Abstract
Bax and Bak are pro-apoptotic factors that are required for cell death by the mitochondrial or intrinsic pathway. Bax is found in an inactive state in the cytosol and upon activation is targeted to the mitochondrial outer membrane where it releases cytochrome c and other factors that cause caspase activation. Although Bak functions in the same way as Bax, it is constitutively localized to the mitochondrial outer membrane. In the membrane, Bak activation is inhibited by the voltage-dependent anion channel isoform 2 (VDAC2) by an unknown mechanism. Using blue native gel electrophoresis, we show that in healthy cells endogenous inactive Bak exists in a 400-kDa complex that is dependent on the presence of VDAC2. Activation of Bak is concomitant with its release from the 400-kDa complex and the formation of lower molecular weight species. Furthermore, substitution of the Bak transmembrane anchor with that of the mitochondrial outer membrane tail-anchored protein hFis1 prevents association of Bak with the VDAC2 complex and increases the sensitivity of cells to an apoptotic stimulus. Our results suggest that VDAC2 interacts with the hydrophobic tail of Bak to sequester it in an inactive state in the mitochondrial outer membrane, thereby raising the stimulation threshold necessary for permeabilization of the mitochondrial outer membrane and cell death.
Keywords: Apoptosis, Cell Death, Intracellular Trafficking, Mitochondria, Mitochondrial Transport, Blue-Native PAGE, Mitochondrial Outer Membrane Permeabilization, VDAC2
Introduction
Apoptosis is a physiological process for eliciting cell death and is essential for the normal growth and development of multicellular organisms. Studies on gene-deleted mice have shown that the presence of either one of the pro-apoptotic Bcl-2 family members, Bak or Bax, is necessary for cell death by the “intrinsic” pathway, which can be inhibited by Bcl-2 (1, 2). In this pathway, also known as the “mitochondrial” pathway, Bak and Bax act on the mitochondrial outer membrane to cause release of molecules such as cytochrome c, which binds to Apaf-1 in the cytosol triggering formation of the apoptosome (2).
The mechanisms that regulate Bak and Bax activation, and how they cause the mitochondrial outer membranes to become permeable, are not well understood. Current models for inducing cytochrome c release include the following: (a) Bak and Bax forming large pores themselves (2); (b) Bak and Bax altering the conformation of existing pores (3); (c) Bak and Bax regulating the production of long chain ceramides that may contribute to pore formation (4, 5); and (d) Bak and Bax regulating mitochondrial morphology machineries to activate MOMP5 (6). Regardless of the mechanism, any of these possible events require Bax/Bak activation. In contrast to Bax, which is redirected from the cytosol to mitochondria upon apoptotic induction (7), Bak is constitutively located within the mitochondrial outer membrane (8, 9). Bak has been reported to interact with a number of other proteins at the mitochondrial outer membrane, including the Bcl-2 anti-apoptotic family members Bcl-xL, Mcl-1 (10, 11), and A1 (12), the pro-apoptotic BH3-only Bcl-2 family proteins tBid, Bad, and Bim (13, 14), the mitochondrial morphology regulators Drp1 and Mitofusin (15, 16), and the tumor suppressor p53 (17). In addition, Cheng et al. (14) showed that the minor voltage-dependent anion channel isoform 2, VDAC2, interacts with the inactive conformer of Bak. VDAC proteins constitute the major gateways for metabolite flux across the mitochondrial outer membrane, and in mammalian cells three isoforms (VDAC1, VDAC2, and VDAC3) can substitute for each other in metabolic exchange (18). Although VDACs have been implicated in regulating apoptosis, conflicting studies have suggested that they may either promote or prevent apoptosis. For example, it was proposed that VDACs are part of the mitochondrial permeability transition complex, which can cause MOMP, resulting in cell death (3). On the other hand, it was reported that Bcl-xL binds directly to VDAC1 to maintain ATP/ADP exchange (19, 20), thereby promoting cell survival. In vitro, VDAC species purified from rat mitochondria could be induced by Bax or Bak to form a channel large enough to release cytochrome c, and this could be prevented by Bcl-xL (21). In contrast, it was found that the BH3-only protein tBid affected gating of VDAC channels rather than Bax (22), although another BH3-only protein Bim was found to directly interact with VDAC, and this was enhanced during apoptosis (23).
The study by Cheng et al. (14) suggested that association of Bak with VDAC2 maintains Bak in an inactive form in healthy cells. Death stimuli, such as treatment with tBid, was shown to abolish the interaction between Bak and VDAC2, allowing apoptosis to proceed (14). Consistent with these observations, vdac1- and vdac3-deficient mice are viable, whereas embryos with a homozygous deletion of vdac2 die during development (14). In vdac2−/− cells activation of Bak was enhanced, and the cells had increased sensitivity to apoptotic stimuli (14). In contrast, others have suggested a pro-apoptotic role for VDAC2 and have reported that it is required for the mitochondrial targeting and activation of Bak (24, 25).
To provide fresh insights into the mechanisms that regulate MOMP, we analyzed Bak complexes in mitochondria using blue native electrophoresis. Our results showed that in healthy cells endogenous Bak exists in a 400-kDa complex in the mitochondrial outer membrane, and release of Bak from this complex coincides with Bak activation, MOMP, and cytochrome c release. The 400-kDa Bak complex is dependent on the presence of VDAC2. Furthermore, replacement of the Bak transmembrane anchor with that of another mitochondrially targeted protein prevented association of Bak with the 400-kDa complex and lowered the threshold for apoptosis. This indicates that the interaction with VDAC2 involves the Bak transmembrane domain, and in the absence of VDAC2, or when the transmembrane domain is replaced, Bak does not spontaneously activate, but the threshold for activation is markedly reduced.
