Abstract
The mechanisms underlying the protective effect of monounsaturated fatty acids (e.g. oleate) against the lipotoxic action of saturated fatty acids (e.g. palmitate) in skeletal muscle cells remain poorly understood. This study aimed to examine the role of mitochondrial long-chain fatty acid (LCFA) oxidation in mediating oleate's protective effect against palmitate-induced lipotoxicity. CPT1 (carnitine palmitoyltransferase 1), which is the key regulatory enzyme of mitochondrial LCFA oxidation, is inhibited by malonyl-CoA, an intermediate of lipogenesis. We showed that expression of a mutant form of CPT1 (CPT1mt), which is active but insensitive to malonyl-CoA inhibition, in C2C12 myotubes led to increased LCFA oxidation flux even in the presence of high concentrations of glucose and insulin. Furthermore, similar to preincubation with oleate, CPT1mt expression protected muscle cells from palmitate-induced apoptosis and insulin resistance by decreasing the content of deleterious palmitate derivates (i.e. diacylglycerols and ceramides). Oleate preincubation exerted its protective effect by two mechanisms: (i) in contrast to CPT1mt expression, oleate preincubation increased the channeling of palmitate toward triglycerides, as a result of enhanced diacylglycerol acyltransferase 2 expression, and (ii) oleate preincubation promoted palmitate oxidation through increasing CPT1 expression and modulating the activities of acetyl-CoA carboxylase and AMP-activated protein kinase. In conclusion, we demonstrated that targeting mitochondrial LCFA oxidation via CPT1mt expression leads to the same protective effect as oleate preincubation, providing strong evidence that redirecting palmitate metabolism toward oxidation is sufficient to protect against palmitate-induced lipotoxicity.
Keywords: Apoptosis, Insulin Resistance, Lipid, Lipid Oxidation, Mitochondria, Skeletal Muscle, Carnitine Palmitoyltransferase 1, Monounsaturated Fatty Acids, Saturated Fatty Acids
Introduction
It has long been recognized that increased plasma free fatty acids are associated with insulin resistance in humans (1). Indeed, plasma free fatty acid concentrations are increased in obese subjects (2) as well as in genetically obese or high fat diet-induced insulin-resistant mice (3, 4). In such conditions, circulating free fatty acid concentrations are elevated, and FA4 metabolism is altered (5–7), leading to ectopic accumulation of FA in the liver, pancreatic β-cells, or skeletal muscle, where they interfere with normal cell function. For instance, FA overload induces skeletal muscle insulin resistance (6), inflammation (8), and cell death via apoptosis (4, 6), a phenomenon commonly referred as “lipotoxicity.” The toxic effects of FA are known to depend on their chain length and degree of saturation. Long-chain saturated FA (SFA), such as palmitate (C16:0) and stearate (C18:0), are the most lipotoxic. Consistently, palmitate induces apoptosis in many cell types (9–12). In muscle cells, palmitate's cytotoxic effect is mediated by increased intracellular concentrations of diacylglycerols (DAG) and ceramides (11). In contrast, monounsaturated FA (MUFA), such as oleate (C18:1), protect against SFA-induced toxicity (8, 13, 14). Whether oleate exerts such a protective effect on palmitate-induced apoptosis in skeletal muscle cells has not been reported.
The mechanisms by which oleate protects cells from palmitate toxicity are not well understood. SFA, which are reported to be less efficiently incorporated into triglycerides (TG) than MUFA, lead to increased accumulation of DAG (8, 14, 15). Oleate has been proposed to protect cells from palmitate-induced lipotoxicity by promoting its esterification into TG, a neutral form of FA storage (8, 15, 16). However, it was recently hypothesized that oleate protects from palmitate-induced insulin resistance and inflammation by increasing its mitochondrial oxidation (as shown by increased CPT1 (carnitine palmitoyltransferase 1) gene expression) (8). CPT1 is a transmembrane enzyme of the mitochondrial outer membrane, which converts long-chain acyl-CoA to acylcarnitine, which enters the mitochondrial matrix and undergoes β-oxidation. Because of its inhibition by malonyl-CoA, an intermediate of lipogenesis synthesized by acetyl-CoA carboxylase (ACC), CPT1 is the key regulatory enzyme of long-chain fatty acid (LCFA) β-oxidation (17). CPT1 exists in at least two isoforms, CPT1A (liver isoform) and CPT1B (muscle isoform), each of which is found in several tissue and cell types (17, 18).
Whether defects in muscle mitochondrial metabolism are a cause or a consequence of insulin resistance has been extensively investigated but remains controversial. In rodents, some argue against the concept that insulin resistance is mediated by muscle mitochondrial dysfunction (19, 20). Others have demonstrated that skeletal muscles from obese and insulin-resistant patients exhibit diminished rates of palmitate oxidation, in association with a decrease in CPT1 activity (21). Obese and insulin-resistant skeletal muscles also have fewer and dysmorphic mitochondria, which may decrease FA oxidative capacity (22, 23). Because accumulation of palmitate-derived metabolites within muscle cells is associated with decreased mitochondrial FA oxidative capacity, increasing LCFA oxidation may exert a protective effect. Additionally, contradictory results have been reported concerning the impact of a modulation of mitochondrial LCFA oxidation on palmitate-induced apoptosis in cardiomyocytes (11, 22) and pancreatic β-cells (13, 24, 25). This question has never been addressed in skeletal muscle cells.
In the present study, we aimed to determine whether oleate protects skeletal muscle cells from palmitate-induced apoptosis and to examine the role of mitochondrial LCFA in mediating any such effect of oleate. To this end, we expressed a mutant form of CPT1A (CPT1 M593S, CPT1mt), which is active but insensitive to malonyl-CoA inhibition (26), in C2C12 myotubes. The metabolic and cellular consequences of an increased LCFA oxidation through CPT1mt expression were compared with the effects of preincubation with oleate (OA) to decipher the mechanisms underlying oleate's protective effect and to determine if direct manipulation of mitochondrial LCFA oxidation would lead to the same protective effect as OA.
EXPERIMENTAL PROCEDURES
Cell Culture
Mouse C2C12 myoblasts (ATCC) were cultured in controlled humidified atmosphere (5% CO2, 37 °C) in Dulbecco's modified Eagle's medium (DMEM, PAA Laboratories) supplemented with 10% (v/v) fetal bovine serum (Invitrogen) and 1% (v/v) amphotericin B, gentamicin (Invitrogen). Confluent myoblasts were induced to differentiate in DMEM containing 2% (v/v) horse serum (Invitrogen) for 4 days prior to all experiments. Adenoviruses (Ad) encoding either β-galactosidase (Ad-LacZ) or rat CPT1A mutated at methionine 593 (Ad-CPT1mt) were constructed and produced as previously described (27). Adenoviral infection was performed in myotubes in serum-free medium containing 5 infectious particles/cell of Ad-LacZ or Ad-CPT1mt for 16 h. Sixteen h following the removal of the infection medium, cells were cultured for 24 h in a medium containing either 5 mm glucose (G5) or 20 mm glucose plus 100 nm insulin (G20+I), as indicated. For insulin treatment, cells were incubated for 10 min in fresh medium in the absence or presence of 100 nm insulin (Novo Nordisk).
