Abstract
Vitamin B12 (cobalamin) is a critical cofactor for animals and protists, yet its biosynthesis is limited to prokaryotes. We previously showed that the symbiotic nitrogen-fixing alphaproteobacterium Sinorhizobium meliloti requires cobalamin to establish a symbiotic relationship with its plant host, Medicago sativa (alfalfa). Here, the specific requirement for cobalamin in the S. meliloti-alfalfa symbiosis was investigated. Of the three known cobalamin-dependent enzymes in S. meliloti, the methylmalonyl CoA mutase (BhbA) does not affect symbiosis whereas disruption of the metH gene encoding the cobalamin-dependent methionine synthase causes a significant defect in symbiosis. Expression of the cobalamin-independent methionine synthase MetE alleviates this symbiotic defect, indicating that the requirement for methionine synthesis does not reflect a need for the cobalamin-dependent enzyme. To investigate the function of the cobalamin-dependent ribonucleotide reductase (RNR) encoded by nrdJ, S. meliloti was engineered to express an Escherichia coli cobalamin-independent (Class Ia) RNR instead of nrdJ. This strain is severely defective in symbiosis. Electron micrographs show that these cells can penetrate alfalfa nodules but are unable to differentiate into nitrogen-fixing bacteroids and instead are lysed in the plant cytoplasm. Flow cytometry analysis indicates that these bacteria are largely unable to undergo endoreduplication. These phenotypes may be due to the inactivation of the Class Ia RNR by reactive oxygen species and/or inadequate oxygen availability in the nodule. These results show that the critical role of the cobalamin-dependent RNR for survival of S. meliloti in its plant host can account for the considerable resources that S. meliloti dedicates to cobalamin biosynthesis.
Keywords: Sinorhizobium meliloti, symbiosis, vitamin B12, cobalamin, ribonucleotide reductase
INTRODUCTION
Vitamin B12 (cobalamin), one of the largest known non-polymeric natural products, is a cofactor synthesized exclusively by prokaryotes that facilitates a variety of radical and methyl transfer reactions (Roth et al. 1996; Ryzhkova 2003). Cobalamin biosynthesis and utilization are distributed unevenly throughout nature. In most animals and protists, cobalamin is an essential cofactor that must be obtained from exogenous sources, whereas plants and fungi do not utilize cobalamin cofactors (Roth et al. 1996). The biosynthesis and utilization of cobalamin vary widely throughout the prokaryotic domains. The majority of sequenced prokaryotes do not possess a complete cobalamin biosynthesis pathway (Raux et al. 2000). Most cobalamin-dependent enzymes in prokaryotes function in metabolic processes for which equivalent cobalamin-independent pathways do not exist such as methanogenesis, antibiotic synthesis, degradation of xenobiotic compounds, and utilization of glycerol, propanediol, ethanolamine, and various amino acids as carbon and/or nitrogen sources (Ryzhkova 2003). In contrast, some bacteria such as Sinorhizobium meliloti use cobalamin cofactors for processes for which cobalamin-independent pathways also exist (Buckel et al. 2005; Rodionov et al. 2003; Roth et al. 1996; Ryzhkova 2003).
S. meliloti is one of many bacteria capable of synthesizing cobalamin de novo through an oxygen-dependent pathway (Campbell et al. 2006; Evans and Kliewer 1964). In S. meliloti, cobalamin functions as a cofactor for three enzymes, all of which have metabolically equivalent, cobalamin-independent counterparts in other organisms. One cobalamin-dependent enzyme in S. meliloti, methionine synthase (MetH), catalyzes the final step in methionine biosynthesis (Matthews 1999; Sato et al. 1974). Nearly all genera in the order Rhizobiales have the metH gene (Dehal et al. 2010) while the cobalamin-independent methionine synthase, MetE, which is present in many organisms, is also present in 43% of the genera in the order Rhizobiales. Methylmalonyl CoA mutase (named BhbA in S. meliloti) is a cobalamin-dependent enzyme that catalyzes a step in the metabolism of branched-chain amino acids (Banerjee and Chowdhury 1999; Charles and Aneja 1999). Organisms that do not require cobalamin utilize the parallel, cobalamin-independent methylcitrate or acryloyl-CoA pathways (Buckel et al. 2005). S. meliloti also utilizes cobalamin for its Class II ribonucleotide reductase (RNR) (Cowles and Evans 1968; Fontecave 1998). RNRs catalyze the synthesis of deoxynucleotides (dNDP or dNTP) from ribonucleotides (NDP or NTP), which is the rate-limiting step in DNA synthesis (Kolberg et al. 2004). Most organisms utilize a cobalamin-independent (Class I or Class III) RNR, and over half of sequenced bacteria possess more than one RNR class (Lundin et al. 2009). It is possible that S. meliloti possesses an additional, unknown class(es) of cobalamin-dependent enzymes.
Given the existence and abundance of these cobalamin-independent pathways, it seems remarkable that many bacteria have retained the ability to synthesize cobalamin, a process that requires the activity of ∼30 genes which comprise 1–7% of a bacterial genome (McCutcheon et al. 2009; Roth et al. 1996). Characterization of a bluB mutant of S. meliloti, which led to the discovery of the enzyme responsible for the synthesis of the lower ligand of cobalamin, demonstrated that cobalamin is essential for S. meliloti for symbiosis with Medicago sativa (alfalfa) but did not specify which cobalamin-dependent enzyme(s) contributed to the cobalamin requirement during symbiosis (Campbell et al. 2006; Taga et al. 2007). One hypothesis that could explain why S. meliloti synthesizes cobalamin for enzymes for which cobalamin-independent counterparts exist in closely related bacteria is that cobalamin-dependent enzymes provide an advantage for survival in the plant host. For example, both MetE and Class Ia RNRs are sensitive to reactive oxygen species (ROS) and/or reactive nitrogen species (RNS), and S. meliloti is exposed to ROS and RNS when infecting the plant (Baudouin et al. 2006; Fontecave 1998; Hondorp and Matthews 2004; Rubio et al. 2004; Santos et al. 2000).