EXPERIMENTAL PROCEDURES
In Vitro Protein Import into Isolated Mitochondria
Bak, Bak-hFis1TM, and Tom22 were amplified from pGEM4Z using M13 forward and reverse primers to generate RNA using the in vitro mMESSAGE mMachine SP6 kit from Ambion. RNA was isolated by LiCl precipitation according to the manufacturer's instructions and applied to in vitro translation reactions using rabbit reticulocyte lysate (Promega) in the presence of [35S]methionine/cysteine protein labeling mix (PerkinElmer Life Sciences) as described previously (26, 27). Mitochondria were isolated fresh prior to import using differential centrifugation as described previously (26). Isolated mitochondria were incubated with translation products in import buffer (20 mm HEPES-KOH, pH 7.4, 250 mm sucrose, 80 mm KOAc, 5 mm MgOAC, 10 mm sodium succinate, 5 mm methionine, 1 mm DTT, and 4 mm ATP) at 37 °C for various times as indicated in the figure legends. Samples subjected to protease treatment were incubated on ice for 10 min with 100 μg/ml proteinase K (Sigma), followed by the addition of 1 mm PMSF and incubation for 10 min on ice. Samples treated by alkaline extraction were resuspended in freshly prepared 0.1 m Na2CO3, pH 11.5, and incubated on ice for 30 min with occasional vortexing. Membranes were isolated by centrifugation at 100,000 × g for 1 h at 4 °C and subsequently solubilized in SDS-PAGE loading dye. For treatment with tBid, recombinant tBid (28) in import buffer was added to samples at a final concentration of 50 nm. Reactions were performed at 37 °C for 10 or 15 min.
Electrophoresis and Immunoblot Analysis
Mitochondrial pellets (50 μg of protein) were solubilized in 50 μl of ice-cold digitonin-containing buffer (1% (w/v) digitonin, 20 mm BisTris, pH 7.0, 0.1 mm EDTA, 50 mm NaCl, 1 mm DTT, 10% (w/v) glycerol) and incubated on ice for 15–20 min. Samples were clarified by centrifugation at 16,000 × g for 10 min at 4 °C, upon which 1/10th the volume of sample buffer (5% (w/v) Coomassie Brilliant Blue G-250, 100 mm BisTris, pH 7.0, 500 mm ϵ-amino-n-caproic acid) was added to the clarified supernatant. Samples were typically separated on 4–13 or 4–16% polyacrylamide gradient gels at 4 °C. Thyroglobulin (669 kDa), ferritin (440 kDa), and bovine serum albumin (134 and 67 kDa) were used as molecular weight markers. SDS-PAGE was performed using gradient Tris-Tricine gels (29). Radiolabeled proteins were detected by digital autoradiography. For Western blot analysis, gels were transferred to PVDF membranes and probed with antibodies against Bak 7D10 (30), Tom40 (31), B17.2L (26), Hsc70 (32), Tim23 (BD Biosciences), cytochrome c (BD Biosciences), and mtHsp70 (provided by Nick Hoogenraad, Melbourne, Australia).
Cell Culture and Generation of Stable Cell Lines
HeLa cells were grown at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 5% (v/v) fetal bovine serum (FBS, Invitrogen). Mouse embryonic fibroblasts (MEFs) derived from bak−/−bax−/− double knock-out (DKO) (33) or vdac2−/− (14) mice were cultured in DMEM supplemented with 10% FBS, 250 μm l-asparagine, and 55 μm 2-mercaptoethanol under an atmosphere of 5% CO2 and 95% air. Wild type (WT) Bak, BakI81T, and Bak-hFis1TM were stably introduced into DKO MEFs by lentiviral infection as described previously (34). Monoclonal populations were selected based on positive protein expression following induction with 4-hydroxytamoxifen (4-HT). For equal protein expression, WT Bak, BakI81T, and Bak-hFis1TM were induced with 18, 5, and 200 nm 4-HT, respectively. No effect on cell viability was observed with 200 nm 4-HT (data not shown).
Cell Death Assays
Both nontransduced and transduced DKO MEFs were seeded on 6-well tissue culture plates at ∼60% confluency and allowed to adhere for 12- 24 h before addition of 4-HT for 16 h. The 4-HT containing medium was removed at which time the cells were supplemented with medium either lacking or containing 10 μm etoposide (Sigma). Cells were incubated with etoposide for 24 h. Cell death was analyzed by annexin V-FITC (Invitrogen) staining and flow cytometry. In each sample, 10,000 events were measured, and cell death (annexin V-FITC-positive cells) was quantified.
RESULTS
Mitochondrial Outer Membrane Permeabilization Is Concomitant with Bak Release from a 400-kDa Complex
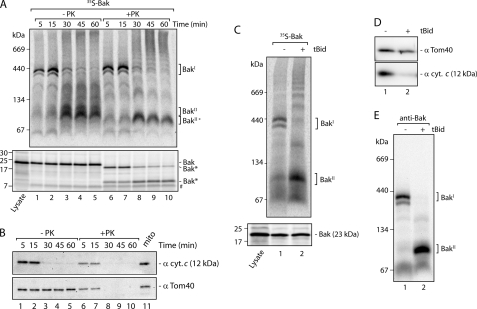
To study mitochondrial import and assembly of Bak, we used an in vitro mitochondrial import assay (35). Bak was translated in vitro using rabbit reticulocyte lysate in the presence of [35S]methionine and incubated with mitochondria that were freshly isolated from HeLa cells. Mitochondria were solubilized in digitonin buffer and analyzed by blue native PAGE (BN-PAGE) so that we could follow precursor import and assembly into complexes. The 35S-labeled Bak was initially found in an ∼400-kDa doublet complex at early time points (Fig. 1A, upper panel, lanes 1 and 2, termed BakI), but after prolonged incubation (between 30 and 60 min) at 37 °C, Bak shifted into a lower species of ∼100 kDa (Fig. 1A, upper panel, lanes 3–5, termed BakII).
FIGURE 1.
