) mice. B, body weight of SIRT6 wild-type and mutant mice from P3 to P60. C, SIRT6 wild-type and mutant mice at P15. D, levels of blood glucose in mutant and wild-type mice (at least 3 pairs of mice were measured at each time point). E, body fat in live animals as measured by using EchoMRI 3-in-1TM (Echo Medical Systems) shown as absolute mass (left) and percent of body weight (right). F, survival profile of SIRT6 mutant (n = 154) and wild-type (n = 143) mice. * represents p < 0.05 by Student's t test.
The Sirt6Δ2–3/Δ2–3 mice were born without obvious defects, but weighed less starting from postnatal day 3 (P3) (Fig. 2B). Some of them became smaller starting from P6, and all were smaller than their wild-type littermates at P15 and onward (Fig. 2C). Sirt6Δ2–3/Δ2–3 mice displayed hypoglycemia as early as P3, and maintained low blood glucose throughout the nursing period (Fig. 2D). They also displayed markedly reduced total body fat as measured in live animal (Fig. 2E), loss of visceral and subcutaneous fat (supplemental Fig. S1, A–D), lordokyphosis, eye abnormality (having discharges and not fully opened), malocclusion, as well as some progeroid syndrome-related phenotypes, including kyphosis, reduced bone density, and increased staining for acidic β-galactosidase (supplemental Fig. S1, E and F and data not shown). Of note, ∼60% of Sirt6Δ2–3/Δ2–3 mice (n>150) with a mixed genetic background (129/Black Swiss/FVB) died at about one month of age and the remaining mice died at various stages within one year of age (Fig. 2F). Out of a smaller number of mice (n = 13) in a pure 129 genetic background, 10 mice died before P30, 1 died at P31, 1 at P39, and another at 7 months of age.
Because SIRT6 plays a role in BER (16), SIRT6 deficiency might trigger p53 activation due to genetic instability associated with impaired DNA damage repair, which may partially contribute to the lethality. To test this, we introduced a p53-null mutation into Sirt6Δ2–3/Δ2–3 mice. Our data indicated that Sirt6Δ2–3/Δ2–3 mice exhibited a similar phenotype irrespective of their p53 status (i.e. p53+/+, p53+/−, and p53−/−) (supplemental Fig. S2A). Sirt6Δ2–3/Δ2–3;p53+/− and Sirt6Δ2–3/Δ2–3;p53−/− also exhibited reduced body weight (supplemental Fig. S2B), low blood glucose (supplemental Fig. S2C), and died invariably during the first year after birth (supplemental Fig. S2D), suggesting that the post-weaning lethality and hypoglycemia are independent of p53.
Despite the slight difference in viability during the first month after birth, all SIRT6 mutant mice regardless of their genetic background showed severe hypoglycemia. Therefore, in our study on glucose metabolism we used Sirt6Δ2–3/Δ2–3 mice with a mixed genetic background.
Hypoglycemia Serves as One of the Major Causes for Lethality Associated with SIRT6 Deficiency
Because the majority of Sirt6Δ2–3/Δ2–3 mice died during the first week after weaning, we suspected that this time might represent a stressful period during which most mutant mice could not adapt to their new environment. In fact, all animals must face a transition of energy intake from mother's milk to their own digestive system after weaning. Our data revealed that although all Sirt6Δ2–3/Δ2–3 mice at P22 had reduced levels of blood glucose, they exhibited a marked variation (Fig. 3A). Therefore, we investigated if there was any correlation between blood glucose concentration and lethality of each animal. Our data revealed that in the group of mice that died before 4 weeks of age, their blood glucose was very low prior to death, while the survivors had obviously higher blood glucose, although their glucose levels were still lower than wild-type mice during this period of time (Fig. 3B). The levels of blood glucose were also positively correlated with the body weight of these mice, i.e. the mutant mice, which died, weighed less than the survivors (Fig. 3C). After P35, some survivor mutant animals gradually reached a normal level of blood glucose although differences in body weight remained obvious at multiple time points (Fig. 3, B and C).
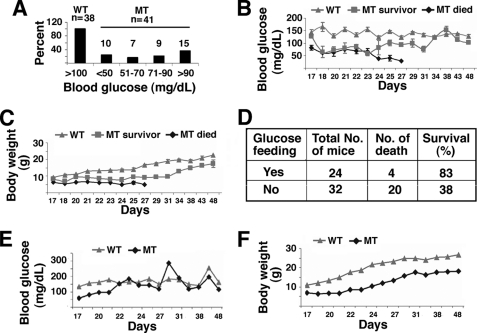
FIGURE 3.
