Abstract
The mitochondrial DNA polymerase from Drosophila embryos lacks dNTP turnover activity. However, a potent 3'----5' exonuclease activity can be detected by a specific assay in which the exonuclease excises mispaired nucleotides at the 3' termini of primed synthetic and natural DNA templates. The excision of a mispaired nucleotide occurs at a significantly greater rate than excision of a correctly paired nucleotide and, under conditions of DNA synthesis, hydrolysis of a mispaired terminal nucleotide occurs prior to primer extension. The 3'----5' exonuclease copurifies quantitatively with DNA polymerase gamma and cosediments with the nearly homogeneous enzyme under native conditions. These results suggest that the 3'----5' exonuclease provides a proofreading function to enhance the fidelity of DNA synthesis during Drosophila mitochondrial DNA replication.
Full text
PDF
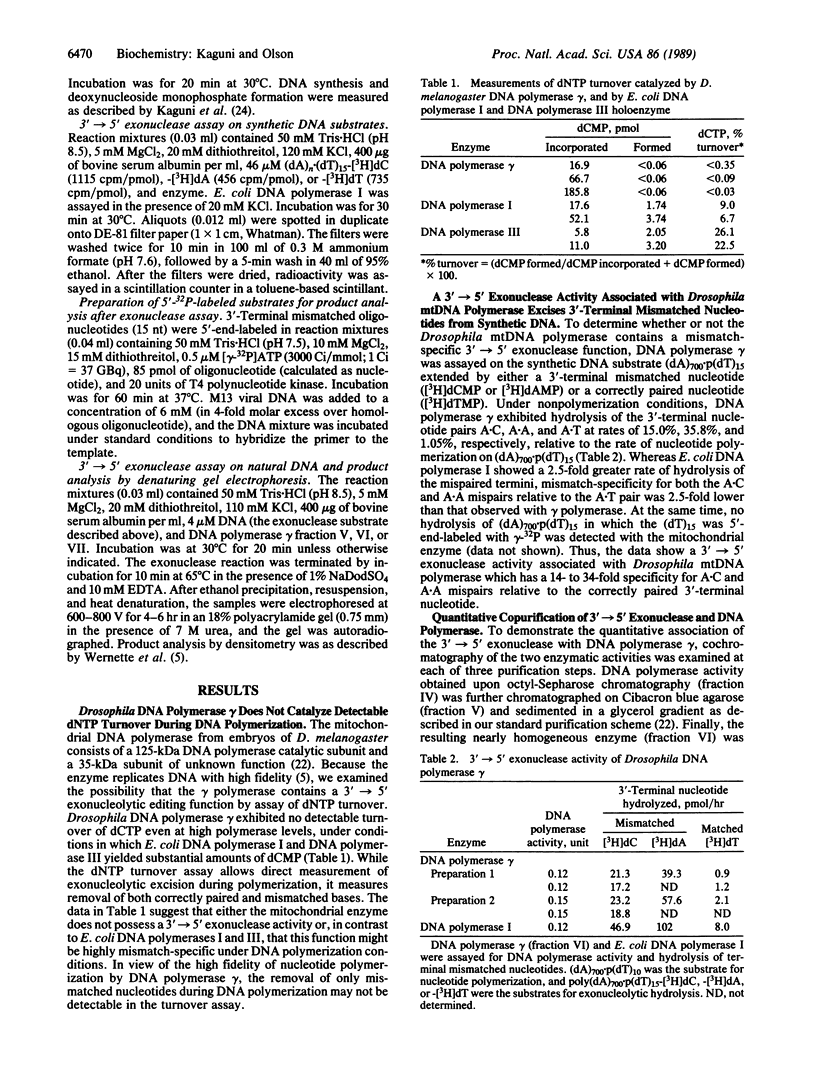
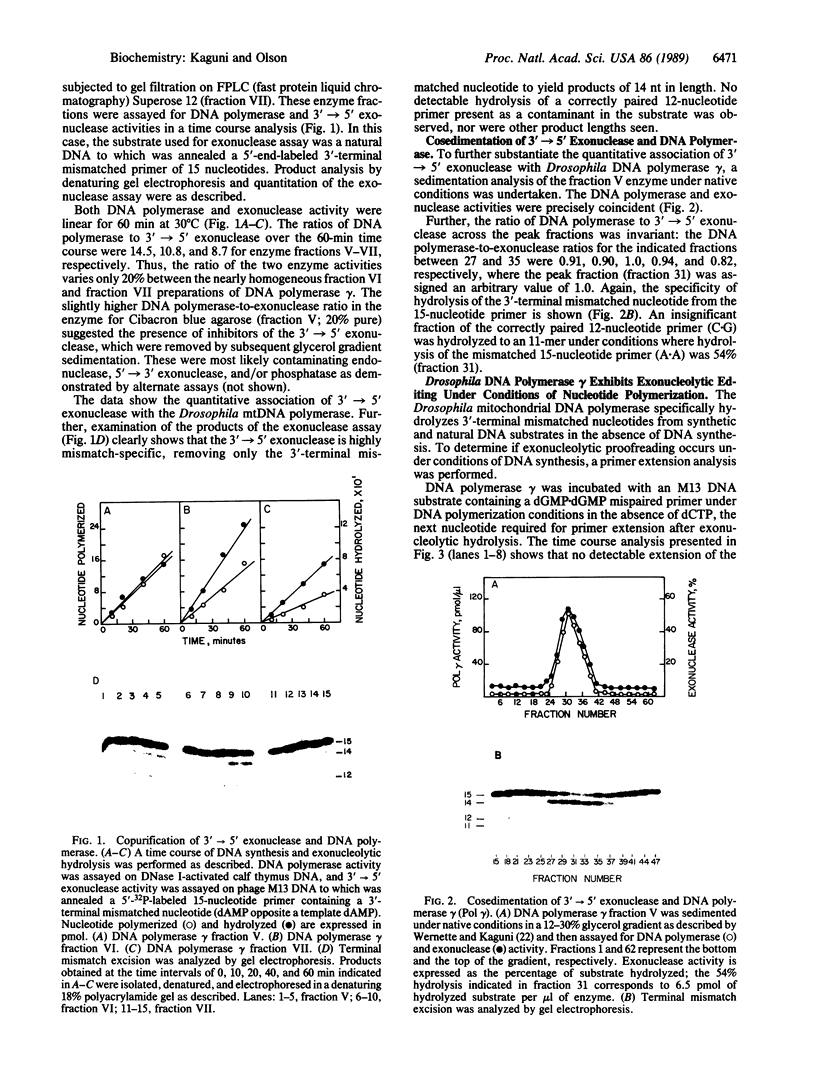
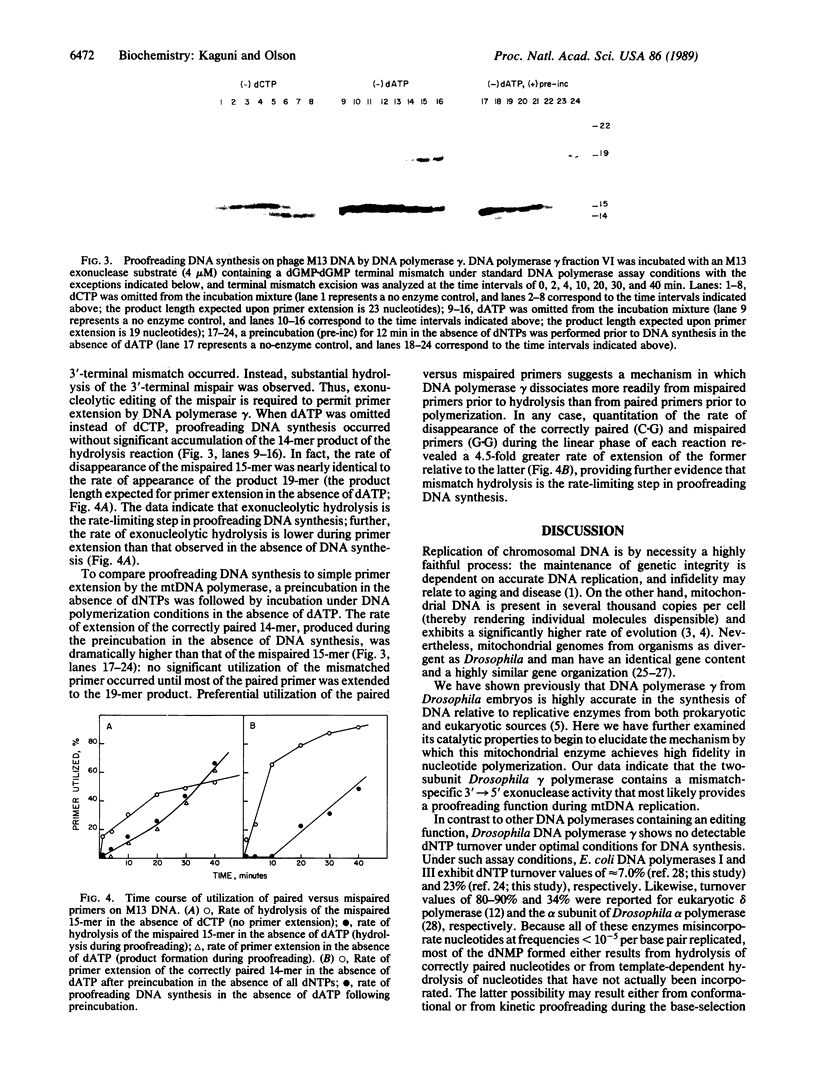

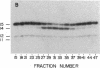
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Brown W. M., George M., Jr, Wilson A. C. Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1967–1971. doi: 10.1073/pnas.76.4.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Black V. L., So A. G. A new mammalian DNA polymerase with 3' to 5' exonuclease activity: DNA polymerase delta. Biochemistry. 1976 Jun 29;15(13):2817–2823. doi: 10.1021/bi00658a018. [DOI] [PubMed] [Google Scholar]
- Clary D. O., Wolstenholme D. R. The mitochondrial DNA molecular of Drosophila yakuba: nucleotide sequence, gene organization, and genetic code. J Mol Evol. 1985;22(3):252–271. doi: 10.1007/BF02099755. [DOI] [PubMed] [Google Scholar]