S. meliloti is a Gram-negative alphaproteobacterium that exists either as a free-living member of the rhizosphere or as an intracellular nitrogen-fixing symbiont of legumes of the genera Medicago, Trigonella, and Melilotus (Gibson et al. 2008). In this mutualistic relationship, bacteria establish long-term residence within the cytoplasm of plant cells in specialized organs called nodules where they convert atmospheric nitrogen to ammonia, a process known as nitrogen fixation (Gibson et al. 2008; Jones et al. 2007). Bacteria initially enter the plant root via an infection thread, a plant-derived tubule formed within the root hair (Gage and Margolin 2000 ). The bacteria replicate in the infection thread and subsequently are internalized by plant cells of the nodule primordium by endocytosis (Gage 2002; Jones et al. 2007). Within the plant cytoplasm, bacteria develop into non-dividing but metabolically active bacteroids (Jones et al. 2007; Oke and Long 1999b). When actively dividing, the bacteria accumulate and store poly-3-hydroxybutyrate (PHB) in granules which are later mobilized as a carbon source during bacteroid development (Lodwig and Poole 2003). During both invasion and bacteroid development, S. meliloti is exposed to plant-derived ROS and RNS which function in both pathogen resistance and signaling (Baudouin et al. 2006; Rubio et al. 2004; Santos et al. 2000). The nodule is a microaerobic environment in which much of the oxygen is sequestered by plant leghemoglobin to allow the oxygen-sensitive nitrogenase enzyme to function while sustaining the obligately aerobic metabolism of the bacteria (Miller et al. 1988; Witty et al. 1987). In indeterminate nodules, a component of the bacteroid differentiation program is the plant-controlled process of endoreduplication, in which both the plant and bacterial cells increase their genomic copy number in the absence of cell division (Maunoury et al. 2010; Paau and Cowles 1978a; Paau et al. 1979). In the host plant Medicago truncatula, a relative of alfalfa, an average of 24 copies of the S. meliloti genome per bacteroid cell are present (Mergaert et al. 2006). The precise genomic copy number of S. meliloti bacteroids in alfalfa nodules has not been determined.
To determine which cobalamin-dependent enzymes in S. meliloti contribute to the cobalamin requirement during symbiosis with alfalfa, we have tested the symbiotic phenotypes of individual mutants in cobalamin-dependent enzymes. In addition, we have engineered S. meliloti strains that express cobalamin-independent enzymes in place of their cobalamin-dependent counterparts to test whether cobalamin-dependent enzymes have properties that enable S. meliloti to survive within nodules. We find that expression of a cobalamin-independent Class Ia RNR cannot support S. meliloti during intracellular infection of its plant host, indicating that the requirement for cobalamin in S. meliloti is due to the function of the Class II RNR.
RESULTS
Contribution of S. meliloti metH and bhbA to symbiosis with alfalfa
Our previous work showed that S. meliloti requires cobalamin for symbiosis with alfalfa (Campbell et al. 2006). This requirement is presumably due to the essential activity of at least one of the three cobalamin-dependent enzymes during symbiosis. To determine the contribution of each of these enzymes to symbiosis with alfalfa, the function of each cobalamin-dependent enzyme was examined individually. Alfalfa seedlings were inoculated with S. meliloti strain Rm1021 derivatives harboring a transposon insertion in bhbA or metH, the genes encoding methylmalonyl CoA mutase and methionine synthase, respectively (Campbell et al. 2006; Charles and Aneja 1999). Plants inoculated with the bhbA∷Tn5 strain exhibited a Fix+ phenotype (i.e., able to fix nitrogen), as the average height and percent pink nodules of these plants were indistinguishable from plants inoculated with Rm1021 (Table 1). Thus, bhbA is not required for symbiosis.
Table 1.
Phenotype of alfalfa inoculated with Rm1021 derivatives
| S. meliloti Strain | Plant Height (cm)a |
Total Nodules Per Planta |
Pink Nodules Per Plant (Percent of Total Nodules)a |
|---|---|---|---|
| Uninoculated (24)b | 1.7 ± 0.1 | 0 ± 0 | N/Ac |
| Rm1021 (57) | 10.4 ± 0.5 | 23 ± 2 | 87 ± 2 |
| bhbA∷Tn5 (12) | 11.0 ± 0.6 | 23 ± 3 | 84 ± 3 |
| metH∷Tn5 (57) | 2.8 ± 0.3 | 21 ± 1 | 5 ± 2 |
| metH∷Tn5/vector (52) | 2.8 ± 0.4 | 21 ± 2 | 8 ± 3 |
| metH∷Tn5/pmetE+ (SmMetE+) (41) | 6.7 ± 0.6 | 20 ± 1 | 59 ± 1 |
| Rm1021/vector (37) | 9.5 ± 0.6 | 22 ± 2 | 85 ± 3 |
| Rm1021/pmetE+ (22) | 10.2 ± 0.7 | 25 ± 3 | 84 ± 3 |
| ΔnrdJ/pnrdJ+ (SmNrdJ+) (26) | 8.6 ± 0.4 | 27 ± 2 | 81 ± 4 |
| ΔnrdJ/pnrdAB+ (SmNrdAB+) (44) | 2.3 ± 0.1 | 23 ± 2 | 1 ± 1 |
| Rm1021/pnrdJ+ (33) | 8.9 ± 0.6 | 21 ± 2 | 83 ± 2 |
| Rm1021/pnrdAB+ (37) | 9.4 ± 0.6 | 19 ± 1 | 86 ± 2 |
Numbers represent mean and standard error.
The number of plants scored is shown in parentheses. Data compiled from multiple independent experiments are presented.
Not applicable; no nodules were present.
In contrast, plants inoculated with the S. meliloti metH∷Tn5 mutant exhibited a Fix−phenotype (i.e., unable to fix nitrogen), as observed by their low average height and scarcity of pink nodules (Table 1). Free-living growth analysis confirmed that the metH∷Tn5 mutant is a methionine auxotroph (Supplementary Fig. 1A). Therefore, it is likely that the Fix− phenotype is due to a lack of free methionine in the nodule. This result is in agreement with a previous study of methionine auxotrophs of S. meliloti strain Rmd201 (Abbas et al. 2002) but not strain 102F34, which can use glycine betaine as an alternative methionine source (Barra et al. 2006).
To test whether a cobalamin-independent methionine synthase could substitute for MetH during symbiosis in the Rm1021 strain background, a plasmid containing the E. coli cobalamin-independent methionine synthase, metE (Chu et al. 1985), was expressed in a metH∷Tn5 mutant. For simplicity, we will refer to this strain as SmMetE+. The E. coli metE gene is able to function in S. meliloti, as expression of metE suppresses the methionine auxotrophy of metH∷Tn5 (Supplementary Fig. 1A). Furthermore, metE complements the Fix− phenotype of the metH∷Tn5 mutant, as the average plant height, percent pink nodules, and nitrogenase activity are dramatically improved (Table 1 and Table 2). The incomplete rescue of the plant height and number of pink nodules per plant and high variability in nitrogen fixation in SmMetE+ is likely due to the 100-fold slower turnover number of MetE compared to MetH (Gonzalez et al. 1992). This result demonstrates that S. meliloti strain Rm1021 must synthesize methionine in order to establish an effective symbiosis, but it is not necessary that the methionine synthase used for this purpose be cobalamin-dependent.