FA Treatment
Sodium salts of palmitic acid and oleic acid (Sigma) were conjugated with FA- and endotoxin-free BSA (PAA Laboratories), and 4 mm stock solution was made in serum-free DMEM containing 5% (w/v) BSA. Myotubes were incubated with carnitine (1 mm), with or without (1% (w/v) BSA) various concentrations of palmitate for the time periods indicated. Preincubation with 0.3 mm oleate was done for 16 h before exposure to palmitate.
Western Blot
Antibodies against CPT1A (28), CPT2 (27), ACC, phospho-ACC, Akt, phospho-Akt, AMP-activated protein kinase (AMPK), phospho-AMPK, caspase-3 (Cell Signaling Technology), cytochrome c, tubulin α (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and mitochondrial respiratory chain subunit B6 of Complex 1 and subunit Fe/S of Complex 3 (gift from Dr. Anne Lombès, INSERM U582, Paris, France) were used.
Subcellular Fractionation
C2C12 myotubes were homogenized in an isolation buffer (0.3 m sucrose, 5 mm Tris-HCl, 1 mm EGTA, pH 7.4) and centrifuged at 1200 × g for 4 min. The supernatant containing mitochondria was then centrifuged at 4900 × g for 10 min. The pellet and the supernatant correspond to the mitochondrial and cytosolic fractions, respectively.
CPT1 Activity Assay
CPT1 activity was determined in mitochondria-enriched fractions (50 μg of protein, 1 mg/ml) using l-[methyl-3H]carnitine (400 μm; 10 Ci/mol) and palmitoyl-CoA (150 μm) as substrates (27). Activity assays were performed with different concentrations of malonyl-CoA to determine the malonyl-CoA concentration required to inhibit CPT1 activity by 50% (IC50).
Determining Cellular Oxygen Consumption
Cells were trypsinized and resuspended in their medium. Measurement of respiration was performed by a polarographic oxygen sensor in a 2-ml glass chamber of an Oxygraph 2K respirometer (Oroboros Instruments). The amplified signal from the oxygen sensor was recorded on a computer at sampling intervals of 1 s. Data were acquired using DatLab3 software and processed with the DatLab2 program, which permits subtraction of oxygen consumption due to the electrode. The respiration medium was equilibrated with air in the oxygraph chambers at 37 °C and stirred until a stable signal was obtained for calibration at air saturation. After calibration, the medium was replaced by 2 ml of aerated cell suspensions, and the chambers were closed with stoppers. Basal respiration was determined after 5–10 min of stabilization. Oligomycin (0.5 μg/ml) was then added to determine the State-4 respiratory rate. Finally, increasing concentrations (40 nm) of carbonyl cyanide m-chlorophenyl hydrazone (CCCP) were used to estimate the maximum (uncoupled) respiratory rate.
FA Metabolism
FA oxidation and esterification were determined during the last 3 h of culture in the presence of either 0.3 mm [1-14C]oleate (0.5 Ci/mol) or 0.8 mm [1-14C]palmitate (2 Ci/mol) bound to 1% (w/v) BSA and 1 mm carnitine. Then medium was collected to determine [14C]CO2 and [14C]acid-soluble products (ASP) (27). ASP consist mainly of shortened acyl-carnitines, Krebs cycle intermediates, and acetyl-CoA. Cells were washed and scraped in PBS to determine [14C]TG, [14C]phospholipids (PL), [14C]DAG, and [14C]non-esterified fatty acid. Lipids were extracted with chloroform/methanol (2:1, v/v) and separated by thin layer chromatography on silica gel plates (Merck) (27).
Immunofluorescence Assay
C2C12 myotubes cultured on coverslips were fixed as described previously (27). Cells were stained with anti-cytochrome c (BD Biosciences) and anti-cleaved caspase-3 (Cell Signaling Technology) antibodies, which were detected by an anti-mouse IgG conjugated with Alexa fluor 594 and an anti-rabbit IgG conjugated with Alexa fluor 488 (Molecular Probes), respectively. After incubation with Hoechst 33342 (2 μm; Molecular Probes) for DNA staining, coverslips were mounted on glass slides with Fluoromount G (Clinisciences).
Caspase-3 Activity Assay
Caspase-3 activity was measured fluorometrically in extracts (100 μg of protein) using the Caspase-3 Cellular Assay Kit Plus with Ac-DEVD-p-nitroanilide as a substrate (BIOMOL International), according to the manufacturer's protocol.
Lipidomic Analysis
Myotubes were homogenized in methanol, 5 mm EGTA (2:1 v/v) with FAST-PREP (MP Biochemicals) and evaporated. The dry pellets were dissolved overnight in 0.1 m NaOH. Lipids were extracted from homogenates according to the method of Bligh and Dyer (29) and analyzed by gas-liquid chromatography for determination of neutral lipid molecular species, ceramides, sphingomyelins (SM) (30) and FA methyl esters (31). Data were normalized to protein concentration.
RT-PCR
Total RNA was extracted using the RNeasy® minikit (Qiagen), following the manufacturer's instructions. RT-PCR was performed with SuperScriptTM II reverse transcriptase (Invitrogen) using 1 μg of total RNA. The resulting cDNA was mixed with a buffer containing 25 mm MgCl2, 15 μm sense and antisense primers, and 0.75 μl of FastStart DNA-MasterSYBR Green I (Roche Applied Science) and analyzed with online quantitative PCR (LightCycler® system), as follows: denaturing at 95 °C for 15 min, annealing at 58 °C for 7 s, elongation at 72 °C for 15 s, and denaturing at 95 °C. Primers (Eurofins, MWG Operon) used are listed in supplemental Table 1. The relative mRNA abundance was calculated using LightCycler® software and normalized to 18 S mRNA.
Statistical Analysis
Data represent at least three independent experiments, are reported as means ± S.E., and were analyzed with analysis of variance using GraphPad Prism 5 software. The Student-Newman-Keuls test was used for post hoc analyses. Differences were considered significant at p < 0.05.
RESULTS
CPT1mt Expression Redirected LCFA Metabolism
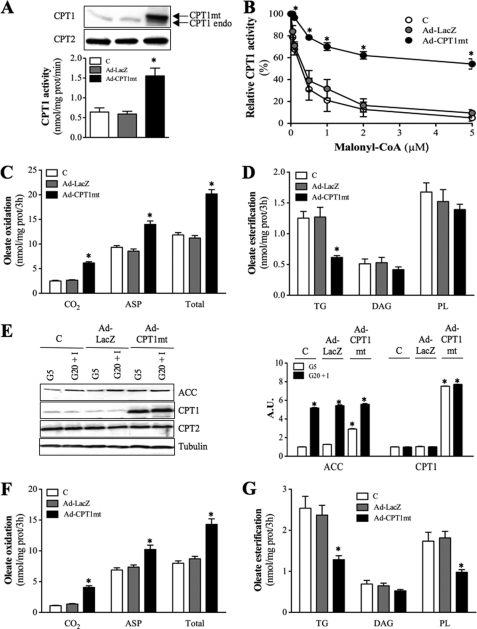
Consistent with previous studies (18), the endogenous CPT1 protein expressed in mouse C2C12 cells was CPT1A, the liver isoform (data not shown). Infection of C2C12 myotubes with Ad-CPT1mt induced a 6-fold increase in mitochondrial CPT1 protein level and a 2.7-fold increase in CPT1 activity compared with control (C) or Ad-LacZ-infected cells (Fig. 1A). Rat CPT1mt was detected as the higher migrating immunoreactive band, and endogenous mouse CPT1A (CPT1endo) was detected as the lower band (Fig. 1A). Expression of CPT2 protein, another enzyme of the CPT system, was not affected by adenofection. In the presence of 5 μm malonyl-CoA, more than 90% of the endogenous mitochondrial CPT1 activity was inhibited, both in control and Ad-LacZ-infected cells, whereas less than 50% inhibition was observed in CPT1mt-expressing cells (Fig. 1B). This decreased inhibitory effect of malonyl-CoA was associated with an 18-fold increase in its IC50 value (control (C), 0.3 ± 0.2 μm; Ad-LacZ, 0.4 ± 0.1 μm; Ad-CPT1mt, 7.10 ± 1.9 μm).