Release of Bak from a 400-kDa complex in isolated HeLa cell mitochondria induces MOMP. A, mitochondria were freshly isolated from HeLa cells and incubated with radiolabeled Bak precursor at 37 °C for varying times. Following import, mitochondria were reisolated and left untreated or treated with proteinase K. Mitochondria were then lysed in digitonin-containing buffer and subjected to BN-PAGE (upper panel) or resuspended in Laemmli sample buffer and subjected to SDS-PAGE (lower panel). Both gels were transferred to PVDF membrane prior to digital autoradiography. Two molecular weight assemblies of Bak are indicated (termed BakI and BakII). Proteolytic fragments of Bak are indicated (Bak*). # indicates nonspecific band. Lysate indicates a sample of the translated 35S-Bak product. B, following autoradiography, the SDS-PAGE PVDF membrane from A was subjected to immunoblot analysis with antibodies against cytochrome c (cyt. c) and Tom40 (as control). 25 μg of total mitochondria protein (shown as mito in lane 11) was loaded as a control. C, 35S-Bak was incubated with isolated HeLa cell mitochondria at 37 °C for 5 min. Mitochondria were isolated and resuspended in import buffer and then left untreated or treated with tBid and incubated for a further 10 min at 37 °C. Samples were split and analyzed by BN-PAGE (upper panel) and SDS-PAGE (lower panel) and then subjected to digital autoradiography. D, HeLa cell mitochondria treated with or without tBid were subjected to SDS-PAGE and probed with antibodies against cytochrome c and Tom40. E, isolated HeLa cell mitochondria were resuspended in import buffer with or without tBid for 10 min at 37 °C. Mitochondria were re-isolated, solubilized in digitonin-containing buffer, and separated by BN-PAGE followed by Western blot analysis using antibodies against Bak.
To determine whether 35S-Bak was buried within the BakΙ and BakΙΙ complexes, we performed a protease protection assay following import. Radiolabeled Bak within the BakΙ complex was largely resistant to externally added protease (Fig. 1A, upper panel, lanes 6 and 7) and therefore likely represents membrane-integrated and assembled Bak. SDS-PAGE analysis of protease-treated mitochondria revealed that the radiolabeled Bak was clipped as judged by its faster migration, and at points where Bak had transitioned to the BakΙΙ complex, further digestion was seen resulting in the accumulation of an ∼10-kDa fragment (Fig. 1A, lower panel, compare lane 5 with 10). It has been previously found that activation of Bak results in it undergoing a conformational change and becoming more susceptible to proteolysis (9, 14, 36). The change in Bak proteolysis is consistent with either a conformational change in Bak from its inactive to active state, an alteration in how it is buried in the membrane, or a change in the proteins with which it was associated.
Different detergents are known to affect the conformation of Bak solubilized from membranes, with digitonin discriminating between active and inactive conformers, although Triton X-100 exposes an epitope in Bak that is also seen in the active form (37, 38). It has also been found that when the detergent CHAPS is used in gel filtration studies, inactive Bak resolves at ∼50 kDa (39, 40). Solubilization of mitochondria in different detergents (digitonin, dodecyl maltoside, Triton X-100, or CHAPS) revealed that the BakΙ complex was only present in digitonin (supplemental Fig. S1). This suggests that the 400-kDa BakΙ complex is quite labile, and Bak-interacting proteins can dissociate in particular detergents. Similar results have been observed with the translocase complex of the mitochondrial outer membrane (41).
We next assessed whether the transition of Bak from the BakΙ to BakΙΙ complex correlated with activation of Bak, leading to MOMP and cytochrome c release. Immunoblot analysis revealed release of cytochrome c from mitochondria at the same time as Bak transitioned to the BakΙΙ complex (Fig. 1B, lanes 1–5), indicating that MOMP was occurring. As a control, we probed the blot with polyclonal antibodies against the mitochondrial outer membrane protein Tom40. Upon MOMP, proteinase K became accessible to the intermembrane space, and Tom40 epitopes were subsequently lost (Fig. 1B, lanes 8–10).
Our findings are similar to those recently reported by Ross et al. (42); however, in their case, Bak remained in the BakΙ doublet species and only shifted when cells were induced to undergo apoptosis. To determine whether the transition we observed of Bak from the BakΙ to BakΙΙ complex was the same as the transitioning observed by Ross et al. (42), we induced MOMP in vitro by adding the membrane-targeted death ligand, tBid. We loaded 35S-Bak into isolated HeLa cell mitochondria for 5 min to generate the 400-kDa BakΙ complex and then subsequently added recombinant tBid. Radiolabeled Bak was found predominantly within the 400-kDa inactive BakΙ complex in untreated samples, although addition of tBid caused it to transition into the BakΙΙ complex (Fig. 1C), and cytochrome c release was observed (Fig. 1D). To ensure the changes observed with the in vitro translated 35S-Bak were mirrored by the endogenous form of Bak, isolated HeLa cell mitochondria were incubated with or without tBid, solubilized in digitonin buffer, and analyzed by BN-PAGE and immunoblot analysis with anti-Bak antibodies. As with in vitro translated Bak, endogenous Bak was found in a 400-kDa complex in nonstimulated samples and, upon treatment with tBid, transitioned to the smaller BakΙΙ complex (Fig. 1E). We conclude that transition of Bak from the 400-kDa BakΙ complex into the lower molecular weight BakΙΙ complex correlated with a conformational change in Bak, as indicated by increased susceptibility of Bak to externally added protease, MOMP induction, and cytochrome c release. Of note is that some endogenous (presumably inactive) Bak was also found at a low molecular weight position suggesting that Bak must not be found in the 400-kDa complex to remain inactive and/or the complex is somewhat labile (Fig. 1E).