Table 2.
Nitrogenase activity in alfalfa inoculated with Rm1021 derivatives
| S. meliloti Strain | Acetylene reduction (percent wild type)a |
|---|---|
| Rm1021/vector (5)b | 86 ± 18 |
| metH∷Tn5 (12) | 14 ± 8 |
| metH∷Tn5/pmetE+ (SmMetE+) (6) | 77 ± 30 |
| ΔnrdJ/pnrdJ+ (SmNrdJ+) (5) | 75 ± 17 |
| ΔnrdJ/pnrdAB+ (SmNrdAB+) (10) | 3 ± 11 |
Acetylene reduction activity for whole plants is presented as the rate of ethylene formation as a percentage of the average rate in plants inoculated with Rm1021. Numbers represent mean and standard error.
The number of plants scored is shown in parentheses. In some cases, data compiled from multiple independent experiments are presented.
The E. coli Class Ia RNR functions in free-living growth of S. meliloti but not in symbiosis
Next, we sought to test the function of the S. meliloti cobalamin-dependent (Class II) RNR, encoded by nrdJ, during symbiosis with alfalfa. The nrdJ gene is essential for DNA synthesis, as no other RNR exists in the S. meliloti genome. Therefore, we tested whether S. meliloti could function when nrdJ is substituted with genes encoding a Class Ia (cobalamin-independent) RNR. The chromosomal nrdJ gene was deleted in the presence of a plasmid expressing nrdA and nrdB from E. coli, which together encode a Class Ia RNR (Carlson et al. 1984). As a control, a Class II RNR, nrdJ from Agrobacterium tumefaciens, was expressed in an S. meliloti ΔnrdJ strain. These strains will be abbreviated as SmNrdAB+ and SmNrdJ+, respectively. Both strains are viable in the free-living state and are not impaired in cobalamin-dependent growth in minimal media. Furthermore, as expected, the Class I RNR-specific inhibitor hydroxyurea (HU) inhibits the growth of SmNrdAB+ (Supplementary Fig. 1B) (Elford 1968). Although the OD600 continues to increase, treatment of SmNrdAB+ with HU results in cell death as in the case of E. coli (Davies et al. 2009) (Supplementary Fig. 1B, C). In contrast, SmNrdJ+, like wild type S. meliloti, is not affected by HU (Supplementary Fig. 1B).
As expected, plants inoculated with SmNrdJ+ have a phenotype indistinguishable from those inoculated with Rm1021 (Fig. 1 A, B, D, E; Table 1, Table 2). In contrast, SmNrdAB+ has a profound defect in symbiosis. This defect is apparent on a gross morphological level, as the plants are short with yellow leaves, and the nodules, though occasionally elongated, are nearly all white (Fig. 1C, F). Like Rm1021 and SmNrdJ+ (Fig. 1G, H), SmNrdAB+ is competent for nodule invasion, as nodules of plants inoculated with gus-expressing bacteria show β-glucuronidase activity throughout the nodule for both elongated (Fig. 1I) and small, round nodules (not shown). The average height of plants inoculated with SmNrdAB+ is only slightly greater than uninoculated plants, and a pink nodule was rarely observed (Table 1). A low level of nitrogenase activity was detectable in some plants, indicating that few bacteria could successfully develop into bacteroids and fix nitrogen (Table 2). This defect in symbiosis was not caused by a lack of nrdA or nrdB expression in the plant, as both NrdA and NrdB proteins were detected in nodule extracts by Western blot (not shown). Moreover, the defect in symbiosis was likely not due to oxygen limitation in the nodule, as co-expression of nrdD and nrdG, which encode the E. coli Class III (anaerobic) RNR (Garriga et al. 1996), with nrdA and nrdB did not improve the symbiotic phenotype (not shown). The plasmids expressing nrdJ or nrdAB did not affect free-living growth or symbiosis when expressed in S. meliloti containing wild type nrdJ (Table 1). Together, these results demonstrate that the cobalamin-independent RNR NrdAB can support free-living growth of S. meliloti but does not function in symbiosis with alfalfa. Furthermore, the requirement for the Class II RNR for symbiosis explains the dependence of S. meliloti on cobalamin biosynthesis.
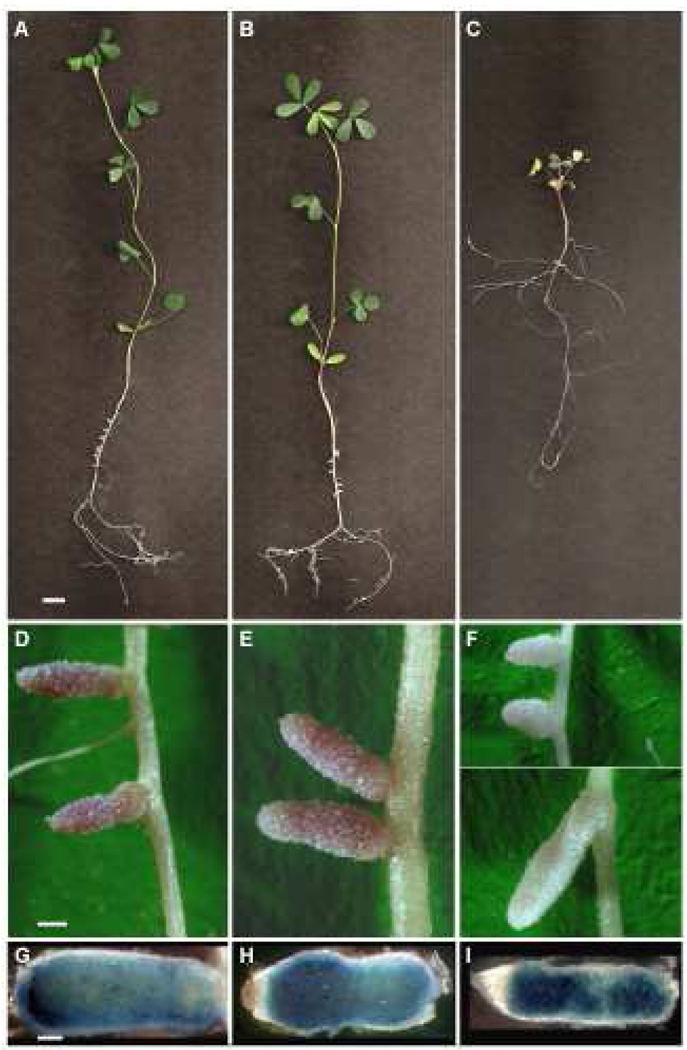
Figure 1.