FIGURE 1.
CPT1mt expression in C2C12 myotubes redirects LCFA metabolism. Uninfected (C) or Ad-LacZ- and Ad-CPT1mt-infected C2C12 myotubes were cultured for 48 h in the presence of G5. Isolated mitochondria were used to determine CPT1 and CPT2 protein levels (A), CPT1 activity in the absence of malonyl-CoA (A), and CPT1 sensitivity to malonyl-CoA inhibition (B). The arrows designate either rat mutant CPT1A (CPT1mt, upper band) or endogenous mouse CPT1A (CPT1endo, lower band). Western blots are representative of four independent experiments. Results are means ± S.E. of four independent experiments. C–G, cells were cultured for 24 h in the presence of either G5 (C–E) or G20+I (E–G), and 0.3 mm [1-14C]oleate bound to 1% (w/v) defatted BSA was added during the last 3 h of culture. C, oleate oxidation to CO2, ASP, and their sum (Total). D, oleate esterification into TG, DAG, and PL at G5. E, immunoblot analysis of cellular extracts using specific antibodies for ACC, CPT1, CPT2, and tubulin. Western blots are representative of three independent experiments. Quantification was done with a Chemigenius apparatus (Syngene), and values are expressed relative to tubulin expression. F, oleate oxidation to CO2, ASP, and total at G20+I. G, oleate esterification into TG, DAG, and PL at G20+I. Data are means ± S.E. (error bars) of three experiments, each performed in triplicate. *, p < 0.05 versus C or Ad-LacZ at G20+I.
In myotubes cultured at low glucose concentration (5 mm; G5), CPT1mt expression increased [1-14C]oleate oxidation to CO2, ASP, and their sum (Total) by 2.5-, 1.5-, and 1.7-fold, respectively, in comparison with control or Ad-LacZ-infected cells (Fig. 1C), despite a 3-fold increase in ACC protein level (Fig. 1E). Furthermore, the ratio of complete (CO2) versus incomplete (ASP) oxidation increased by 38% in CPT1mt-expressing cells, although it remained unchanged in Ad-LacZ-infected cells (control (C), 0.27 ± 0.02; Ad-LacZ, 0.32 ± 0.02; Ad-CPT1mt, 0.44 ± 0.02). In contrast to control and Ad-LacZ-infected cells, [1-14C]oleate esterification into TG was decreased by 2-fold in CPT1mt-expressing cells; DAG and PL were not changed significantly (Fig. 1D). CPT1mt expression also specifically increased by 52% the total amount of metabolized [1-14C]oleate (control (C), 16.07 ± 0.56; Ad-LacZ, 15.22 ± 0.36; Ad-CPT1mt, 23.12 ± 0.92). In contrast to oleate, the metabolic fate of octanoate, a medium-chain FA that enters mitochondria independently of the CPT system, was unaffected by CPT1mt expression (supplemental Fig. S1).
We next examined whether the CPT1mt-mediated increase in [1-14C]oleate oxidation flux was maintained in the presence of high glucose (20 mm) and insulin (100 nm) concentrations (G20+I), which are known to promote lipogenesis and to inhibit LCFA oxidation (32). In control cells, G20+I led to a 5.2-fold increase in ACC protein expression (Fig. 1E). [1-14C]oleate oxidation to CO2, ASP, and total oxidation were decreased by 50, 26, and 30%, respectively, when compared with cells cultured at G5 (Fig. 1F). Conversely, G20+I induced a 2-fold increase in [1-14C]oleate esterification into TG, whereas its incorporation into PL and DAG was not significantly affected (Fig. 1G). Adenofection with Ad-LacZ did not modify the specific effects of G20+I. In contrast, although G20+I led to a similar increase in ACC protein expression in CPT1mt-expressing cells (Fig. 1E), [1-14C]oleate oxidation was 1.2-fold higher than in control cells cultured at G5 (Fig. 1F). Furthermore, CPT1mt expression abolished the stimulatory effect of G20+I on [1-14C]oleate esterification into TG and reduced its esterification into PL (Fig. 1G).
CPT1mt Expression Prevented Palmitate-induced Apoptosis
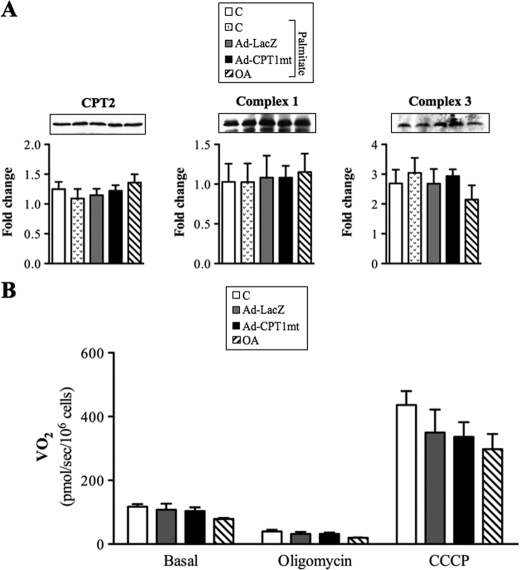
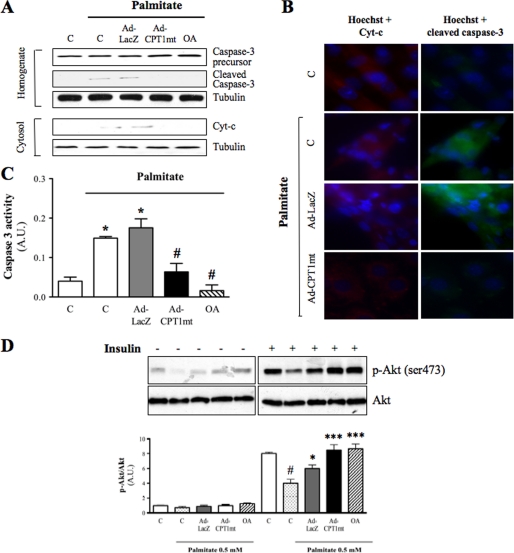
We first determined the optimal conditions for palmitate-induced apoptosis in C2C12 myotubes. In the presence of an FA/BSA molar ratio of 5.3, palmitate induced caspase-3 cleavage in a concentration-dependent (supplemental Fig. S2A) and time-dependent (supplemental Fig. S2B) manner. In the presence of 0.8 mm palmitate, caspase-3 cleavage was enhanced when the FA/BSA molar ratio increased from 1.33 to 5.3 (supplemental Fig. S2C). Thus, for tests we used 24-h exposure with 0.8 mm palmitate and a FA/BSA molar ratio of 5.3. Under these conditions, 57% of cells showed caspase-3 activation, with 34% of them positive for propidium iodide, a marker of late apoptosis (supplemental Fig. S2D). Because a protective effect of oleate on palmitate-induced apoptosis in skeletal muscle cells has not yet been reported, we compared the effect of CPT1mt expression with a preincubation with 0.3 mm oleate for 16 h. First, because mitochondria play a pivotal role during apoptosis, we investigated the effects of adenoinfection with Ad-LacZ or Ad-CPT1mt and OA on mitochondrial content and function (Fig. 2). Neither the amount of mitochondrial protein (Fig. 2A) nor the respiratory rate was changed, either in basal conditions or in the presence of oligomycin or the mitochondrial uncoupler CCCP (Fig. 2B). In both control and Ad-LacZ-infected myotubes, palmitate exposure induced mitochondrial cytochrome c release into the cytosol, as shown by Western blot (Fig. 3A) and immunofluorescence assays (Fig. 3B). Indeed, upon palmitate exposure, the cytochrome c fluorescent staining shifted from a characteristic mitochondrial pattern to a more diffuse one, which is typical of a cytosolic location (33). As expected, palmitate-induced cytochrome c release was associated with caspase-3 cleavage (Fig. 3, A and B) and a 3.5-fold increase in its activity (Fig. 3C). Interestingly, CPT1mt expression protected myotubes from palmitate-induced cytochrome c release (Fig. 3A), which was further confirmed by the recovery of its mitochondrial pattern (Fig. 3B). Moreover, in CPT1mt-expressing cells, palmitate-induced caspase-3 cleavage (Fig. 3, A and B) and caspase-3 activity (Fig. 3C) were decreased by 71 and 63%, respectively. Similarly to CPT1mt expression, OA abolished palmitate-induced cytochrome c release (Fig. 3, A and B), caspase-3 cleavage (Fig. 3, A and B) and caspase-3 activity (Fig. 3C). We also investigated the effect of CPT1mt expression and OA on insulin resistance induced by a lower palmitate concentration. As shown previously (18, 34), insulin induced a 90% increase in Ser473 phosphorylation of Akt, which was decreased by 60% by exposure to 0.5 mm palmitate (Fig. 3D). Neither CPT1mt expression nor OA decreased Akt phosphorylation in C2C12 myotubes, suggesting protection against palmitate-induced insulin resistance (Fig. 3D).