Activation of Bak in HeLa Cell Mitochondria Is Temperature-dependent and Influenced by Bak Levels and Soluble Factors
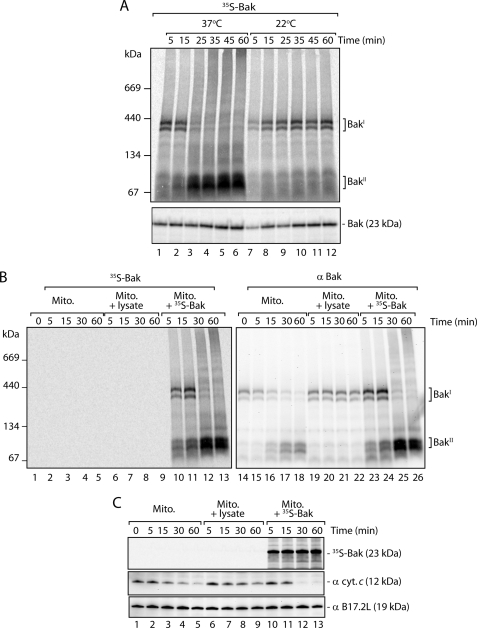
Our results indicate that MOMP can occur in isolated HeLa cell mitochondria under our in vitro import conditions, and this activation mirrors tBid-inducible MOMP through Bak activation. Bak has previously been reported to undergo spontaneous activation following incubation at 43 °C (43). It is possible that prolonged incubation of isolated HeLa cell mitochondria at 37 °C may also induce this activation. Indeed, transition of Bak from BakΙ to the BakΙΙ complex was not observed when the assay was performed at 22 °C (Fig. 2A, lanes 7–12).
FIGURE 2.
Bak activation in HeLa cell mitochondria is temperature-dependent and influenced by Bak levels and soluble factors. A, mitochondria (Mito.) were freshly isolated from HeLa cells and incubated with 35S-Bak at 37 or 22 °C for varying times. Following import, mitochondria were re-isolated, lysed in digitonin-containing buffer, and subjected to BN-PAGE (upper panel) or resuspended in Laemmli sample buffer and subjected to SDS-PAGE (lower panel). Gels were visualized by autoradiography. B, isolated HeLa cell mitochondria in import buffer were either left alone or supplemented with rabbit reticulocyte lysate or 35S-Bak containing rabbit reticulocyte lysate and incubated at 37 °C for the indicated times. Samples were solubilized in digitonin-containing buffer, separated by BN-PAGE and transferred to PVDF membrane. Following digital autoradiography (left panel), immunoblotting with antibodies against Bak was performed (right panel). C, experiment was performed as described in B; however, the samples were separated by SDS-PAGE and subsequently transferred to PVDF. Following digital autoradiography (upper panel), immunoblotting was performed with antibodies against cytochrome c (cyt. c, middle panel) and B17.2L (lower panel).
Next we tested whether activation of MOMP in isolated HeLa cell mitochondria required newly imported Bak. We took isolated HeLa cell mitochondria and incubated them at 37 °C for varying times either alone, in the presence of reticulocyte lysate lacking translated Bak, or with reticulocyte lysate containing translated 35S-Bak. Samples were analyzed by BN-PAGE to monitor Bak transitions and SDS-PAGE to follow cytochrome c release. Incubation of mitochondria alone at 37 °C was sufficient to induce transitioning of endogenous Bak to its active form (Fig. 2B, lanes 14–18), and this correlated with cytochrome c release (Fig. 2C, lanes 1–5). However, in the presence reticulocyte lysate, the BakΙ complex was stable (Fig. 2B, lanes 19–22), and cytochrome c release was blocked (Fig. 2C, lanes 6–9). Conversely, the addition of 35S-Bak in reticulocyte lysate (Fig. 2B, lanes 10–13) induced both Bak transitioning (Fig. 2B, lanes 23–26) and complete release of cytochrome c from mitochondria (Fig. 2C, lanes 10–13). Our results indicate that although isolated HeLa cell mitochondria can undergo spontaneous Bak activation in vitro, this is inhibited when mitochondria are incubated in the presence of rabbit reticulocyte lysate lacking newly translated Bak. This inhibition might be due to the presence of anti-apoptotic proteins such as Bcl-xL in the reticulocyte lysate (43). However, the inhibitory property of the lysate was overcome when the lysate contained newly translated Bak molecules. Indeed, the Western blot analysis showed that the radiolabeled Bak contributed to the overall increased reactivity to Bak antibodies (Fig. 2B, compare lane 14 with 23) indicating that relatively high amounts of Bak were synthesized in the reticulocyte lysate. Thus, the integration of additional Bak molecules into the outer membrane appears to disturb an equilibrium existing between Bak and its regulatory proteins, causing Bak activation and MOMP.
VDAC2 Is Required for Formation of the Inactive Bak Complex
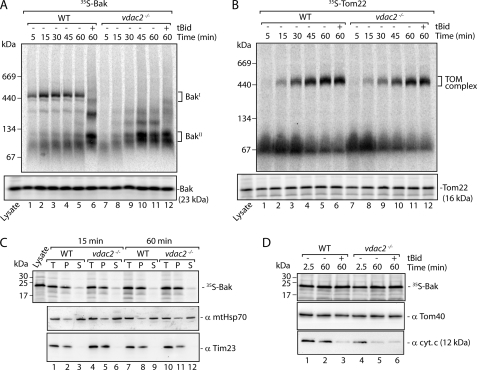
We observed that the size of the 400-kDa Bak complex resembled VDAC complexes previously seen by BN-PAGE (42, 44, 45). To determine whether VDAC2 influenced the formation of the Bak complex, we compared import and assembly of Bak in mitochondria isolated from wild type and vdac2−/− MEFs. In mitochondria from wild type MEFs, radiolabeled Bak integrated into the 400-kDa BakΙ complex, but unlike HeLa cell mitochondria, spontaneous transition into the BakΙΙ complex following extended incubation at 37 °C was not observed. Nevertheless, Bak still transitioned into the smaller complex following addition of tBid (Fig. 3A, lanes 1–6). The transition of Bak from inactive to active form may therefore be dependent on the steady-state levels of other anti-apoptotic proteins in mitochondria. After addition of 35S-Bak to mitochondria isolated from vdac2−/− MEFs, we did not observe the 400-kDa BakΙ complex. Instead, Bak immediately formed the BakΙΙ complex (Fig. 3A, lanes 7–12). To ensure that other large molecular weight assemblies were not affected in vdac2−/− mitochondria, we assessed the import and assembly of a control mitochondrial outer membrane protein, Tom22, which proceeded normally into the TOM complex in mitochondria isolated from vdac2−/− cells (Fig. 3B).