Hypoglycemia and partial rescue of SIRT6 mutant mice by glucose. A, blood glucose levels in mutants (n = 41) and wild-type controls at P22 (n = 38). Mutant mice exhibit significant variation ranging from <50 mg/dL to >90 mg/dL. B and C, blood glucose (B) and body weight (C) of mice during a period from P17 to P48. D, rescue of SIRT6 lethality by feeding the mutant mice with water containing 10% glucose. E–F, blood glucose (E) and body weight (F) of mice that were supplied with water containing 10% glucose starting at P22.
Because glucose is a major source of energy, we suspected that the low level of blood glucose could be a cause for lethality. To test this, we fed Sirt6Δ2–3/Δ2–3 mice with water supplemented with 10% glucose starting from P22 when the animals were weaned. After feeding with glucose, only 4 out of 24 (17%) died, while in the control group of 32 mice fed with regular water, 20 (62%) died (Fig. 3D). These data indicate that hypoglycemia is one of the major causes for the early post-weaning lethality.
Next, we compared the body weight and glucose levels of live mice. With the presence of 10% glucose in the water, the blood glucose of Sirt6Δ2–3/Δ2–3 mice quickly increased, reaching a level similar to that of wild-type mice at P30 and maintained comparable levels thereafter (Fig. 3E). Mutant mice also increased body weight but they did not reach the same level as the controls during the same period (Fig. 3F). We also studied the fertility of Sirt6Δ2–3/Δ2–3 mice by mating them with wild-type mice of the opposite sex. Out of a total of 12 females tested, six gave birth but none of them could nurse their pups. On the other hand, all Sirt6Δ2–3/Δ2–3 males tested (n = 6) could not impregnate females although their sperms fertilized eggs equally well compared with wild-type sperms in an in vitro fertilization study (data not shown). These observations suggest that although Sirt6Δ2–3/Δ2–3 mice survived through the “stress period,” they were not completely normal because of the absence of SIRT6.
Enhanced Glucose Uptake and Insulin Sensitivity in SIRT6 Mutant Mice
Hypoglycemia could be caused by a number of factors, including impaired gluconeogenesis, reduced food intake, and increased glucose uptake into peripheral organs/tissues. Our data ruled out the first two possibilities (supplemental Fig. S3, A and B), therefore, we studied glucose uptake by performing a 2-deoxyglucose-uptake experiment. Our data showed that under physiological conditions, without giving exogenous insulin to the mice, there was slightly increased glucose uptake in all tissues examined in Sirt6Δ2–3/Δ2–3 mice including the quadriceps (white muscle), gastrocnemius (red muscle), brown fat, white fat, spleen, liver, thymus, lung, and skin, although the increased uptake reached a statistically significant level only in the spleen as compared with controls (Fig. 4A).
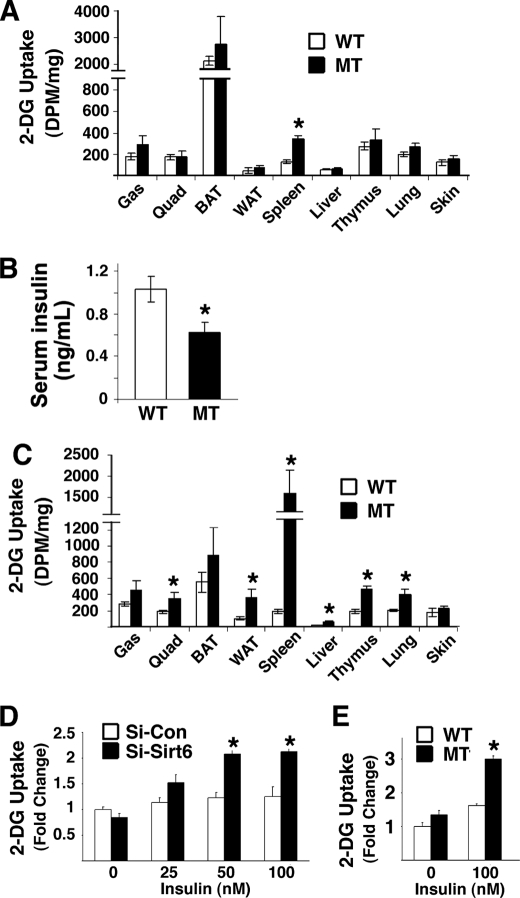
FIGURE 4.