FIGURE 2.
CPT1 expression and OA had no impact on mitochondrial content and function. Uninfected cells (C) and Ad-LacZ- or Ad-CPT1mt-infected myotubes and oleate-preincubated myotubes were cultured for 24 h in the presence of G5. A, immunoblot analysis of protein extracts using specific antibodies against CPT2, Complex 1, and Complex 3. Western blots are representative of four independent experiments. Quantification was done with a Chemigenius apparatus (Syngene), and the values are expressed relative to tubulin expression. B, oxygen consumption (VO2) of cells resuspended in their culture medium was determined in basal conditions and in the presence of 0.5 μg/ml oligomycin. The maximum O2 consumption rate was evaluated in the presence of an optimal concentration of the uncoupler CCCP. The histograms represent data from three independent experiments in which measurements were performed in duplicate. Error bars, S.E.
FIGURE 3.
CPT1mt expression and OA similarly decreased palmitate-induced apoptosis in C2C12 myotubes. Results are shown for uninfected cells (C) and Ad-LacZ- or Ad-CPT1mt-infected myotubes and oleate-preincubated myotubes. Cells cultured in G5 were treated with either vehicle (1% (w/v) BSA) or palmitate (0.8 mm) for 24 h. A, immunoblotting was performed to determine caspase-3 cleavage in homogenates and cytochrome c (Cyt-c) release from mitochondria in cytosolic fractions. Tubulin was used as a loading control. Western blots are representative of three independent experiments. B, detection of cytochrome c and cleaved caspase-3 by immunofluorescence analysis. Hoechst 33342 was used to stain myotube nuclei (magnification ×40). C, determination of caspase-3 activity. Data are means ± S.E. (error bars) of three independent experiments. D, determination of Akt and phospho-Akt (Ser473) (p-Akt) protein levels. Cells cultured in G5 were treated with either vehicle (1% (w/v) BSA) or palmitate (0.5 mm) for 24 h and exposed to insulin (100 nm) as indicated. Western blots are representative of three independent experiments. #, p < 0.001 versus C without palmitate; *, p < 0.05; ***, p < 0.001 versus C exposed to palmitate. A.U., arbitrary units.
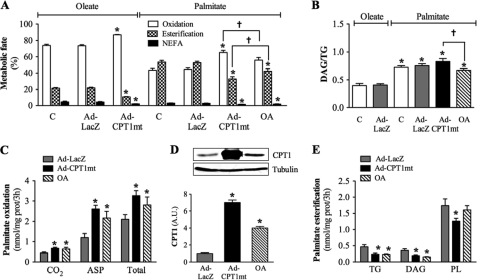
CPT1mt Expression and Oleate Preincubation Differentially Affected the Metabolic Fate of Palmitate
To determine how CPT1mt expression and OA protected myotubes from palmitate-induced apoptosis, we studied their impact on the metabolic fate of [1-14C]palmitate. We first checked that 0.3 and 0.8 mm palmitate were oxidized and esterified in similar proportions (data not shown). This allowed us to compare the metabolic fates of 0.3 mm oleate and 0.8 mm palmitate, the latter being the cytotoxic concentration. In control and Ad-LacZ-infected cells (Fig. 4A), only 44% of the metabolized palmitate was oxidized, in contrast to 74% for oleate, with no difference in the CO2/ASP ratio (palmitate, 0.28 ± 0.02; oleate, 0.27 ± 0.02). The percentage of esterified palmitate (TG + DAG + PL) was 2.4-fold higher than for oleate (Fig. 4A). Moreover, the DAG/TG ratio was 2-fold higher for [1-14C]palmitate in comparison with [1-14C]oleate (Fig. 4B), suggesting less efficient incorporation of palmitate into TG. CPT1mt expression and OA increased [1-14C]palmitate oxidation by 1.6- and 1.4-fold, respectively (Fig. 4C). The OA-induced increase in palmitate oxidation was associated with 3.7-fold higher CPT1 protein expression (Fig. 4D). Esterification of [1-14C]palmitate into TG and DAG was decreased by 2-fold in both CPT1mt-expressing and OA-treated cells, but its incorporation into PL was decreased (by 28%) only in CPT1mt-expressing cells (Fig. 4E). When compared with control and Ad-LacZ-infected cells, neither CPT1mt expression nor OA modified the total amount of metabolized [1-14C]palmitate (data not shown), but they did affect its metabolic fate. Following CPT1mt expression and OA, the percentage of oxidized palmitate was increased by 47 and 26%, respectively, whereas the percentage of esterified palmitate was decreased by 38 and 16%, respectively (Fig. 4A). The percentage of non-metabolized [1-14C]palmitate (NEFA) was also reduced by 41 and 31% by CPT1mt expression and OA, respectively (Fig. 4A). It is noteworthy that CPT1mt expression affected the metabolic fate of [1-14C]palmitate significantly more than did OA. Furthermore, whereas CPT1mt expression had no impact on the DAG/TG for palmitate, OA significantly reduced this ratio (Fig. 4B).
FIGURE 4.