FIGURE 3.
VDAC2 is required for formation of the inactive 400-kDa Bak complex. A, mitochondria were isolated from wild type (WT) and vdac2−/− MEFs, incubated with 35S-Bak for the indicated times, then analyzed by both BN-PAGE (upper panel) and SDS-PAGE (lower panel), and visualized by autoradiography. Mitochondria receiving tBid treatment were first incubated for 45 min with 35S-Bak at 37 °C, upon which 50 nm recombinant tBid was added and samples were incubated for a further 15 min at 37 °C. B, wild type (WT) and vdac2−/− mitochondria were incubated with 35S-Tom22 at 37 °C for the indicated times. tBid-treated samples were incubated for 45 min with 35S-Tom22 at 37 °C, followed by a further 15 min at 37 °C with 50 nm recombinant tBid. Samples were analyzed by both BN-PAGE (upper panel) and SDS-PAGE (lower panel) as in A. C, 35S-Bak was incubated with mitochondria isolated from wild type (WT) and vdac2−/− MEFs for 15 min or 60 min. Mitochondria were reisolated following import, resuspended in Na2CO3 (0.1 m, pH 11.5), and then incubated on ice for 30 min. Membrane sheets were isolated by ultracentrifugation, and total (T), pellet (P), and supernatant (S) samples were solubilized in Laemmli buffer and separated by SDS-PAGE. The gel was transferred to PVDF membrane, subjected to digital autoradiography (upper panel), and subsequently analyzed by immunoblotting using the antibodies indicated (lower panels). D, 35S-Bak was incubated with mitochondria isolated from wild type (WT) and vdac2−/− MEFs for the indicated times at 37 °C. tBid-treated samples were first incubated at 37 °C for 45 min upon which 50 nm recombinant tBid was added, and samples were incubated for a further 15 min at 37 °C. Mitochondria were isolated and solubilized in Laemmli buffer, separated by SDS-PAGE, and subsequently analyzed by digital autoradiography (upper panel) and immunoblotting using antibodies against Tom40 and cytochrome c (lower panels).
Some studies have suggested that rather than influencing the activity of Bak, VDAC2 is needed for recruitment of new Bak molecules to mitochondria (24, 25). To determine whether lack of association of Bak with the BakΙ complex in vdac2−/− mitochondria was simply due to ineffective recruitment of Bak to the mitochondrial outer membrane, we assessed membrane integration of 35S-Bak following import into wild type and vdac2−/− MEFs by alkaline extraction of soluble and peripheral membrane proteins (35, 46). Membrane integrated species were isolated by ultracentrifugation and separated by SDS-PAGE. After incubation for 15 or 60 min, the membrane integration of 35S-Bak was equally observed in wild type and vdac2−/− mitochondria, similar to the control integral inner membrane protein Tim23 (Fig. 3C). These results suggest that Bak can integrate into the outer mitochondrial membrane in the absence of VDAC2 just as efficiently, but bypasses the BakΙ complex to directly enter the BakΙΙ complex. Because this complex is seen when mitochondria undergo MOMP, we hypothesized that upon Bak import, isolated vdac2−/− mitochondria would be more susceptible than wild type mitochondria to MOMP and cytochrome c release. We therefore assessed cytochrome c release in mitochondria from wild type and vdac2−/− mitochondria after the import of radiolabeled Bak at short time points (2.5 min) or longer time points (60 min). Indeed, the release of cytochrome c took place faster in mitochondria from vdac2−/− cells than those from wild type cells (Fig. 3D). These results suggest that VDAC2 is involved in sequestering Bak in an inactive state in the BakΙ complex, consistent with previous findings (14).
Transmembrane Anchor in Bak Is Required for Sequestration with VDAC2
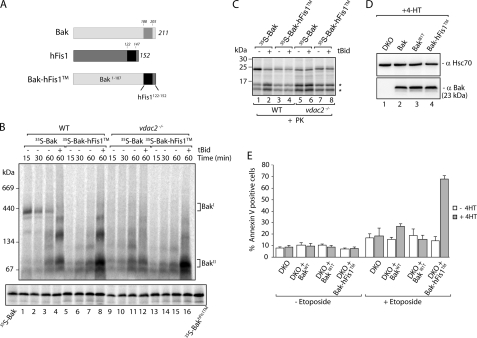
Based on the β-barrel structures of refolded VDAC1 (20, 47, 48) and previous biochemical data, it is likely that VDAC2 (49) is almost entirely buried within the mitochondrial outer membrane. We hypothesized that the transmembrane domain of Bak might mediate its interaction with VDAC2. To test this, we replaced the C-terminal transmembrane anchor of Bak with that of hFis1, a mitochondrial outer membrane protein that, like Bak, contains mitochondrial targeting information at its C terminus (Fig. 4A) (50). Import and assembly assays showed that 35S-Bak-hFis1TM failed to assemble into the 400-kDa BakΙ complex in control mitochondria, but rather appeared to directly assemble into the BakΙΙ complex (Fig. 4B, lanes 5–8). Furthermore, in mitochondria isolated from vdac2−/− cells, both Bak and Bak-hFis1TM assembled directly into the BakΙΙ complex. Efficient binding of both Bak and Bak-hFis1TM to mitochondria was seen in both cases (Fig. 4B, lower panel). To ascertain whether Bak-hFis1TM efficiently integrated within the outer membrane in its correct orientation, Bak and Bak-hFisTM were imported into wild type and vdac2−/− mitochondria with or without tBid treatment and were treated with proteinase K. An identical profile consisting of two characteristic proteolytic fragments was observed for both proteins, suggesting the hFis1 transmembrane domain did not influence either the active or inactive conformations of Bak (Fig. 4C).
FIGURE 4.