Glucose uptake test in vivo and in vitro. A, 2-deoxyglucose-uptake experiment in vivo without injecting insulin. Slightly increased glucose uptake was observed in all nine tissues in Sirt6Δ2–3/Δ2–3 mice compared with wild-type control mice. Five pairs of P25–26 mice at fed condition were used in the assay. B, insulin levels of the mice used in the 2-DG uptake test in A. C, 2-deoxyglucose-uptake experiment conducted 40 min. after injection of insulin (0.75 units/kg body weight). Significantly increased glucose uptake was observed in most organs/tissues examined in Sirt6Δ2–3/Δ2–3 mice compared with wild-type control mice. At least five pairs of fed mice at P25–28 were used in the assay. D, 2-deoxyglucose-uptake conducted in C2C12 cells that were infected with lentiviral shRNA specific to SIRT6 or to luciferase. (SIRT6 knockdown efficiency was similar to that shown in Fig. 5E.) E, 2-deoxyglucose-uptake conducted in SIRT6 mutant and control MEFs. * represents p < 0.05 by Student's t test.
Insulin is a major factor that controls blood glucose level. We found that the mutant mice had significantly lower levels of insulin (Fig. 4B). While the cause for this reduction remains elusive, the slightly higher glucose uptake suggests that the mutant mice are more sensitive to insulin. To investigate this, we performed a 2-deoxyglucose-uptake experiment after injection of insulin in the mice. Our data showed significantly increased levels of glucose uptake in the spleen, liver, white muscle, white fat, thymus, and lung, although the increased uptake in the red muscle, brown fat and skin was moderate compared with controls (Fig. 4C). Thus, Sirt6Δ2–3/Δ2–3 mice maintained higher levels of glucose uptake from the blood into many organs, which might be responsible for the hypoglycemia. To rule out the possibility that the increased glucose uptake in Sirt6Δ2–3/Δ2–3 mice is a secondary effect due to other physiological abnormalities of these mice, we measured glucose uptake in C2C12 cells, a cell line widely used for studying the effect of insulin on glucose uptake in muscle cells, which carry shRNA-mediated knockdown of SIRT6. Our data showed significantly increased insulin-stimulated glucose uptake in these cells that have been transduced with lentiviral shRNA specific for SIRT6 compared with control shRNA transduced C2C12 cells (Fig. 4D). A similar increase in glucose uptake was also found in SIRT6 mutant MEFs as compared with their controls (Fig. 4E). These data provide strong evidence that SIRT6 deficiency enhanced glucose uptake both in vitro and in vivo.
SIRT6 Deficiency Activates AKT Both in Vitro and in Vivo
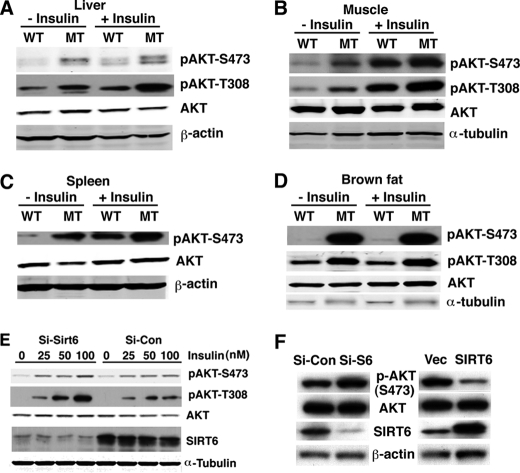
Next, we studied the potential molecular mechanism underlying enhanced insulin sensitivity and glucose uptake in the mutant mice. Insulin treatment induces tyrosine phosphorylation of insulin receptor substrates (IRSs), which interact with the p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3K), leading to the activation of a cascade of phosphorylation events, resulting in activation of AKT, which plays an important role in cellular glucose uptake (23, 24). Therefore, we analyzed pAKT levels in SIRT6 mutant mice. We observed that pAKT levels at both pAKT-S473 and -T380 were increased in the liver, muscle, spleen, and brown fat of SIRT6 mutant mice as compared with control mice prior to insulin treatment (Fig. 5, A–D). There were increased pAKT levels in both wild-type and mutant mice after insulin treatment (Fig. 5, A–D), indicating both SIRT6 wild-type and mutant mice were responsive to exogenous insulin treatment.
FIGURE 5.
SIRT6 deficiency increases AKT activation in vivo and in vitro. A–D, SIRT6 deficiency increases phosphorylation of AKT in liver (A), muscle (B), spleen (C), and brown fat (D). E, levels of AKT phosphorylation in C2C12 cells after infection with shRNA constructs that are specific to SIRT6 or luciferase. F, levels of AKT phosphorylation in Hepa1–6 cells carrying overexpression of SIRT6 or vector control.