Effects of CPT1mt expression and OA on the metabolic fate of palmitate. Myotubes were treated as described in the legend to Fig. 3 and cultured for 3 h in the presence of 0.3 mm [1-14C]oleate or 0.8 mm [1-14C]palmitate bound to 1% (w/v) BSA. A, metabolism of oleate and palmitate toward oxidation, esterification, or non-esterified fatty acid (NEFA). B, DAG/TG ratio for oleate and palmitate. C, palmitate oxidation to CO2, ASP, and total. D, CPT1 protein level was determined by immunoblotting using specific antibody against CPT1A. Western blots are representative of three independent experiments performed in duplicate. Quantification was done with a Chemigenius apparatus (Syngene), and the values are expressed relative to tubulin expression. E, palmitate esterification into TG, DAG, and PL. Data are means ± S.E. (error bars) of three experiments, each performed in triplicate. *, p < 0.05 versus control (C) or Ad-LacZ exposed to oleate; †, p < 0.05 versus Ad-CPT1mt.
CPT1mt Expression and Oleate Preincubation Affected Lipid Content and Nature
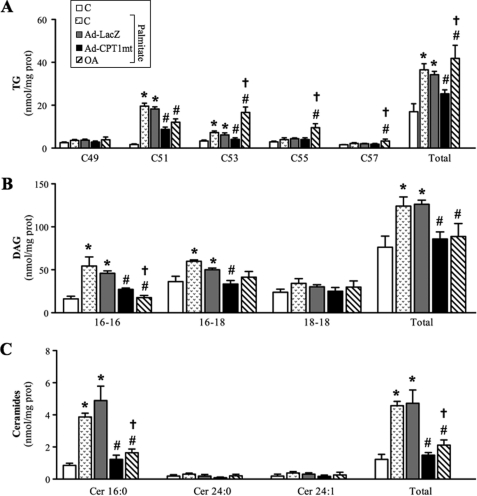
Lipidomic analyses were performed to further investigate the metabolic effects of CPT1mt expression and OA. Exposure to palmitate led to a 2-fold increase in the total TG content of myotubes, with marked increases in C51 (glycerol esterified with C16-C16-C16) and C53 (glycerol esterified with either C16-C16-C18 or C18-C18-C14) (Fig. 5A). The total concentrations of DAG (especially 16-16 and 16-18) (Fig. 5B) and ceramides (specifically Cer16:0) (Fig. 5C) were also increased, by 1.6- and 4.5-fold, respectively. CPT1mt expression markedly or totally decreased palmitate-induced increases in TG (Fig. 5A), DAG (Fig. 5B), and ceramides (Fig. 5C). Surprisingly, whereas OA also abolished palmitate-induced increase in DAG (Fig. 5B) and ceramides (Fig. 5C), its effect on TG differed from that of CPT1mt expression. In comparison with palmitate-exposed cells, only C51 was decreased, by 38%, by OA, whereas C53, C55 (glycerol esterified with C16-C18-C18), and C57 (glycerol esterified with C18-C18-C18) were increased by 128, 141, and 53%, respectively, leading to a further 24% increase in total TG (Fig. 5A). Of all of the FA methyl esters analyzed (Table 1), palmitate exposure resulted only in an increase in palmitate and a decrease in oleate, which remained unaffected by CPT1mt expression. In contrast, OA induced a 2-fold increase in oleate content and a 2-fold decrease in palmitoleate (C16:1) content. Thus, palmitate exposure led to a 20% increase in SFA at the expense of MUFA (Table 1). Only OA was able to restore a percentage similar to that of control cells. Of note, the amounts of cholesterol, cholesterol esters, and SM were unaffected by palmitate treatment, CPT1mt expression, and OA (Table 1).
FIGURE 5.
Effect of CPT1mt expression and OA on lipid composition in C2C12 myotubes exposed to palmitate. Cells were treated and cultured as described in the legend to Fig. 3. Lipids were extracted from myotubes and analyzed as described under “Experimental Procedures” to determine cellular TG (A), DAG (B), and ceramide (C) contents. Data are means ± S.E. (error bars) of three experiments performed in duplicate. *, p < 0.05 versus control (C) without palmitate; #, p < 0.05 versus C or Ad-LacZ exposed to palmitate; †, p < 0.05 versus Ad-CPT1mt.
TABLE 1.
Effect of CPT1mt expression and OA on lipid content and nature
Cells were treated and cultured as described in the legend to Fig. 2. Lipids were extracted from myotubes and analyzed as described under “Experimental Procedures.” Data are means ± S.E. of three experiments performed in duplicate. C, uninfected cells; OA, cells preincubated for 16 h with oleate; PUFA, polyunsaturated fatty acid(s).
| Without palmitate (C) | With palmitate |
||||
|---|---|---|---|---|---|
| C | Ad-LacZ | Ad-CPT1mt | OA | ||
| Total FA methyl esters (nmol/mg protein) | 615 ± 113 | 975 ± 64a | 892 ± 56a | 1110 ± 112a | 824 ± 69 |
| SFA (%) | 43.0 ± 3.2 | 51.6 ± 0.7a | 50.0 ± 0.7a | 50.7 ± 0.8a | 47.4 ± 0.5b,c |
| C16:0 (%) | 23.5 ± 2.3 | 34.3 ± 1.0a | 34.2 ± 0.8a | 31.7 ± 0.9a | 29.3 ± 0.6a,b |
| C18:0 (%) | 19.4 ± 0.7 | 16.3 ± 0.7 | 14.7 ± 0.6 | 17.5 ± 1.6 | 17.0 ± 0.7 |
| C24:0 (%) | 1.12 ± 0.05 | 0.84 ± 0.08 | 0.92 ± 0.04 | 0.88 ± 0.08 | 0.81 ± 0.07 |
| MUFA (%) | 42.5 ± 1.5 | 36.2 ± 0.6a | 38.1 ± 1.4 | 37.0 ± 1.3 | 41.7 ± 0.5b,c |
| C16:1ω7 (%) | 13.4 ± 1.2 | 16.7 ± 0.7 | 16.2 ± 2.1 | 16.6 ± 1.8 | 6.6 ± 0.5a,b,c |
| C18:1ω9 (%) | 17.5 ± 0.8 | 12.8 ± 1.0a | 13.7 ± 0.4a | 13.0 ± 0.2a | 29.9 ± 0.9a,b,c |
| C18:1ω7 (%) | 9.5 ± 0.5 | 5.7 ± 0.5a | 7.1 ± 0.3a | 6.3 ± 0.1a | 3.9 ± 0.1a,b,c |
| C24:1ω9 (%) | 0.69 ± 0.15 | 0.58 ± 0.06 | 0.69 ± 0.64 | 0.55 ± 0.01 | 0.70 ± 0.03 |
| PUFA (%) | 14.5 ± 2.3 | 12.2 ± 0.5 | 11.9 ± 0.8 | 12.3 ± 0.7 | 10.9 ± 0.2 |
| C18:2ω6 (%) | 5.2 ± 0.4 | 3.9 ± 0.1a | 3.8 ± 0.2a | 3.3 ± 0.2a | 2.8 ± 0.0a |
| C20:2ω6 (%) | 1.9 ± 0.3 | 1.6 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 |
| C20:3ω6 (%) | 1.3 ± 0.2 | 1.0 ± 0.0 | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.0 |
| C20:4ω6 (%) | 2.3 ± 0.3 | 1.9 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 |
| C20:5ω3 (%) | 2.7 ± 0.6 | 2.5 ± 0.2 | 3.0 ± 0.3 | 3.3 ± 0.3 | 2.6 ± 0.1 |
| C22:6ω3 (%) | 0.60 ± 0.14 | 0.47 ± 0.02 | 0.43 ± 0.02 | 0.49 ± 0.05 | 0.49 ± 0.03 |
| MUFA/SFA | 1.01 ± 0.09 | 0.70 ± 0.02a | 0.76 ± 0.10a | 0.73 ± 0.04a | 0.88 ± 0.02b |
| Total cholesterol (nmol/mg protein) | 645 ± 94 | 812 ± 56 | 742 ± 76 | 729 ± 30 | 666 ± 52 |
| Total cholesterol esters (nmol/mg protein) | 4.9 ± 0.7 | 7.3 ± 0.7 | 5.3 ± 0.6 | 5.4 ± 0.8 | 6.3 ± 1.2 |
| C16 (%) | 45.0 ± 0.8 | 27.7 ± 0.3 | 35.3 ± 0.5 | 37.8 ± 0.5 | 36.5 ± 0.5 |
| C18 (%) | 31.1 ± 0.1 | 24.2 ± 0.3 | 39.6 ± 0.7 | 36.6 ± 0.6 | 42.8 ± 0.8 |
| C20:4 (%) | 24.0 ± 0.8 | 48.0 ± 0.5 | 25.2 ± 0.6 | 25.7 ± 0.6 | 20.6 ± 0.9 |
| Total sphingomyelins (nmol/mg protein) | 86.8 ± 19.8 | 98.6 ± 11.9 | 115.4 ± 13.7 | 76.5 ± 15.3 | 130.5 ± 13.0 |
a p < 0.05 versus uninfected cells without palmitate.