- Clayton D. A., Doda J. N., Friedberg E. C. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotterill S. M., Reyland M. E., Loeb L. A., Lehman I. R. A cryptic proofreading 3'----5' exonuclease associated with the polymerase subunit of the DNA polymerase-primase from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5635–5639. doi: 10.1073/pnas.84.16.5635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawid I. B. Evolution of mitochondrial DNA sequences in Xenopus. Dev Biol. 1972 Oct;29(2):139–151. doi: 10.1016/0012-1606(72)90051-6. [DOI] [PubMed] [Google Scholar]
- Echols H., Lu C., Burgers P. M. Mutator strains of Escherichia coli, mutD and dnaQ, with defective exonucleolytic editing by DNA polymerase III holoenzyme. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2189–2192. doi: 10.1073/pnas.80.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fansler B. S., Loeb L. A. Sea urchin nuclear DNA polymerase. Methods Enzymol. 1974;29:53–70. doi: 10.1016/0076-6879(74)29009-8. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W., Tsui W. C. Kinetic basis of spontaneous mutation. Misinsertion frequencies, proofreading specificities and cost of proofreading by DNA polymerases of Escherichia coli. J Mol Biol. 1982 Mar 25;156(1):37–51. doi: 10.1016/0022-2836(82)90457-0. [DOI] [PubMed] [Google Scholar]