We showed earlier that C2C12 cells carrying shRNA-mediated knockdown of SIRT6 exhibited increased glucose uptake upon insulin treatment (Fig. 4D). These cells also showed higher levels of phosphorylation of AKT at S473 and T308 than control shRNA-treated cells upon insulin treatment (Fig. 5E). A similar increase in phosphorylation of AKT was also observed in another cell line, Hepa1–6 with acute suppression of SIRT6 by shRNA (Fig. 5F, left), and consistently, overexpression of SIRT6 in these cells decreased pAKT (Fig. 5F, right). As the phosphorylated AKT is the activated form, these data indicate that SIRT6 deficiency enhanced AKT activity, which enhances glucose uptake both in vitro and in vivo.
SIRT6 Deficiency Enhanced Membrane Recruitment of GLUT1 and GLUT4
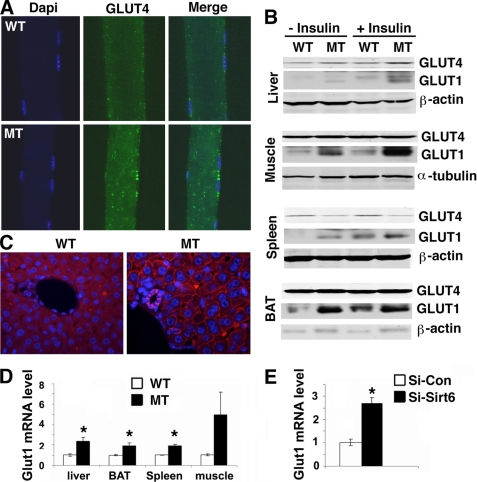
Glucose is transported into cells through glucose transporters (GLUTs) (25–27). It is known that glucose uptake into muscle and fat are primarily regulated by insulin/AKT through their functions that recruit GLUT4 to the plasma membrane. Our analysis revealed markedly increased GLUT4 intensity on the membrane of muscle cells of SIRT6 mutant mice compared with wild-type cells under both conditions without (Fig. 6A) and with insulin treatment (data now shown), whereas no change of GLUT4 protein level was detected (Fig. 6B).
FIGURE 6.
Location and expression of GLUT1 and GLUT4 in vivo. A, SIRT6 deficiency increases membrane association of GLUT4 in the muscle under physiological conditions. B, Western blot analysis of GLUT1 and GLUT4 in the liver, muscle, spleen, and brown fat from mice without or with insulin treatment (0.75 units/kg body weight, 30 min after IP injection). C, SIRT6 deficiency increases membrane association of GLUT1 in the liver. D and E. SIRT6 deficiency increases Glut1 transcription in the liver, muscle, spleen, and brown fat (D) and C2C12 cells carrying shRNA-mediated knockdown of SIRT6 (E). * represents p < 0.05 by Student's t test.
It is also known that GLUT1 is widely expressed in many tissues and provides these tissues with their basal glucose requirement. Therefore, we next examined GLUT1 levels in multiple tissues by immunohistochemical and immunofluorescent staining and found it was also markedly increased in these tissues (Fig. 6C, supplemental Fig. S4, and data not shown). Western blot analysis revealed increased total protein of GLUT1 in multiple organs/tissues (Fig. 6B). To examine whether the increased GLUT1 is caused by transcription, we examined mRNA levels by real time RT-PCR. Our data detected an obvious increase of Glut1 transcript in multiple organs (Fig. 6D), and in C2C12 cells carrying shRNA-mediated knockdown of SIRT6 (Fig. 6E), suggesting that the increased GLUT1 is caused by transcriptional activation of this gene. Because GLUT1 is widely expressed in many organs/tissues (25), its increased level may account for the enhanced basal level of glucose uptake in SIRT6 mutant mice without insulin treatment. On the other hand, we found that insulin treatment increased membrane association of both GLUT1 and GLUT4 (Fig. 6, A and C), and total protein level of GLUT1 (Fig. 6B), this may account for the marked increase in glucose uptake in SIRT6 mutant mice after insulin treatment. These data indicate that SIRT6 deficiency causes both insulin dependent and independent glucose uptake, which is responsible for the hypoglycemia in the mutant mice.