Phenotype of alfalfa inoculated with S. meliloti expressing different RNRs. Whole plants (A–C), nodules (D–F), and nodules stained with gus-expressing S. meliloti (G–I) are shown for alfalfa inoculated with Rm1021 (A, D, G), SmNrdJ+ (B, E, H), and SmNrdAB+ (C, F, I). Scale bars represent 5 mm (A–C), 0.5 mm (D–F), and 0.25 mm (G–I).
Bacteria isolated from nodules of plants inoculated with SmNrdAB+ appear morphologically similar to bacteroids
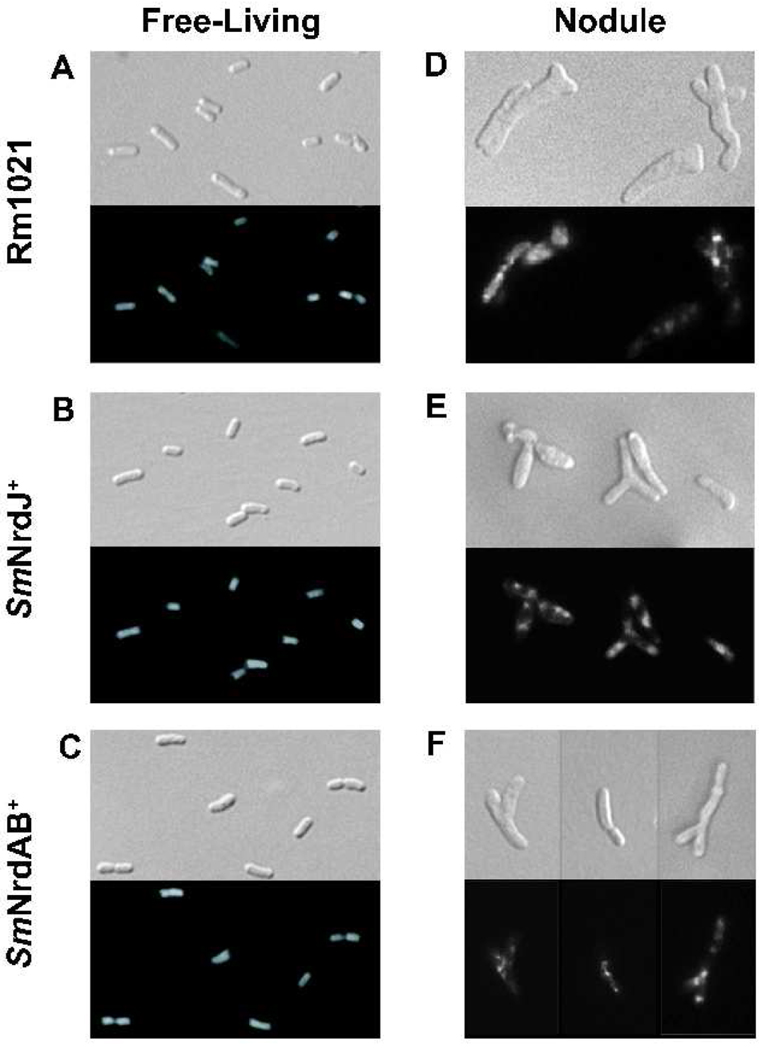
In the normal development of the S. meliloti-alfalfa symbiosis, S. meliloti cells are released from infection threads into the plant cytoplasm following invasion of the nodule. The cells then undergo a series of physiological and morphological changes including cell elongation and branching as they differentiate into mature, nitrogen-fixing bacteroids. SmNrdAB+ is apparently unable to complete this process, as its nitrogen fixing activity is very low despite the ability to invade the nodule (Table 2 and Fig. 1I). To investigate the nature of this defect, bacterial cells isolated from nodules were examined by microscopy. As a control, we first visualized free-living Rm1021, SmNrdJ+, and SmNrdAB and observed no detectable difference in cell morphology or DAPI staining pattern (Fig. 2 A–C). To examine bacterial cells from nodules, nodules were crushed and the released bacteria were visualized by microscopy. Surprisingly few bacterial cells were isolated from nodules of plants inoculated with SmNrdAB+ considering that the level of bacterially expressed β-glucuronidase appeared comparable to the level in nodules containing Rm1021 or SmNrdJ+ (Fig. 1 D–F). However, the few bacterial cells isolated from these nodules are morphologically indistinguishable from those isolated from Rm1021 and SmNrdJ+ (Fig. 2 D–F). The cells contain hallmarks of mature bacteroids, including their elongated, Y-shaped morphology and punctate DAPI staining. The low level of nitrogenase activity observed in plants inoculated with SmNrdAB+ (Table 2) could be explained by the presence of these very few bacteroids in the nodule. However, the normal β-glucuronidase activity present throughout these nodules (Fig. 1I) does not reflect this reduction in bacterial cell number and instead could indicate that some cell lysis of SmNrdAB+ occurs in the nodule.
Figure 2.
Cellular morphology and DNA distribution of free-living bacteria and alfalfa nodule extracts. DAPI-stained bacteria visualized by Nomarski (upper panels) and fluorescence microscopy (lower panels) are shown for Rm1021 (A, D), SmNrdJ+ (B, E), and SmNrdAB+ (C, F). Free-living bacteria grown to exponential phase (A–C) and bacterial cells extracted from nodules (D–F) are shown.
Endoreduplication is incomplete in S. meliloti containing a Class Ia RNR
Given that RNRs are necessary for providing the dNTP substrates for DNA replication, we addressed the possibility that the defect in nitrogen fixation observed in plants inoculated with SmNrdAB+ could be explained by incomplete endoreduplication. To analyze the DNA content in the bacterial cells quantitatively, cells were fixed with ethanol, treated with the nucleic acid binding dye Sytox Green, and analyzed by flow cytometry. As a control, the DNA content of free-living bacteria was examined. As observed previously (Mergaert et al. 2006), the DNA content in free-living bacteria shows two distinct peaks corresponding to one and two copies of the genome (1n and 2n; Fig. 3, vertical dotted lines), and no difference was observed among the three strains (Fig. 3 A–C). The size and shape distributions show a homogeneous population and are nearly identical among the three strains (Fig. 3 D–F).