- Kaguni L. S., DiFrancesco R. A., Lehman I. R. The DNA polymerase-primase from drosophila melanogaster embryos. Rate and fidelity of polymerization on single-stranded DNA templates. J Biol Chem. 1984 Jul 25;259(14):9314–9319. [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Changes in DNA polymerases alpha, beta, and gamma during the replicative life span of cultured human fibroblasts. Biochemistry. 1982 Mar 2;21(5):1002–1009. doi: 10.1021/bi00534a027. [DOI] [PubMed] [Google Scholar]
- Krauss S. W., Linn S. Fidelity of fractionated deoxyribonucleic acid polymerases from human placenta. Biochemistry. 1980 Jan 8;19(1):220–228. doi: 10.1021/bi00542a033. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. Fidelity of mammalian DNA polymerases. Science. 1981 Aug 14;213(4509):765–767. doi: 10.1126/science.6454965. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Schaaper R. M., Beckman R. A., Loeb L. A. On the fidelity of DNA replication. Effect of the next nucleotide on proofreading. J Biol Chem. 1981 Oct 10;256(19):9883–9889. [PubMed] [Google Scholar]
- Kunkel T. A., Soni A. Exonucleolytic proofreading enhances the fidelity of DNA synthesis by chick embryo DNA polymerase-gamma. J Biol Chem. 1988 Mar 25;263(9):4450–4459. [PubMed] [Google Scholar]
- Kunkel T. A. The mutational specificity of DNA polymerases-alpha and -gamma during in vitro DNA synthesis. J Biol Chem. 1985 Oct 15;260(23):12866–12874. [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- McHenry C. S., Crow W. DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem. 1979 Mar 10;254(5):1748–1753. [PubMed] [Google Scholar]
- McHenry C., Kornberg A. DNA polymerase III holoenzyme of Escherichia coli. Purification and resolution into subunits. J Biol Chem. 1977 Sep 25;252(18):6478–6484. [PubMed] [Google Scholar]