SIRT6 Deficiency Enhances Insulin Signaling Upstream of AKT and Inhibition of AKT Activation Blocks Insulin-stimulated Glucose Uptake
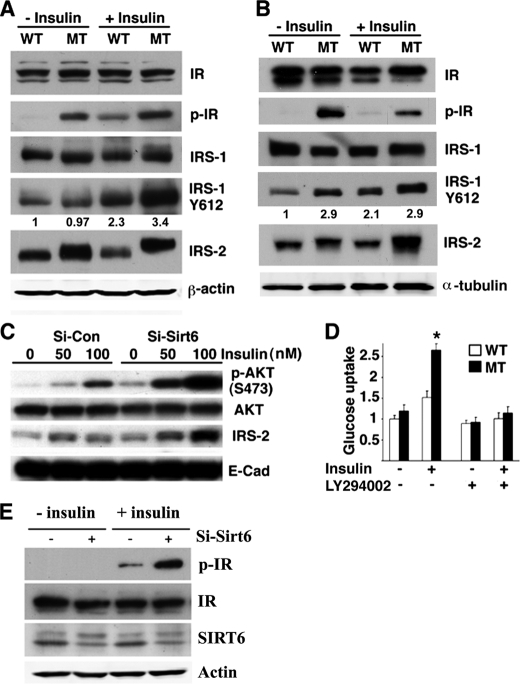
Next, we investigated how the absence of SIRT6 increased phosphorylation of AKT. Levels of pAKT can be affected by many factors, including multiple growth factors, kinases, protein phosphatases, and pAKT stability. To investigate this, we examined expression of several proteins that are upstream of AKT in SIRT6 mutant mice. These experiments detected significantly increased phosphorylation of IRS1 and insulin receptor (IR) upon insulin treatment in the liver (Fig. 7A) and IRS2 total level both in the liver (Fig. 7A) and muscle (Fig. 7B), and phosphorylation of IR in the muscle (Fig. 7B). An increased level of IRS2 was also observed in the C2C12 cells carrying acute suppression of SIRT6 by shRNA (Fig. 7C).
FIGURE 7.
SIRT6 deficiency enhances insulin signaling upstream of AKT and inhibition of AKT activation blocks insulin-stimulated glucose uptake. A and B, levels of expression and phosphorylation of insulin receptor, IRS1, and IRS2 in the liver (A) and muscle (B) in SIRT6 mutant and wild-type mice before and after insulin treatment (0.75 units/kg body weight, 30 min after IP injection). Numbers in A and B indicated the ratios of p-IRS1(Y612)/IRS-1. C, shRNA-mediated knockdown of SIRT6 increases levels of IRS2 before and after insulin treatment for 20 min in C2C12 cells. (Sirt6 knockdown efficiency is similar to that shown in Fig. 5E.) D, LY294002 inhibits insulin-stimulated glucose uptake in SIRT6 mutant MEF cells. E, shRNA-mediated knockdown of SIRT6 in Hepa1–6 cells increases pIR levels upon insulin treatment. Insulin treatment in D and E was 100 nm for 20 min.
It has been shown that insulin treatment, through insulin receptor, IRS1, IRS2, and the PI3K cascade, activates AKT, which enhances glucose uptake (23, 24). To test if this is the case, we treated MEF cells with LY294002, an inhibitor of PI3K activity, and observed that inhibition of PI3K activity blocked glucose uptake by insulin treatment (Fig. 7D). These data suggest that SIRT6 negatively regulates AKT activity through modulation of the insulin signaling pathway and the absence of SIRT6, consequently, causes increased insulin sensitivity and activation of AKT, leading to enhanced glucose uptake. In accordance with the in vivo data, we also observed increased phosphorylation of IR in the hepatocyte cell line Hepa1–6 when SIRT6 was knocked down by lentiviral shRNA (Fig. 7E).
DISCUSSION
In this study, we have studied the potential role of SIRT6 in insulin sensitivity and glucose metabolism in mice carrying targeted disruption of SIRT6. We found that all SIRT6 mutant mice were all very sick and exhibited a significant reduction in blood glucose, which we suspect to be a major cause for the early post-weaning lethality of about 60% of the mutant mice. Consistently, feeding the mutant mice with glucose containing water reduced the lethality to about 17%. We further demonstrate that the hypoglycemia in the SIRT6 mutant mice is caused by enhanced glucose uptake in multiple organs/tissues that is accompanied by altered expression, cellular location, or phosphorylation of several key factors, including insulin receptor, IRS1, IRS2, AKT, GLUT1, and GLUT4, which play an essential role in glucose uptake (23, 25–27). Similar molecular changes and enhanced glucose uptake were also observed in cultured cells carrying shRNA-mediated acute suppression of SIRT6. These data uncover an important role of SIRT6 in modulation of glucose homeostasis through inhibiting the insulin signaling pathway, and provide evidence that the hypoglycemia phenotype in SIRT6 mutant mice is directly caused by the absence of SIRT6.