Substitution of the Bak transmembrane anchor blocks VDAC2 association and enhances Bak activation. A, schematic representation of the Bak-hFis1TM construct. B, 35S-Bak and 35S-Bak-hFis1TM were incubated with mitochondria isolated from wild type (WT) and vdac2−/− MEFs for the indicated times at 37 °C. Following 45 min of import, tBid-treated samples received 50 nm of recombinant tBid and were incubated for 15 min at 37 °C. Samples were analyzed by BN-PAGE (upper panel) and SDS-PAGE (lower panel) and then subjected to digital autoradiography. C, mitochondria were isolated from wild type (WT) and vdac2−/− MEFs and incubated with 35S-Bak and 35S-Bak-hFis1TM precursors for 60 min at 37 °C. Following import, samples were either left untreated or treated with 50 nm tBid for 10 min at 37 °C. All samples were then treated with 100 μg/ml proteinase K for 10 min on ice. Mitochondria were reisolated, solubilized in Laemmli buffer, and separated by SDS-PAGE. Proteolytic fragments of Bak are indicated (*). D, Bax/Bak double knock-out (DKO) MEFs were stably transfected with wild type Bak, BakI81T, and Bak-hFis1TM. Protein expression was induced by the addition of 4-HT for 16 h. A sample of whole cell extract was separated by SDS-PAGE and analyzed by immunoblotting using antibodies against Hsc70 (as loading control) and Bak. E, Bax/Bak double knock-out MEFs (double knock-out) containing Bak, BakI81T, or Bak-hFis1TM were incubated with 4-HT for 16 h to induce protein expression, followed by incubation either with or without 10 μm etoposide for 24 h. Cells were isolated and stained with annexin V and analyzed by flow cytometry (error bars, S.E.; n = 3).
Our data suggest that the C-terminal transmembrane domain of Bak is not only required for its integration into the mitochondrial outer membrane but is also required for the assembly of Bak into the 400-kDa VDAC2 complex. Because Bak entered the BakΙΙ complex directly in the absence of VDAC2 and caused isolated mitochondria to undergo MOMP, we hypothesized that cellular expression of Bak-hFis1TM would induce apoptosis more rapidly and potently than wild type Bak. To test this, we used an inducible lentiviral system to express wild type Bak and Bak-hFis1TM in Bax/Bak DKO MEFs and compared them to an inactive Bak mutant, BakI81T (38). Using this system, expression of the constructs can be induced with 4-HT (34).
We isolated monoclonal cell populations and monitored protein expression and cell death. We were not able to isolate a clonal population that was capable of expressing Bak-hFis1TM to the same levels as wild type Bak (data not shown), and therefore we could not monitor cell death in a constitutively expressing system. We therefore adjusted 4-HT concentrations such that similar levels of the three proteins following 4-HT induction were present (Fig. 4D). The cells were viable, indicating that the levels of Bak were below the threshold of auto-activating apoptosis. To test their susceptibility to induction of apoptosis, cells were treated with etoposide for 24 h, and the percentage of dead cells was determined by annexin V staining using flow cytometry (Fig. 4E). The Bax/Bak DKO cells were resistant to cell death as reported previously (33, 51). Similarly, cells expressing the BakI81T mutant displayed an overall resistance to etoposide treatment (38). In this time period, about 25% of the cells expressing Bak alone were dead, although nearly 70% of the cells expressing Bak-hFis1TM were dead.
DISCUSSION
In this study, we analyze the complexes formed by the pro-apoptotic protein Bak in its active and inactive forms using BN-PAGE. We find that the C-terminal transmembrane anchor of Bak does not simply act to target and insert the protein in the mitochondrial outer membrane but that it is also involved in maintaining Bak in an inactive conformation by sequestration in a complex dependent on VDAC2. As inactive Bak can be cross-linked directly with VDAC2 (14) and it forms a 400-kDa doublet complex on BN-PAGE, which resembles that formed by VDAC2 (44), it is likely that VDAC2 is found within this complex. The structure of VDAC1, which shares significant homology to VDAC2, was found to form a β-barrel and would be almost entirely embedded in the outer membrane. However, it has been suggested that the refolding conditions employed have led to an artifact because biochemical approaches argue for additional regions of VDAC1 extruding from the membrane (49). Nevertheless, it is likely that all VDAC species resemble one another with the majority of the protein embedded in the outer membrane in a β-barrel conformation. As the Bak transmembrane anchor is critical for its association with the VDAC2 complex, it suggests that direct contacts occur in the membrane region. Nevertheless, we cannot exclude that additional cytosolic contacts also take place to stabilize Bak within the VDAC2 complex. As the gene duplications that generated the three VDAC isoforms occurred early in vertebrate evolution (52), it will be interesting to determine when in evolution the interaction between VDACs and Bak arose and why Bak appears to interact only with VDAC2.
Because the transmembrane anchor of Bak is not lost following Bak activation, it is likely that structural changes in Bak (or associating proteins) displace Bak from the VDAC2 complex. Importantly, dissociation of Bak from the VDAC2 complex by swapping the transmembrane anchor of Bak with hFis1 does not automatically result in activation of Bak but renders cells more sensitive to induction of apoptosis. These results are consistent with previous conclusions that VDAC2 acts as a brake to prevent inadvertent Bak activation (39). We propose that other anti-apoptotic molecules such as Bcl-xL and Mcl-1 help keep Bak in check following its release from the VDAC2 complex (10, 11). In addition, we cannot rule out that other proteins besides VDAC2 and Bak are present in the ∼400-kDa complex. It is also interesting that the complex is found in a doublet form on BN-PAGE and may indicate the presence of other proteins whose association may be labile to the conditions employed. A similar scenario has been observed for the translocase complex of the outer mitochondrial membrane where the Tom20 receptor is lost from the complex following solubilization in high concentrations of digitonin and BN-PAGE (53). Furthermore, we note that when other detergents were used, Bak does not resolve in the VDAC2 complex (supplemental Fig. S1). Other studies have reported Bak to exist in different sized complexes, and this may be due to the various conditions and detergents employed (39, 40, 54).