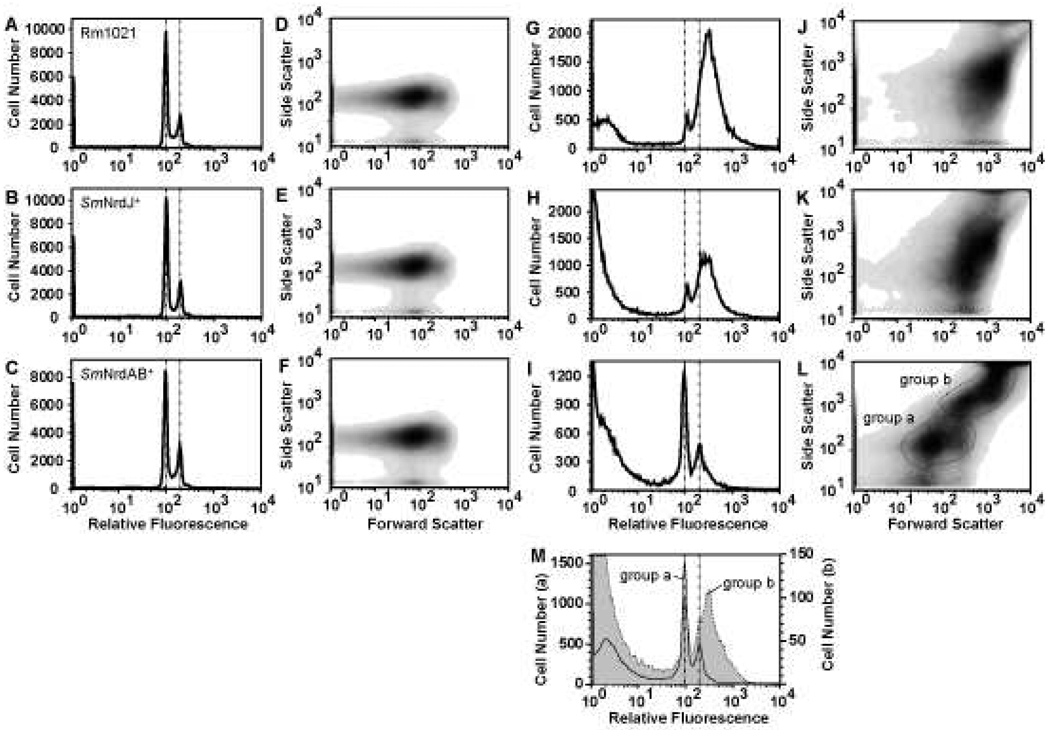
Figure 3.
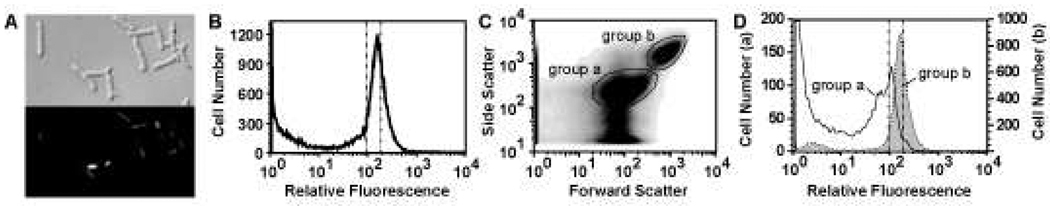
DNA content of free-living bacteria and alfalfa nodule extracts. Flow cytometry of Sytox Green-stained stationary phase free-living bacteria (A–F) and bacterial cells isolated from nodules (G–L) are shown. Histogram plots (A–C, G–I) and corresponding density plots showing the relative size and shape distribution (D–F, J–L) are presented for Rm1021 (A, D, G, J), SmNrdJ+ (B, E, H, K), and SmNrdAB+ (C, F, I, L). Genomic DNA content corresponding to 1n and 2n are marked with vertical dotted lines. The presence of objects with little or no Sytox Green staining (G–I) is partially due to degradation of the samples. Histogram plots of the two populations indicated in (L) are represented in (M) (group a, solid line; group b, shaded). Note the 10-fold difference in the scale of the y-axes in (M).
Flow cytometry analysis of bacterial cells isolated from alfalfa nodules inoculated with Rm1021 shows a broad dominant peak with DNA content centered around 3n and a minor peak at 1n (Fig. 3G). The SmNrdJ+ nodule extracts show a similar pattern (Fig. 3H). In contrast, the distribution of DNA content of SmNrdAB+ cells extracted from nodules shows a dominant peak at 1n and a broad peak centered at 2n (Fig. 3I). The distribution of DNA content in Rm1021 nodule extracts is more similar to previous flow microfluorometry analyses of S. meliloti extracted from alfalfa nodules (Paau and Cowles 1978b; Paau et al. 1979) than flow cytometry analysis of purified bacteroids extracted from nodules of M. truncatula, a relative of alfalfa (Mergaert et al. 2006). This difference can be explained by a difference between alfalfa and M. truncatula and/or a difference in the bacteroid purification method.
The size and shape distributions of cells extracted from nodules containing Rm1021 or SmNrdJ+ (Fig. 3J, K) are distinct from those of free-living bacteria (Fig. 3D, E) and reflect the differences in size and morphology between free-living bacteria and bacteroids. The DNA content and size and shape distributions of these strains differ significantly from SmNrdAB+ (Fig. 3I, L). The SmNrdAB+ nodule extracts contain a mixture of two populations of equivalent cell number with distinct size and shape distributions (Fig. 3L). The DNA content of one population (group a, Fig. 3L) is similar to free-living stationary phase cells, suggesting that these cells have not initiated the endoreduplication process (compare group a, Fig. 3M to Fig. 3 A–C). The other population (group b, Fig. 3L) largely consists of unstained particles possibly including cells lacking DNA, as there are relatively few cells with significant DNA content (group b, Fig. 3M). Some cells in this population contain higher than 2n DNA content, suggesting that endoreduplication may occur in a minority population (compare group b, Fig. 3M to Fig. 3G). The low nitrogenase activity detected in plants inoculated with SmNrdAB+ (Table 2) could be due to this population.