In humans, hypoglycemia is relatively common in patients with diabetes treated with insulin (28) although it can be also triggered by a combination of many other factors (29). However, SIRT6 mutant mice have a much lower level of blood insulin, which should in theory reduce glucose uptake into peripheral organs/tissues, leading to higher levels of blood glucose (23). Paradoxically, these mice exhibit an opposite phenotype, i.e. much lower glucose in blood caused by an increased basal level of glucose uptake regardless of their low blood insulin concentration. Several lines of evidence also indicate that SIRT6-deficient mice are much more sensitive to insulin. First, we showed that SIRT6 deficiency increases AKT phosphorylation at Ser-473 and Thr-308, which is linked to the activation of this protein (30, 31). The increase in pAKT levels occurs in multiple organs of SIRT6 mutant mice even without treatment of exogenous insulin. Second, we detected increased phosphorylation of insulin receptor, IRS1, and IRS2. Previous studies indicated that the phosphorylation of insulin receptor and its adaptor proteins is solely due to ligand-dependent activation (32). It is generally believed that muscle and adipose tissues are insulin sensitive organs/tissues in terms of glucose uptake (23, 25–27). However, the increased phosphorylation of these proteins occurs in many other organs/tissues of SIRT6 mutant mice, suggesting that the absence of SIRT6 sensitizes SIRT6 mice as a whole organism to insulin action. Third, enhanced insulin-stimulated glucose uptake was found in all organs/tissues of SIRT6 mutant mice examined, and in cultured SIRT6-deficient MEFs as well as in C2C12 cells carrying shRNA-mediated knockdown of SIRT6. These data indicate that SIRT6 negatively regulates insulin action through modulation of multiple signaling molecules upstream of AKT.
We showed that SIRT6 deficiency enhances membrane association of two major glucose transporters, GLUT1 and GLUT4. Both transporters are stored internally in membrane vesicles after they are made (23, 25–27). It is known that insulin-AKT signaling stimulates fusion of vesicles carrying GLUT4 with the plasma membrane and causes the transport of glucose into cells (23). Insulin can also increase glucose transport activity due to biosynthetic elevation of GLUT1 in L6 muscle cells (33) and in rat cardiac muscle in vivo (34), and increase the membrane association of GLUT1 (35, 36). Previous investigations demonstrated that members in the insulin signaling pathway, such as IRS2 and AKT can also recruit GLUT1 to the cell membrane and maintain glucose uptake (37, 38). The increased levels of IRS2 and pAKT could conceivably recruit surface bound GLUT1, leading to the enhanced glucose uptake. Our data showed that there are increased GLUT1 protein and RNA levels, as well as enhanced membrane association of both GLUT1 and GLUT4 in multiple tissues/organs of the Sirt6Δ2–3/Δ2–3 mice (Fig. 6, A–D and not shown), which is consistent with these reports. Of note, SIRT6 deficiency also increased transcription of the Glut1 gene. The transcription of Glut1 can be regulated by many factors, such as hypoxia-inducible factor-1α (HIF-1α) (39), transcription factor EPAS1 (40), insulin (41), and altered glucose concentration (42). Indeed, a recent study showed that SIRT6 regulates glucose homeostasis by functioning as a corepressor of HIF-1α (43). Our study revealed a correlation between enhanced insulin/pIR/pAKT signaling and GLUT1 as well as GLUT4, which may be complementary to the finding that increased GLUT1 in SIRT6 mutant mice could also result from increased HIF-1α levels (43).
It was shown that all mice carrying a targeted deletion of SIRT6 exon 1 through exon 6 (Sirt6Δ1–6/Δ1–6) in a 129SvJ genetic background died before 4 weeks of age (16). We found that among 13 mutant mice in 129SvEv background, 10 died before P30, 1 died at P31, 1 at P39 and another died at 7 months. Our data clearly indicate that genetic background can be a factor that affects viability of SIRT6 mutant mice as up to 40% of our mutant mice in 129/FVB/Black Swiss background were alive at 4 weeks of age. Nonetheless, the fact that most SIRT6 mutant mice, irrespective of their genetic background, died during the first week after weaning suggests there is a stressful period that is not compatible with their viability. While our data suggest that hypoglycemia is one of the major factors for the early post-weaning lethality of SIRT6 mutant mice, we cannot rule out the involvement of other factors, such as a dramatic transition from nursing by mother to the pup's self-feeding. A recent study also showed that SIRT6 deficiency causes activation of NF-κB signaling and the deletion of one allele of RelA/p65 overcame lethality of ∼40% of SIRT6 mutant mice (18). As neither the feeding with glucose water nor the reduced NF-κB signaling could completely rescue the early post-weaning lethality of SIRT6 mutant mice, it is possible both factors are involved in the phenotypic development of SIRT6 mutant mice. This is an interesting issue and should be addressed further.