b p < 0.05 versus uninfected cells or Ad-LacZ exposed to palmitate.
c p < 0.05 versus Ad-CPT1mt.
CPT1mt Expression and Oleate Preincubation Affected the Expression of Key Enzymes of FA Metabolism
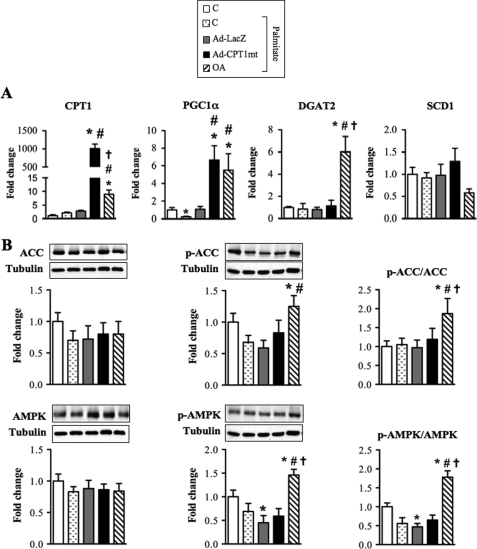
We first investigated the gene expression of DGAT2 (diacylglycerol acyltransferase 2), which catalyzes the final step of TG esterification in the cytosol. OA induced a 6-fold increase in DGAT2 gene expression (Fig. 6A), which could explain the increased TG content observed in these cells. The decreased palmitoleate content in OA-treated cells was associated with a trend (p = 0.054) for a decrease in the mRNA level of SCD1 (stearoyl-CoA desaturase 1), the enzyme that converts SFA to MUFA. Moreover, the increased LCFA oxidation in OA-treated cells was associated to an increase in both gene (Fig. 6A) and protein (data not shown) expressions of CPT1. Interestingly, we observed not only that OA counteracted the previously reported palmitate down-regulation of PGC1α (peroxisome proliferator-activated receptor γ coactivator 1α) expression (8), a key player in the induction of gene expression of FA oxidation enzymes, but also that OA stimulated PGC1α expression (Fig. 6A). AMPK is known to increase mitochondrial LCFA oxidation through inactivation of ACC by phosphorylation, hence protecting CPT1 activity from malonyl-CoA inhibition. Although the total AMPK and ACC protein levels were unaffected, OA increased AMPK phosphorylation on threonine 172, which reflects its activation, and ACC phosphorylation on serine 79, which inhibits its activity (Fig. 6B). Thus, both phospho-ACC/ACC and phospho-AMPK/AMPK ratios were increased in OA-treated cells exposed to palmitate (Fig. 6B). In contrast to OA, CPT1mt expression affected only PGC1α expression, with a 6.7-fold increase in its mRNA level (Fig. 6A).
FIGURE 6.
Effects of CPT1mt expression and OA on gene and protein expression of key enzymes involved in lipid metabolism. Myotubes were treated and cultured as described in the legend to Fig. 3. A, mRNA levels of CPT1, PGC1α, SCD1, and DGAT2 were determined by quantitative RT-PCR and normalized to 18 S mRNA level. Results are means ± S.E. (error bars) of four experiments and are expressed relative to untreated cells (C). B, protein expression of ACC, AMPK, phospho-ACC (Ser79) (p-ACC), and phospho-AMPK (Thr172) (p-AMPK) was analyzed by immunoblotting. Western blots are representative of five independent experiments. Quantification was done with a Chemigenius apparatus (Syngene), and the values are expressed relative to tubulin expression. *, p < 0.05 versus C without palmitate; #, p < 0.05 versus C or Ad-LacZ exposed to palmitate; †, p < 0.05 versus Ad-CPT1mt.
DISCUSSION
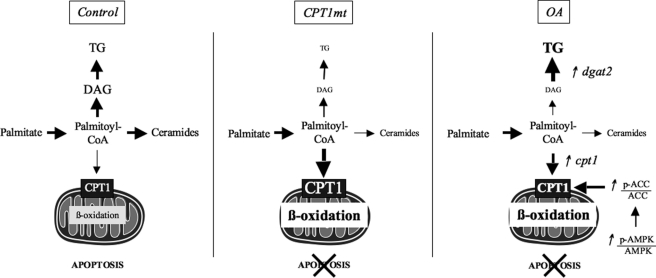
Our findings indicate that oleate preincubation protects skeletal muscle cells from palmitate-induced apoptosis by acting on its metabolism through two mechanisms. It promotes the clearance of DAG-containing palmitate moieties toward TG and palmitate oxidation. Second, because targeting mitochondrial LCFA oxidation directly, via the expression of a malonyl-CoA-insensitive CPT1 (CPT1mt), also has a protective effect, redirection of palmitate metabolism toward oxidation appears to be sufficient for protection against its lipotoxic effect (Fig. 7).
FIGURE 7.
CPT1mt expression and OA reach similar final effects via different mechanisms. Control, the SFA palmitate induces apoptosis in skeletal muscle cells by promoting intracellular TG, DAG, and ceramide accumulation. CPT1mt, CPT1mt expression reduces palmitate-induced apoptosis by increasing mitochondrial oxidation of palmitate and decreasing the production of TG, DAG, and ceramides. OA, preincubation with oleate prevents palmitate-induced apoptosis by promoting mitochondrial LCFA oxidation, secondary to increased CPT1mt expression and phospho-ACC/ACC ratio, and by channeling palmitate toward TG as a result of an enhanced DGAT2 expression. These two mechanisms reduce the availability of palmitate for incorporation into DAG and de novo ceramide synthesis. In conclusion, OA induces palmitate oxidation and storage into TG, whereas CPT1mt expression increases mitochondrial oxidation of palmitate.