SmNrdAB+ bacteria are unable to persist in the nodule
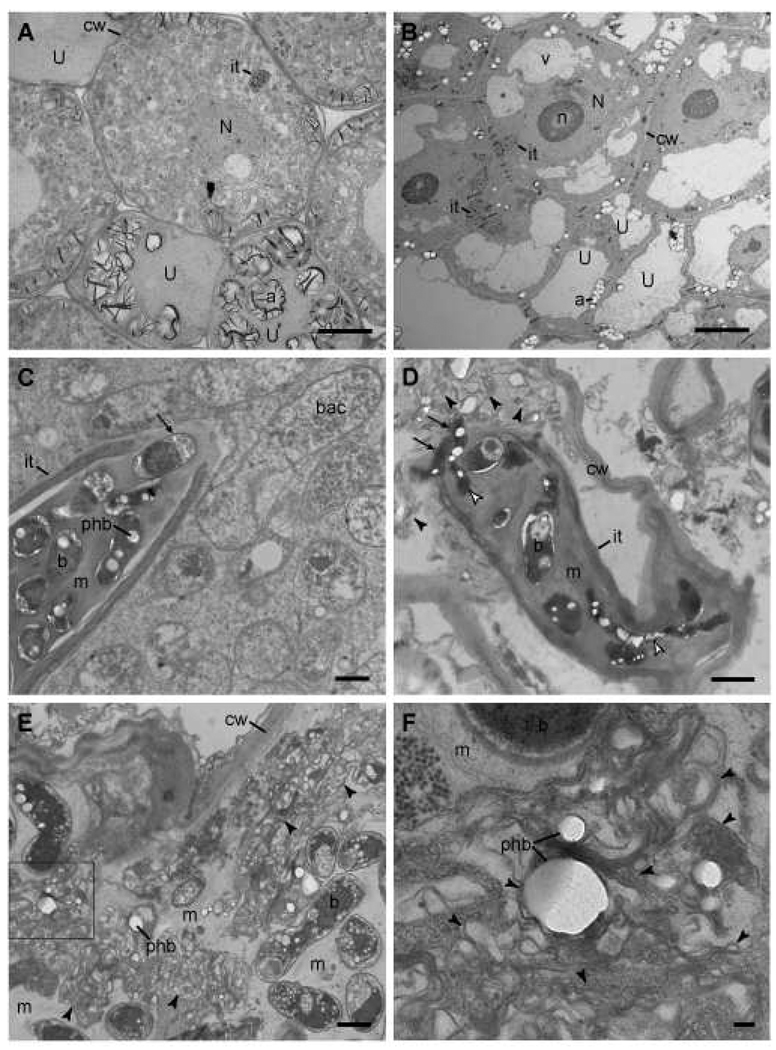
The scarcity of bacterial cells extracted from nodules of alfalfa inoculated with SmNrdAB+ and their defect in endoreduplication prompted a closer examination of these nodules by transmission electron microscopy. In nodules containing SmNrdJ+, which are indistinguishable from those containing Rm1021 (not shown), the plant cells are filled with bacteroids (Fig. 4A), whereas those inoculated with SmNrdAB+ contain infection threads, but bacteroids are not visible in the plant cytoplasm (Fig. 4B). Consistent with a lack of nitrogen-fixing bacteroids, numerous amyloplasts are present in the nodule cells of plants inoculated with SmNrdAB+ (Fig. 4B). Unlike in infection threads containing SmNrdJ+ (Fig. 4C), some cells with morphological irregularities are present in infection threads containing SmNrdAB+ (Fig. 4D). Moreover, SmNrdJ+ cells are capable of differentiating into mature bacteroids (Fig. 4C), while in nodules containing SmNrdAB+ bacterial cells are initially released into the plant cytoplasm but do not differentiate into bacteroids (Fig. 4D). Instead, membranes that appear to have been derived from bacteria accumulate in the plant cytoplasm of nodules inoculated with SmNrdAB+ (Fig. 4 D–F, arrowheads). Many of these structures appear to contain PHB granules, suggesting a bacterial origin (Fig. 4F). The presence of bacteria in infection threads containing SmNrdAB+ demonstrates that this strain is competent for replication in the infection thread, albeit with slightly altered cell morphology. These cells appear to be killed shortly after release into the cytoplasm, as they have not depleted their PHB supply. The abundance of these bacterial cell remnants could explain the normal level of β-glucuronidase activity we observed, as the Gus protein is stable enough to persist in nodules after the cells have been damaged.
Figure 4.
Transmission electron micrographs of sectioned nodules. Micrographs of alfalfa nodules inoculated with SmNrdJ+ (A, C) or SmNrdAB+ (B, D–F) are shown. F represents a higher magnification image of the boxed area in E. Scale bars represent 10 µm (A, B), 1 µm (C–E), and 0.1 µm (F). SmNrdAB+ micrographs shown in B, D, and E are from three different nodules fixed in two independent experiments. Abbreviations: cw, plant cell wall; U, uninfected cell; it, infection thread; v, vacuole; a, amyloplast; N, nucleus; n, nucleolus; b, undifferentiated bacterium; bac, bacteroid; m, infection thread matrix; phb, polyhydroxybutyrate granule. Arrows in C and D, bacteria exiting infection thread; white arrowheads in D, elongated bacteria in infection thread; black arrowheads in D–F, membrane fragments possibly derived from lysed bacteria.
SmNrdAB+ cells in nodules are distinct from free-living SmNrdAB+ treated with HU
The phenotype of SmNrdAB+ that we observed by electron microscopy suggests that inhibition of RNR activity, and thus reduction in dNTP pools, in the nodule environment results in the accumulation of empty or PHB-containing membranes in the plant cytoplasm. To understand the physiological basis of this phenotype, we tested whether inhibition of RNR activity by treatment with HU in free-living SmNrdAB+ could mimic the phenotype of SmNrdAB+ within the nodule. The cellular morphology of SmNrdAB+ following growth with HU for 6 h appears similar to that of cells isolated from nodules, as the cells become elongated and branched (Fig. 5A, upper panel). However, unlike cells extracted from nodules (Fig. 2 D–F), the DNA in HU-treated cells is diffusely distributed in most cells (Fig. 5A, lower panel). HU treatment causes loss of viability in 99% of cells without a corresponding loss in culture turbidity, indicating that, unlike in nodules, extensive cell lysis does not occur (Supplementary Fig. 1B, C). Flow cytometry analysis of the DNA in SmNrdAB+ treated with HU for 20 h shows that most cells fall within a broad distribution centered at a DNA content slightly lower than 2n (Fig. 5B). These cells can be partitioned into two populations with distinct size and shape distributions (Fig. 5C). The DNA content in one of these populations (group a, Fig. 5C, D) is broadly distributed, with the majority containing incomplete genomic content (less than 1n). The other population (group b, Fig. 5C, D) contains cells whose DNA content is centered near 2n, but this distribution is broader than that of untreated cells (compare group b, Fig. 5D to Fig. 3 A–C). Although HU-treated cells appear morphologically similar to bacteroids, their DNA content and their size and shape distributions are distinct from both untreated free-living bacteria and bacteria extracted from nodules (compare Fig. 5C, D to Fig. 3). Together, these results demonstrate that inhibition of RNR activity in free-living S. meliloti results in physiological changes distinct from those resulting from the loss of RNR activity in the nodule.