In summary, we showed that SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. The absence of SIRT6 triggers a marked increase in GLUT1 abundance, which may be responsible for the enhanced basal level of glucose uptake. Concurrently, it also results in activation of multiple members of the insulin signaling pathway, including insulin receptor, IRS1, IRS2, and pAKT in many organs/tissues, which normally do not respond to insulin. This should contribute to the widespread and enhanced basal level and insulin-stimulated glucose uptake in the mutant mice. Our finding that the absence of SIRT6 sensitizes mice to insulin signaling is of great significance as insulin resistance is a major cause for type II diabetes, one of the most common chronic diseases in humans, affecting over 124 million individuals worldwide (44). Approaches for the partial inhibition of SIRT6 or its related proteins may be beneficial for the prevention and/or therapeutic treatment of type II diabetes in the near future.
Supplementary Material
Acknowledgments
We thank Dr. Samuel Cushman for providing an anti-GLUT4 antibody, and Drs. Cuiling Li, Tatyana Chanturiya, Hisashi Hasumi, and Yunping Wu for technical assistance. We thank members of the Deng laboratory for helpful discussion.
This work was supported, in whole or in part, by the Intramural Research Program of NIDDK, National Institutes of Health.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- Sir2
- silent information regulator 2
- BER
- base excision repair
- GLUT
- glucose transporter.
REFERENCES
- 1.Blander G., Guarente L. (2004) Annu. Rev. Biochem. 73, 417–435 [DOI] [PubMed] [Google Scholar]
- 2.Guarente L., Kenyon C. (2000) Nature 408, 255–262 [DOI] [PubMed] [Google Scholar]
- 3.Saunders L. R., Verdin E. (2007) Oncogene 26, 5489–5504 [DOI] [PubMed] [Google Scholar]
- 4.Vaquero A., Scher M., Erdjument-Bromage H., Tempst P., Serrano L., Reinberg D. (2007) Nature 450, 440–444 [DOI] [PubMed] [Google Scholar]
- 5.Haigis M. C., Guarente L. P. (2006) Genes Dev. 20, 2913–2921 [DOI] [PubMed] [Google Scholar]
- 6.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng C. X. (2009) Int. J. Biol. Sci. 5, 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T., Deng C. X., Mostoslavsky R. (2009) Nature 460, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarente L. (2007) Cold Spring Harb. Symp. Quant. Biol. 72, 483–488 [DOI] [PubMed] [Google Scholar]
- 10.Kim H. S., Patel K., Muldoon-Jacobs K., Bisht K. S., Aykin-Burns N., Pennington J. D., van der Meer R., Nguyen P., Savage J., Owens K. M., Vassilopoulos A., Ozden O., Park S. H., Singh K. K., Abdulkadir S. A., Spitz D. R., Deng C. X., Gius D. (2010) Cancer Cell 17, 41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs K. M., Pennington J. D., Bisht K. S., Aykin-Burns N., Kim H. S., Mishra M., Sun L., Nguyen P., Ahn B. H., Leclerc J., Deng C. X., Spitz D. R., Gius D. (2008) Int. J. Biol. Sci. 4, 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn B. H., Kim H. S., Song S., Lee I. H., Liu J., Vassilopoulos A., Deng C. X., Finkel T. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 14447–14452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R. H., Sengupta K., Li C., Kim H. S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B., Jia R., Zheng Z. M., Appella E., Wang X. W., Ried T., Deng C. X. (2008) Cancer Cell 14, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H. L., Mostoslavsky R., Saito S., Manis J. P., Gu Y., Patel P., Bronson R., Appella E., Alt F. W., Chua K. F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 10794–10799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBurney M. W., Yang X., Jardine K., Hixon M., Boekelheide K., Webb J. R., Lansdorp P. M., Lemieux M. (2003) Mol. Cell. Biol. 23, 38–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mostoslavsky R., Chua K. F., Lombard D. B., Pang W. W., Fischer M. R., Gellon L., Liu P., Mostoslavsky G., Franco S., Murphy M. M., Mills K. D., Patel P., Hsu J. T., Hong A. L., Ford E., Cheng H. L., Kennedy C., Nunez N., Bronson R., Frendewey D., Auerbach W., Valenzuela D., Karow M., Hottiger M. O., Hursting S., Barrett J. C., Guarente L., Mulligan R., Demple B., Yancopoulos G. D., Alt F. W. (2006) Cell 124, 315–329 [DOI] [PubMed] [Google Scholar]