Consistent with previous studies (11, 35), we showed that palmitate induced apoptosis in C2C12 myotubes. First, prolonged exposure of myotubes to palmitate promoted intracellular accumulation of TG (specifically TG species with at least two C16: C51 and C53), DAG (specifically 16-16 and 16-18), and ceramides (specifically Cer16:0). As previously suggested (11), these deleterious metabolites result from enhanced de novo ceramide synthesis rather than from SM degradation because cellular SM content was unaffected by palmitate treatment. Second, palmitate exposure increased SFA and decreased MUFA, leading to a decreased MUFA/SFA ratio. Previous studies have suggested that palmitate-induced DAG and TG accumulation might result from a higher esterification flux for palmitate than for oleate (15, 16), as we also observed. However, our metabolic investigations also revealed that palmitate is much less prone to oxidation than oleate, which could favor directing its metabolism toward esterification.
We found that preincubation with oleate decreased palmitate esterification into DAG and TG after 3 h of palmitate exposure, resulting in a decreased DAG/TG ratio. However, oleate preincubation promoted intracellular TG accumulation, in particular TG species containing at least one C18 (C53, C55, and C57) after a 24-h palmitate exposure. This difference probably results from an enhanced expression of DGAT2, whose affinity is 50% higher for oleyl-CoA than for palmitoyl-CoA (36, 37). Indeed, oleate preincubation decreased TG and DAG species that contain solely C16 (i.e. C51 and DAG 16-16), suggesting that preincubation with oleate diverts DAG 16-16 to TG enriched with C18. Additionally, the lipidomic analysis revealed that oleate preincubation reduced palmitate content and increased the MUFA/SFA ratio. Interestingly, preincubation with oleate also reduced palmitoleate content in palmitate-exposed cells, which might be due to decreased SCD1 gene expression. Because oleate is a product of SCD1, we hypothesized that it can induce a negative feedback on SCD1 gene expression. It has been reported that oleate preincubation led to an increase in CPT1 mRNA level in skeletal muscle cells (8, 38). We demonstrated that oleate preincubation enhanced mitochondrial oxidation of palmitate, as a consequence of increased CPT1 gene and protein expressions. Our findings also indicate that preincubation with oleate increases mitochondrial LCFA oxidation through a second mechanism, namely activation of AMPK, which leads to ACC inhibition and, thus, to increased CPT1 activity.
We investigated whether acting directly on mitochondrial oxidation, by expressing CPT1mt, leads to the same protective effect as oleate preincubation. In agreement with the work of Sebastián et al. (34), we showed that CPT1mt expression induced an increase in LCFA oxidation at the expense of their esterification. Like oleate preincubation, DAG and ceramide contents are reduced by CPT1mt expression. In contrast to preincubation with oleate, CPT1mt expression decreased the rate of esterification of palmitate into TG (regarding the two species raised by palmitate) and had no impact on the nature or content of FA in myotubes. These results contrast with those of Perdomo et al. (18), who reported that expression of the wild-type CPT1 did not modify TG, DAG, or ceramide contents. This difference highlights the importance of malonyl-CoA in the regulation of CPT1 activity. It has been suggested that increased mitochondrial LCFA oxidation can lead to an accumulation of ASP (39). However, in our study, the CO2/ASP ratio for palmitate was increased, not decreased, following CPT1mt expression, both in the presence of low glucose and high glucose/insulin concentrations. This indicates that CPT1mt expression permits the maintenance of complete oxidation of palmitate, thus avoiding accumulation of incompletely oxidized molecules.
It is well established that skeletal muscle insulin resistance is associated with intramuscular lipid accumulation. Inhibition of CPT1 by etomoxir, an irreversible inhibitor, increased lipid deposition in skeletal muscle and exacerbated insulin resistance in high fat-fed animals (40), indicating that alteration in LCFA flux into mitochondria is critical for regulating the deleterious effect of lipids on insulin sensitivity. Furthermore, it has been recently reported that in vivo wild-type CPT1 overexpression in rat skeletal muscle enhanced mitochondrial LCFA oxidation and decreased high fat diet-induced insulin resistance (41, 42). These effects were associated with a moderate decrease in both TG level and palmitate incorporation into DAG. We hypothesize that these mild effects may be due to the expression of wild-type CPT1, which is sensitive to malonyl-CoA inhibition. Indeed, it has been shown in muscle from obese and diabetic subjects that malonyl-CoA level is increased, leading to a decrease in mitochondrial LCFA oxidation (41, 42). Here we demonstrated that targeting mitochondrial LCFA oxidation, via the expression of a malonyl-CoA-insensitive CPT1, led to the same protective effect as preincubation with oleate, providing strong evidence that redirecting palmitate metabolism toward oxidation is sufficient to protect against palmitate-induced apoptosis and insulin resistance. In conclusion, we propose that increased CPT1 activity together with decreased malonyl-CoA sensitivity may be a promising strategy for the treatment of the deleterious effects of lipid accumulation caused by obesity, insulin resistance, or aging.
Supplementary Material
Acknowledgments
The C2C12 mouse skeletal muscle cell line was kindly provided by Dr. Chantal Wrutniak (INRA, Montpellier, France). We thank Dr. Anne Lombes (INSERM U582, Paris, France) for antibodies against complexes 1 and 3 and Dr. Catherine Postic (INSERM U1016, Paris, France) for critical reading of the manuscript and for selected primers. We thank Justine Bertrand Michel (Lipidomic Core, IFR 150, Toulouse, France) for help with and expertise about lipid content analysis. We appreciate the assistance of the Flow Cytometry Core of the Cochin Institute, and we thank the Vector Core of the University Hospital of Nantes supported by the Association Francaise contre les Myopathies for providing the adenovirus vectors.
This research was funded by the Agence Nationale de la Recherche, ANR Mithycal and by Grant no. 13714 from the Association Française contre les Myopathies (AFM).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. S1 and S2.
- FA
- fatty acid(s)
- ASP
- acid-soluble product(s)
- CCCP
- carbonyl cyanide m-chlorophenyl hydrazone
- CPT1mt
- mutant CPT1A M593S
- DAG
- diacylglycerol(s)
- LacZ
- β-galactosidase
- LCFA
- long-chain fatty acid
- MUFA
- monounsaturated fatty acid(s)
- OA
- preincubation with oleate
- PL
- phospholipid(s)
- SFA
- saturated fatty acid(s)
- SM
- sphingomyelin(s)
- TG
- triglyceride(s)
- G5
- 5 mm glucose
- G20+I
- 20 mm glucose plus 100 nm insulin.