Figure 5.
Cellular morphology and DNA content of SmNrdAB+ treated with HU. A. Free-living SmNrdAB+ treated with 1 mM HU was analyzed by microscopy as in Fig. 4 and (B–D) by flow cytometry as in Fig. 5. The two populations in (C) are represented in (D) (note the 5-fold difference in the scale of the y-axes).
SmNrdAB+ cells are sensitive to ROS
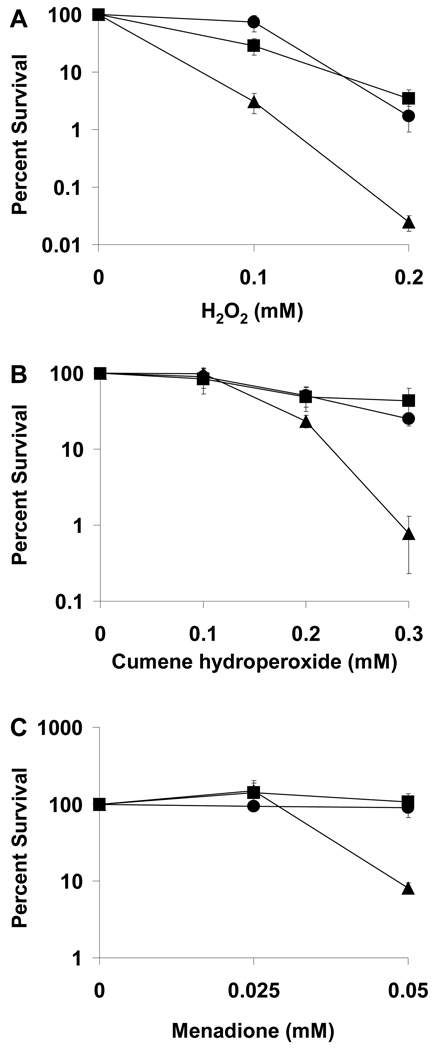
Our observation that the Class II RNR (NrdJ) can function in the plant while the Class Ia RNR (NrdAB) cannot suggests either that the NrdAB enzyme is inhibited in the plant or that a factor required for its activity is absent from the plant. Although Class I and Class II RNRs both catalyze the synthesis of deoxynucleotides, the catalytic mechanisms differ between the two classes. Specifically, the stable tyrosyl radical in Class Ia RNRs, which is used to generate a transient cysteinyl radical, can be quenched by HU or ROS (Fontecave and Gerez 2002), whereas Class II RNRs use adenosylcobalamin to generate a transient cysteinyl radical and hence are not sensitive to HU or ROS. S. meliloti encounters ROS produced by the plant during infection, and therefore inactivation of the Class Ia RNR by ROS could explain the inability of SmNrdAB+ to survive in the plant. To determine whether SmNrdAB+ is indeed sensitive to ROS, its survival in the presence of ROS was tested. Compared to strains expressing nrdJ, SmNrdAB+ is sensitive to hydrogen peroxide and the ROS-generating compounds menadione and cumene hydroperoxide, as significantly fewer colonies grew on plates containing these agents (Fig. 6). This result suggests that the sensitivity of SmNrdAB+ to ROS could contribute to its failure to survive in the plant.
Figure 6.
Heterologous expression of E. coli Class Ia RNR causes sensitivity to ROS. Plating efficiency of Rm1021 (squares), SmNrdJ+ (circles), and SmNrdAB+ (triangles) on LBMC plates containing hydrogen peroxide (A), cumene hydroperoxide (B), or menadione (C) is expressed as a percentage of the colony number in the absence of treatment. Each point represents the mean of three independent cultures, and error bars represent standard error.
DISCUSSION
In a previous study, we found that the biosynthesis of cobalamin is necessary for S. meliloti to form a productive symbiotic relationship with alfalfa. The primary objective of the present study was to determine which cobalamin-dependent enzyme(s) in S. meliloti is necessary during symbiosis with alfalfa. Here, we find that a cobalamin-dependent (Class II) RNR is specifically required for symbiosis, whereas the cobalamin-dependent methylmalonyl-CoA mutase (BhbA) is not required at all, and S. meliloti can function in the nodule with either a cobalamin-dependent or cobalamin-independent methionine synthase. Substitution of NrdJ with the E. coli cobalamin-independent (Class Ia) RNR results in an unusual symbiotic defect characterized by irregular cell morphology in infection threads and cell lysis in the nodule. Thus, the Class Ia RNR apparently loses its enzyme activity in the infection thread and/or in the plant cytoplasm.
This study also raises a broader question regarding why certain bacteria such as S. meliloti use cobalamin exclusively for metabolic functions for which other organisms do not require cobalamin. Given that cobalamin-independent counterparts of MetH and NrdJ exist in bacteria closely related to S. meliloti (Dehal et al. ; Lundin et al. 2009), it might seem more evolutionarily favorable for S. meliloti not to have retained its requirement for cobalamin biosynthesis and instead utilized only cobalamin-independent enzymes. However, the inability of a cobalamin-independent RNR to function in S. meliloti during symbiosis which we observe here could explain why S. meliloti makes the substantial genetic investment necessary to synthesize cobalamin when metabolically equivalent cobalamin-independent processes are available.
The phenotype of SmNrdAB+ in the nodule, in which empty or PHB-containing bacterial cell membranes accumulate in the plant cytoplasm, is distinct from previously observed phenotypes of S. meliloti lacking the ability to fix nitrogen, detoxify ROS, or form a normal outer membrane (Campbell et al. 2002; Glazebrook et al. 1993; Hirsch et al. 1983; Jamet et al. 2003). The morphology of SmNrdAB+ in alfalfa nodules appears similar to wild type S. meliloti in nodules of an M. truncatula dnf1 mutant; in both cases cells released from infection threads do not differentiate into mature bacteroids, although the dnf1 mutant does not induce bacterial cell lysis (Wang et al. 2010). We assume that the phenotype of SmNrdAB+ is entirely due to inactivation of the RNR within the plant, as the only genetic difference between SmNrdAB+ and SmNrdJ+ is the RNR. Our observation that the phenotype of SmNrdAB+ in the nodule is distinct from free-living SmNrdAB+ treated with HU suggests that factors in the plant cytoplasm that induce bacterial endoreduplication, alteration of the bacterial cell envelope, cell enlargement, and/or other physiological changes associated with bacteroid differentiation influence whether cells lyse in response to RNR inactivation.