- Ottiger H. P., Hübscher U. Mammalian DNA polymerase alpha holoenzymes with possible functions at the leading and lagging strand of the replication fork. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3993–3997. doi: 10.1073/pnas.81.13.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland M. E., Lehman I. R., Loeb L. A. Specificity of proofreading by the 3'----5' exonuclease of the DNA polymerase-primase of Drosophila melanogaster. J Biol Chem. 1988 May 15;263(14):6518–6524. [PubMed] [Google Scholar]
- Vishwanatha J. K., Coughlin S. A., Wesolowski-Owen M., Baril E. F. A multiprotein form of DNA polymerase alpha from HeLa cells. Resolution of its associated catalytic activities. J Biol Chem. 1986 May 15;261(14):6619–6628. [PubMed] [Google Scholar]
- Wernette C. M., Conway M. C., Kaguni L. S. Mitochondrial DNA polymerase from Drosophila melanogaster embryos: kinetics, processivity, and fidelity of DNA polymerization. Biochemistry. 1988 Aug 9;27(16):6046–6054. doi: 10.1021/bi00416a033. [DOI] [PubMed] [Google Scholar]
- Wernette C. M., Kaguni L. S. A mitochondrial DNA polymerase from embryos of Drosophila melanogaster. Purification, subunit structure, and partial characterization. J Biol Chem. 1986 Nov 5;261(31):14764–14770. [PubMed] [Google Scholar]
- Wolstenholme D. R., Clary D. O. Sequence evolution of Drosophila mitochondrial DNA. Genetics. 1985 Apr;109(4):725–744. doi: 10.1093/genetics/109.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn M. H. Drosophila melanogaster mitochondrial DNA, a novel organization and genetic code. Nature. 1983 Jul 21;304(5923):234–241. doi: 10.1038/304234a0. [DOI] [PubMed] [Google Scholar]