REFERENCES
- 1.Schalch D. S., Kipnis D. M. (1965) J. Clin. Invest. 44, 2010–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams J. M., 2nd, Pratipanawatr T., Berria R., Wang E., DeFronzo R. A., Sullards M. C., Mandarino L. J. (2004) Diabetes 53, 25–31 [DOI] [PubMed] [Google Scholar]
- 3.Dentin R., Benhamed F., Hainault I., Fauveau V., Foufelle F., Dyck J. R., Girard J., Postic C. (2006) Diabetes 55, 2159–2170 [DOI] [PubMed] [Google Scholar]
- 4.Bonnard C., Durand A., Peyrol S., Chanseaume E., Chauvin M. A., Morio B., Vidal H., Rieusset J. (2008) J. Clin. Invest. 118, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGarry J. D. (2002) Diabetes 51, 7–18 [DOI] [PubMed] [Google Scholar]
- 6.Hulver M. W., Berggren J. R., Cortright R. N., Dudek R. W., Thompson R. P., Pories W. J., MacDonald K. G., Cline G. W., Shulman G. I., Dohm G. L., Houmard J. A. (2003) Am. J. Physiol. Endocrinol. Metab. 284, E741–E747 [DOI] [PubMed] [Google Scholar]
- 7.Kelley D. E., Goodpaster B., Wing R. R., Simoneau J. A. (1999) Am. J. Physiol. 277, E1130–E1141 [DOI] [PubMed] [Google Scholar]
- 8.Coll T., Eyre E., Rodríguez-Calvo R., Palomer X., Sánchez R. M., Merlos M., Laguna J. C., Vázquez-Carrera M. (2008) J. Biol. Chem. 283, 11107–11116 [DOI] [PubMed] [Google Scholar]
- 9.Sparagna G. C., Hickson-Bick D. L., Buja L. M., McMillin J. B. (2001) Antioxid. Redox Signal. 3, 71–79 [DOI] [PubMed] [Google Scholar]
- 10.Shimabukuro M., Zhou Y. T., Levi M., Unger R. H. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2498–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turpin S. M., Lancaster G. I., Darby I., Febbraio M. A., Watt M. J. (2006) Am. J. Physiol. Endocrinol. Metab. 291, E1341–E1350 [DOI] [PubMed] [Google Scholar]
- 12.Paumen M. B., Ishida Y., Muramatsu M., Yamamoto M., Honjo T. (1997) J. Biol. Chem. 272, 3324–3329 [DOI] [PubMed] [Google Scholar]
- 13.Miller T. A., LeBrasseur N. K., Cote G. M., Trucillo M. P., Pimentel D. R., Ido Y., Ruderman N. B., Sawyer D. B. (2005) Biochem. Biophys. Res. Commun. 336, 309–315 [DOI] [PubMed] [Google Scholar]
- 14.Chavez J. A., Summers S. A. (2003) Arch. Biochem. Biophys. 419, 101–109 [DOI] [PubMed] [Google Scholar]
- 15.Montell E., Turini M., Marotta M., Roberts M., Noé V., Ciudad C. J., Macé K., Gómez-Foix A. M. (2001) Am. J. Physiol. Endocrinol. Metab. 280, E229–E237 [DOI] [PubMed] [Google Scholar]
- 16.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGarry J. D., Brown N. F. (1997) Eur. J. Biochem. 244, 1–14 [DOI] [PubMed] [Google Scholar]
- 18.Perdomo G., Commerford S. R., Richard A. M., Adams S. H., Corkey B. E., O'Doherty R. M., Brown N. F. (2004) J. Biol. Chem. 279, 27177–27186 [DOI] [PubMed] [Google Scholar]
- 19.Turner N., Bruce C. R., Beale S. M., Hoehn K. L., So T., Rolph M. S., Cooney G. J. (2007) Diabetes 56, 2085–2092 [DOI] [PubMed] [Google Scholar]
- 20.Hancock C. R., Han D. H., Chen M., Terada S., Yasuda T., Wright D. C., Holloszy J. O. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J. Y., Hickner R. C., Cortright R. L., Dohm G. L., Houmard J. A. (2000) Am. J. Physiol. Endocrinol. Metab. 279, E1039–E1044 [DOI] [PubMed] [Google Scholar]
- 22.Kelley D. E., He J., Menshikova E. V., Ritov V. B. (2002) Diabetes 51, 2944–2950 [DOI] [PubMed] [Google Scholar]
- 23.He J., Watkins S., Kelley D. E. (2001) Diabetes 50, 817–823 [DOI] [PubMed] [Google Scholar]
- 24.Kong J. Y., Rabkin S. W. (2002) Am. J. Physiol. Heart Circ. Physiol. 282, H717–H725 [DOI] [PubMed] [Google Scholar]
- 25.Sol E. M., Sargsyan E., Akusjärvi G., Bergsten P. (2008) Biochem. Biophys. Res. Commun. 375, 517–521 [DOI] [PubMed] [Google Scholar]
- 26.Morillas M., Gómez-Puertas P., Bentebibel A., Sellés E., Casals N., Valencia A., Hegardt F. G., Asins G., Serra D. (2003) J. Biol. Chem. 278, 9058–9063 [DOI] [PubMed] [Google Scholar]
- 27.Akkaoui M., Cohen I., Esnous C., Lenoir V., Sournac M., Girard J., Prip-Buus C. (2009) Biochem. J. 420, 429–438 [DOI] [PubMed] [Google Scholar]
- 28.Prip-Buus C., Cohen I., Kohl C., Esser V., McGarry J. D., Girard J. (1998) FEBS Lett. 429, 173–178 [DOI] [PubMed] [Google Scholar]
- 29.Bligh E. G., Dyer W. J. (1959) Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 30.Vieu C., Tercé F., Chevy F., Rolland C., Barbaras R., Chap H., Wolf C., Perret B., Collet X. (2002) J. Lipid Res. 43, 510–522 [PubMed] [Google Scholar]
- 31.Lillington J. M., Trafford D. J., Makin H. L. (1981) Clin. Chim. Acta 111, 91–98 [DOI] [PubMed] [Google Scholar]
- 32.Guillet-Deniau I., Pichard A. L., Koné A., Esnous C., Nieruchalski M., Girard J., Prip-Buus C. (2004) J. Cell Sci. 117, 1937–1944 [DOI] [PubMed] [Google Scholar]
- 33.Galluzzi L., Zamzami N., de La Motte Rouge T., Lemaire C., Brenner C., Kroemer G. (2007) Apoptosis 12, 803–813 [DOI] [PubMed] [Google Scholar]
- 34.Sebastián D., Herrero L., Serra D., Asins G., Hegardt F. G. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E677–E686 [DOI] [PubMed] [Google Scholar]
- 35.Rachek L. I., Musiyenko S. I., LeDoux S. P., Wilson G. L. (2007) Endocrinology 148, 293–299 [DOI] [PubMed] [Google Scholar]
- 36.Cases S., Stone S. J., Zhou P., Yen E., Tow B., Lardizabal K. D., Voelker T., Farese R. V., Jr. (2001) J. Biol. Chem. 276, 38870–38876 [DOI] [PubMed] [Google Scholar]
- 37.Lardizabal K. D., Mai J. T., Wagner N. W., Wyrick A., Voelker T., Hawkins D. J. (2001) J. Biol. Chem. 276, 38862–38869 [DOI] [PubMed] [Google Scholar]
- 38.Wensaas A. J., Rustan A. C., Just M., Berge R. K., Drevon C. A., Gaster M. (2009) Diabetes 58, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muoio D. M., Newgard C. B. (2006) Annu. Rev. Biochem. 75, 367–401 [DOI] [PubMed] [Google Scholar]
- 40.Dobbins R. L., Szczepaniak L. S., Bentley B., Esser V., Myhill J., McGarry J. D. (2001) Diabetes 50, 123–130 [DOI] [PubMed] [Google Scholar]
- 41.Bruce C. R., Brolin C., Turner N., Cleasby M. E., van der Leij F. R., Cooney G. J., Kraegen E. W. (2007) Am. J. Physiol. Endocrinol. Metab. 292, E1231–E1237 [DOI] [PubMed] [Google Scholar]
- 42.Bruce C. R., Hoy A. J., Turner N., Watt M. J., Allen T. L., Carpenter K., Cooney G. J., Febbraio M. A., Kraegen E. W. (2009) Diabetes 58, 550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.