In our studies to characterize the Bak complex, we found that mitochondria isolated from HeLa cells and incubated at 37 °C for prolonged periods (45–60 min) underwent Bak activation, MOMP, and cytochrome c release. However, mitochondria isolated from MEFs were less prone to such activation. It has been previously found that heat-shocking mitochondria at 43 °C for 15 min before a chase at 37 °C caused Bak activation and MOMP (43). Thus, Bak is heat-labile and has the propensity to undergo heat-induced conformational changes. Because we only used physiological temperatures and auto-activation of Bak was not seen in mitochondria from MEFs, it suggests that other limiting factors are present in HeLa cell mitochondria. Indeed, we found that the addition of rabbit reticulocyte lysate to HeLa cell mitochondria had an inhibitory effect on Bak activation. Likewise, Pagliari et al. (43) found that cytosolic Bcl-xL prevented the heat-induced activation of Bak. It is therefore likely that mitochondrial preparations from different cell types harbor variable levels of anti- and pro-apoptotic molecules that regulate Bak activation at 37 °C. Nevertheless, our results raise a note of caution about experiments that employ isolated mitochondria that are incubated at 37 °C for prolonged times where MOMP may ensue and potentially cause erroneous results.
Under our conditions of BN-PAGE, we did not observe active Bak to form large oligomers that would be consistent with it forming a pore-like structure. However, we did find that Bak oligomers could be seen when Bak was imported into mitochondria containing already active Bak (Fig. 2B) or following protease treatment of mitochondria containing active Bak (e.g. Fig. 1A). However, analysis of endogenous, active Bak showed it to be predominantly present in the lower BakII complex. It is plausible that the BakII species we observe represents the active dimeric form previously reported (30) and that assembles into a labile pore that cannot be resolved under the conditions employed here but can be seen using cross-linking approaches (30, 55). Although additional studies using complementary techniques are required to clarify this, it is clear that BN-PAGE is a useful tool to analyze the complex made by inactive Bak and also to monitor the transition of Bak to its active form.
In conclusion, this study not only provides new mechanistic insights into apoptotic regulation but also indicates that studies on the entire Bak protein and at the mitochondrial outer membrane need to be taken into consideration. In light of this study, it will be important to analyze the contributions made by the transmembrane anchors of other Bcl-2 family in the regulation of apoptosis.
Supplementary Material
Acknowledgments
We thank Gareth Apswoude, Ian Gentle, and David Huang for assistance and reagents.
This work was supported in part by grants from the National Health and Medical Research Council, Association for International Cancer Research, the Clive and Vera Ramaciotti Foundation, and the Australian Research Council Centre of Excellence for Coherent X-ray Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- MOMP
- mitochondrial outer membrane permeabilization
- VDAC
- voltage-dependent anion channel
- tBid
- truncated Bid
- BN-PAGE
- Blue-Native PAGE
- 4-HT
- 4-hydroxytamoxifen
- DKO
- double knock-out
- MEFs
- mouse embryonic fibroblasts
- TOM
- translocase of outer membrane
- TM
- transmembrane
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1.Chipuk J. E., Green D. R. (2008) Trends Cell Biol. 18, 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 3.Shoshan-Barmatz V., Keinan N., Zaid H. (2008) J. Bioenerg. Biomembr. 40, 183–191 [DOI] [PubMed] [Google Scholar]
- 4.Ganesan V., Colombini M. (2010) FEBS Lett. 584, 2128–2134 [DOI] [PubMed] [Google Scholar]
- 5.Siskind L. J., Mullen T. D., Romero Rosales K., Clarke C. J., Hernandez-Corbacho M. J., Edinger A. L., Obeid L. M. (2010) J. Biol. Chem. 285, 11818–11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karbowski M., Norris K. L., Cleland M. M., Jeong S. Y., Youle R. J. (2006) Nature 443, 658–662 [DOI] [PubMed] [Google Scholar]
- 7.Wolter K. G., Hsu Y. T., Smith C. L., Nechushtan A., Xi X. G., Youle R. J. (1997) J. Cell Biol. 139, 1281–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffiths G. J., Dubrez L., Morgan C. P., Jones N. A., Whitehouse J., Corfe B. M., Dive C., Hickman J. A. (1999) J. Cell Biol. 144, 903–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei M. C., Lindsten T., Mootha V. K., Weiler S., Gross A., Ashiya M., Thompson C. B., Korsmeyer S. J. (2000) Genes Dev. 14, 2060–2071 [PMC free article] [PubMed] [Google Scholar]
- 10.Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., Huang D. C. (2005) Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuconati A., Mukherjee C., Perez D., White E. (2003) Genes Dev. 17, 2922–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits C., Czabotar P. E., Hinds M. G., Day C. L. (2008) Structure 16, 818–829 [DOI] [PubMed] [Google Scholar]
- 13.Korsmeyer S. J., Wei M. C., Saito M., Weiler S., Oh K. J., Schlesinger P. H. (2000) Cell Death Differ. 7, 1166–1173 [DOI] [PubMed] [Google Scholar]
- 14.Cheng E. H., Sheiko T. V., Fisher J. K., Craigen W. J., Korsmeyer S. J. (2003) Science 301, 513–517 [DOI] [PubMed] [Google Scholar]
- 15.Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., Youle R. J. (2002) J. Cell Biol. 159, 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks C., Wei Q., Feng L., Dong G., Tao Y., Mei L., Xie Z. J., Dong Z. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 11649–11654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chipuk J. E., Kuwana T., Bouchier-Hayes L., Droin N. M., Newmeyer D. D., Schuler M., Green D. R. (2004) Science 303, 1010–1014 [DOI] [PubMed] [Google Scholar]