- 17.Lombard D. B., Schwer B., Alt F. W., Mostoslavsky R. (2008) J. Intern. Med. 263, 128–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawahara T. L., Michishita E., Adler A. S., Damian M., Berber E., Lin M., McCord R. A., Ongaigui K. C., Boxer L. D., Chang H. Y., Chua K. F. (2009) Cell 136, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng C., Wynshaw-Boris A., Zhou F., Kuo A., Leder P. (1996) Cell 84, 911–921 [DOI] [PubMed] [Google Scholar]
- 20.Deng C. X., Xu X. (2004) Methods Mol. Biol. 280, 185–200 [DOI] [PubMed] [Google Scholar]
- 21.Lakso M., Pichel J. G., Gorman J. R., Sauer B., Okamoto Y., Lee E., Alt F. W., Westphal H. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 5860–5865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X., Li C., Garrett-Beal L., Larson D., Wynshaw-Boris A., Deng C. X. (2001) Genesis 30, 1–6 [DOI] [PubMed] [Google Scholar]
- 23.Chang L., Chiang S. H., Saltiel A. R. (2004) Mol. Med. 10, 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 25.Mueckler M. (1994) Eur. J. Biochem. 219, 713–725 [DOI] [PubMed] [Google Scholar]
- 26.Scheepers A., Joost H. G., Schürmann A. (2004) JPEN J. Parenter Enteral Nutr. 28, 364–371 [DOI] [PubMed] [Google Scholar]
- 27.Wood I. S., Trayhurn P. (2003) Br. J. Nutr. 89, 3–9 [DOI] [PubMed] [Google Scholar]
- 28.Johnson E. S., Koepsell T. D., Reiber G., Stergachis A., Platt R. (2002) J. Clin. Epidemiol. 55, 253–259 [DOI] [PubMed] [Google Scholar]
- 29.Guettier J. M., Gorden P. (2006) Endocrinol. Metab. Clin. North Am. 35, 753–766, viii–ix [DOI] [PubMed] [Google Scholar]
- 30.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 31.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 32.Saltiel A. R., Kahn C. R. (2001) Nature 414, 799–806 [DOI] [PubMed] [Google Scholar]
- 33.Taha C., Mitsumoto Y., Liu Z., Skolnik E. Y., Klip A. (1995) J. Biol. Chem. 270, 24678–24681 [DOI] [PubMed] [Google Scholar]
- 34.Laybutt D. R., Thompson A. L., Cooney G. J., Kraegen E. W. (1997) Am. J. Physiol. 273, H1309–H1316 [DOI] [PubMed] [Google Scholar]
- 35.Hausdorff S. F., Bennett A. M., Neel B. G., Birnbaum M. J. (1995) J. Biol. Chem. 270, 12965–12968 [DOI] [PubMed] [Google Scholar]
- 36.Taha C., Liu Z., Jin J., Al-Hasani H., Sonenberg N., Klip A. (1999) J. Biol. Chem. 274, 33085–33091 [DOI] [PubMed] [Google Scholar]
- 37.Wieman H. L., Wofford J. A., Rathmell J. C. (2007) Mol. Biol. Cell 18, 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pankratz S. L., Tan E. Y., Fine Y., Mercurio A. M., Shaw L. M. (2009) J. Biol. Chem. 284, 2031–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marín-Hernández A., Gallardo-Pérez J. C., Ralph S. J., Rodríguez-Enríquez S., Moreno-Sánchez R. (2009) Mini Rev. Med. Chem. 9, 1084–1101 [DOI] [PubMed] [Google Scholar]
- 40.Wada T. (2007) Yakugaku Zasshi 127, 143–151 [DOI] [PubMed] [Google Scholar]
- 41.Díaz M., Vraskou Y., Gutiérrez J., Planas J. V. (2009) Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R794–800 [DOI] [PubMed] [Google Scholar]
- 42.Klip A., Tsakiridis T., Marette A., Ortiz P. A. (1994) FASEB J. 8, 43–53 [DOI] [PubMed] [Google Scholar]
- 43.Zhong L., D'Urso A., Toiber D., Sebastian C., Henry R. E., Vadysirisack D. D., Guimaraes A., Marinelli B., Wikstrom J. D., Nir T., Clish C. B., Vaitheesvaran B., Iliopoulos O., Kurland I., Dor Y., Weissleder R., Shirihai O. S., Ellisen L. W., Espinosa J. M., Mostoslavsky R. (2010) Cell 140, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinn L. (2001) Nurs. Clin. North Am. 36, 175–192, v [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.