- 18.Craigen W. J., Graham B. H. (2008) J. Bioenerg. Biomembr. 40, 207–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vander Heiden M. G., Chandel N. S., Schumacker P. T., Thompson C. B. (1999) Mol. Cell 3, 159–167 [DOI] [PubMed] [Google Scholar]
- 20.Hiller S., Garces R. G., Malia T. J., Orekhov V. Y., Colombini M., Wagner G. (2008) Science 321, 1206–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu S., Narita M., Tsujimoto Y. (1999) Nature 399, 483–487 [DOI] [PubMed] [Google Scholar]
- 22.Rostovtseva T. K., Antonsson B., Suzuki M., Youle R. J., Colombini M., Bezrukov S. M. (2004) J. Biol. Chem. 279, 13575–13583 [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T., Shimizu S., Matsuoka Y., Yoneda Y., Tsujimoto Y. (2002) Oncogene 21, 4944–4956 [DOI] [PubMed] [Google Scholar]
- 24.Setoguchi K., Otera H., Mihara K. (2006) EMBO J. 25, 5635–5647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy S. S., Ehrlich A. M., Craigen W. J., Hajnóczky G. (2009) EMBO Rep. 10, 1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lazarou M., McKenzie M., Ohtake A., Thorburn D. R., Ryan M. T. (2007) Mol. Cell. Biol. 27, 4228–4237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker M. J., Webb C. T., Stroud D. A., Palmer C. S., Frazier A. E., Guiard B., Chacinska A., Gulbis J. M., Ryan M. T. (2009) Mol. Biol. Cell 20, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uren R. T., Dewson G., Chen L., Coyne S. C., Huang D. C., Adams J. M., Kluck R. M. (2007) J. Cell Biol. 177, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schägger H., von Jagow G. (1987) Anal. Biochem. 166, 368–379 [DOI] [PubMed] [Google Scholar]
- 30.Dewson G., Kratina T., Czabotar P., Day C. L., Adams J. M., Kluck R. M. (2009) Mol. Cell 36, 696–703 [DOI] [PubMed] [Google Scholar]
- 31.Humphries A. D., Streimann I. C., Stojanovski D., Johnston A. J., Yano M., Hoogenraad N. J., Ryan M. T. (2005) J. Biol. Chem. 280, 11535–11543 [DOI] [PubMed] [Google Scholar]
- 32.Lithgow T., Ryan M., Anderson R. L., Høj P. B., Hoogenraad N. J. (1993) FEBS Lett. 332, 277–281 [DOI] [PubMed] [Google Scholar]
- 33.Lindsten T., Ross A. J., King A., Zong W. X., Rathmell J. C., Shiels H. A., Ulrich E., Waymire K. G., Mahar P., Frauwirth K., Chen Y., Wei M., Eng V. M., Adelman D. M., Simon M. C., Ma A., Golden J. A., Evan G., Korsmeyer S. J., MacGregor G. R., Thompson C. B. (2000) Mol. Cell 6, 1389–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunning C. J., McKenzie M., Sugiana C., Lazarou M., Silke J., Connelly A., Fletcher J. M., Kirby D. M., Thorburn D. R., Ryan M. T. (2007) EMBO J. 26, 3227–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stojanovski D., Pfanner N., Wiedemann N. (2007) Methods Cell Biol. 80, 783–806 [DOI] [PubMed] [Google Scholar]
- 36.Ruffolo S. C., Shore G. C. (2003) J. Biol. Chem. 278, 25039–25045 [DOI] [PubMed] [Google Scholar]
- 37.Hsu Y. T., Wolter K. G., Youle R. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3668–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dewson G., Kratina T., Sim H. W., Puthalakath H., Adams J. M., Colman P. M., Kluck R. M. (2008) Mol. Cell 30, 369–380 [DOI] [PubMed] [Google Scholar]
- 39.Ren D., Kim H., Tu H. C., Westergard T. D., Fisher J. K., Rubens J. A., Korsmeyer S. J., Hsieh J. J., Cheng E. H. (2009) Sci. Signal. 2, ra48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim H., Tu H. C., Ren D., Takeuchi O., Jeffers J. R., Zambetti G. P., Hsieh J. J., Cheng E. H. (2009) Mol. Cell 36, 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dekker P. J., Ryan M. T., Brix J., Müller H., Hönlinger A., Pfanner N. (1998) Mol. Cell. Biol. 18, 6515–6524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross K., Rudel T., Kozjak-Pavlovic V. (2009) Cell Death Differ. 16, 697–707 [DOI] [PubMed] [Google Scholar]
- 43.Pagliari L. J., Kuwana T., Bonzon C., Newmeyer D. D., Tu S., Beere H. M., Green D. R. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17975–17980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kozjak-Pavlovic V., Ross K., Götz M., Goosmann C., Rudel T. (2010) J. Mol. Biol. 397, 219–232 [DOI] [PubMed] [Google Scholar]
- 45.Krimmer T., Rapaport D., Ryan M. T., Meisinger C., Kassenbrock C. K., Blachly-Dyson E., Forte M., Douglas M. G., Neupert W., Nargang F. E., Pfanner N. (2001) J. Cell Biol. 152, 289–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. (1982) J. Cell Biol. 93, 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bayrhuber M., Meins T., Habeck M., Becker S., Giller K., Villinger S., Vonrhein C., Griesinger C., Zweckstetter M., Zeth K. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 15370–15375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ujwal R., Cascio D., Colletier J. P., Faham S., Zhang J., Toro L., Ping P., Abramson J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 17742–17747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colombini M. (2009) Trends Biochem. Sci. 34, 382–389 [DOI] [PubMed] [Google Scholar]
- 50.Stojanovski D., Koutsopoulos O. S., Okamoto K., Ryan M. T. (2004) J. Cell Sci. 117, 1201–1210 [DOI] [PubMed] [Google Scholar]
- 51.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. (2001) Science 292, 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Young M. J., Bay D. C., Hausner G., Court D. A. (2007) BMC Evol. Biol. 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meisinger C., Ryan M. T., Hill K., Model K., Lim J. H., Sickmann A., Müller H., Meyer H. E., Wagner R., Pfanner N. (2001) Mol. Cell. Biol. 21, 2337–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valentijn A. J., Upton J. P., Gilmore A. P. (2008) Biochem. J. 412, 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mikhailov V., Mikhailova M., Degenhardt K., Venkatachalam M. A., White E., Saikumar P. (2003) J. Biol. Chem. 278, 5367–5376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.