The difference between the phenotypes of S. meliloti expressing a Class Ia versus a Class II RNR is presumably a result of an important difference in the catalytic properties of these two RNR classes. The known differences between these enzymes are the requirement for adenosylcobalamin by the Class II RNR and the requirements for iron, oxygen, and the formation of a tyrosyl radical in the Class Ia RNR. Thus, the inability of the Class Ia RNR to function in the plant is likely due to an insufficient supply of iron and/or oxygen or a deficiency in the formation or stability of the tyrosyl radical. Iron limitation is not likely to be the source of the symbiotic defect of SmNrdAB+ because the plant growth medium contains sufficient iron, and wild type S. meliloti can survive in nodules even in the presence of an iron chelator (Battistoni et al. 2002). Moreover, previous transcriptomics and proteomics studies of S. meliloti in M. truncatula and alfalfa nodules had indicated that an iron transporter is induced in the nodule, which had appeared to suggest that iron is limiting, however this transporter was later shown to be specific to manganese (Ampe et al. 2003; Davies and Walker 2007; Djordjevic 2004). Oxygen limitation is a more plausible explanation for the SmNrdAB+ phenotype, as the nodule environment is microaerobic. Although we observed that co-expression of the E. coli Class Ia and Class III (oxygen sensitive) RNRs in S. meliloti did not result in symbiotic proficiency, it is possible that the Class III RNR does not function in the nodule environment. An additional explanation for the inability of NrdAB to function in the nodule is that the stable tyrosyl radical of a Class Ia RNR is inactivated by ROS during infection, subsequently leading to DNA damage and eventually cell death. This is based on our observation that SmNrdAB+ is sensitive to ROS, coupled with previous evidence that plant-derived ROS are abundant during infection (Baudouin et al. 2006; Rubio et al. 2004; Santos et al. 2000). Regardless of the mode of inactivation of NrdAB in S. meliloti, it is important to recognize that the plant host also relies on a Class Ia RNR for cell division and endoreduplication, and this enzyme is functional in the same environment in which the bacterial enzyme is not (Fontecave 1998).
RNR activity is presumably required at several stages during infection of the nodule when DNA synthesis or repair occurs. DNA synthesis occurs when certain populations of cells proliferate in the infection thread (Gage 2002), and later when cells undergo endoreduplication during bacteroid differentiation (Mergaert et al. 2006; Paau and Cowles 1978b; Paau et al. 1979). The presence of DNA strand breaks and the localization of double strand break DNA repair proteins suggest that DNA repair is common in bacteroids (Kobayashi et al. 2008; Kobayashi et al. 2001). Our observation that numerous bacteria are present in infection threads containing SmNrdAB+ indicates that DNA synthesis and cell division occur relatively normally in infection threads. However, the presence of elongated cells in these infection threads indicates that the cells could be experiencing a degree of DNA stress similar to the effect of treatment of SmNrdAB+ with HU or exposure of wild type S. meliloti to DNA damaging agents (Latch and Margolin 1997). This phenotype suggests that the Class Ia RNR may progressively lose activity in the infection thread, causing the dNTP reserves of the bacterial cells to be depleted. Bacterial cell lysis may occur upon release from the infection thread as the bacteria attempt to initiate genomic endoreduplication with very limited dNTP pools. Production of ROS and RNS is a common antimicrobial strategy in eukaryotic cells. For example, mammalian leukocytes localize ROS, RNS and other antimicrobial compounds to phagosomes in order to kill pathogens that have been engulfed (Fialkow et al. 2007). Similarly, plants produce ROS and RNS as part of the defense response against pathogens (Apel and Hirt 2004). A common survival mechanism employed by both pathogenic and mutualistic bacteria involves destroying ROS by increasing the production of catalases and superoxide dismutases (Day et al. 2000; De Groote et al. 1997; Lindgren et al. 2007). Despite the importance of catalase activity for symbiosis with alfalfa (Jamet et al. 2003), destroying ROS and RNS entirely would not be a suitable mechanism for S. meliloti to overcome the oxidative stress encountered in the plant because ROS and RNS are critical for both signaling and structural maintenance of the infection thread (Jamet et al. 2007; Peleg-Grossman et al. 2007). Indeed, overexpression of catalase has been shown to cause infection thread cell wall anomalies (Jamet et al. 2007). Consistent with a critical role of ROS and RNS in nodule development, we have observed that treatment of roots with compounds that quench nitric oxide or prevent its production inhibits nodule formation (not shown). A different strategy for survival in the presence of ROS and RNS is to decrease the permeability of the cell to small molecules (Palazzolo-Ballance et al. 2008). S. meliloti employs this strategy by increasing the production of exopolysaccharide in the infection thread which is moderately protective against oxidative stress; in addition exopolysaccharide acts by modulating the plant’s defense response (Davies and Walker 2007; Jones et al. 2008; Nogales et al. 2006). An additional mechanism of avoiding killing by ROS and RNS is the production of proteins that are less vulnerable to inactivation by ROS and RNS. S. meliloti apparently employs this strategy by expression of the Class II RNR.
Many pathogenic bacteria reside within a eukaryotic host cytoplasm for months or even years. Several mammalian pathogens, including those of the genera Chlamydia, Mycobacterium, Salmonella, Rickettsia, Burkholderia, and Brucella, are engulfed by the mammalian host cell and survive a defense response in which ROS and RNS are produced by the host (Fang 2004; Roop et al. 2009; Walker et al. 2003). Therefore, each of these bacteria must have mechanisms to survive in an environment that is able to eliminate most other bacteria. Some intracellular pathogens, including Mycobacterium, Brucella, and Burkholderia, possess both a Class I and Class II RNR (Dawes et al. 2003; Lundin et al. 2009). Interestingly, the Class Ic RNR, an ROS-and RNS-resistant variant of the Class Ia RNR of E. coli, was discovered recently in Chlamydia trachomatis and is thought to facilitate survival in the host cytoplasm (Hogbom et al. 2004; Jiang et al. 2008). Further studies on the ROS-resistant RNRs of symbiotic and pathogenic bacteria may define the roles of these enzymes in bacterial survival within the host and may lead to the discovery of new methods of microbial control.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions
All S. meliloti strains are derivatives of wild type strain Rm1021 (Meade et al. 1982). S. meliloti strains were grown at 30 °C in LB supplemented with 2.5 mM magnesium sulfate and 2.5 mM calcium chloride (LBMC) or M9 sucrose medium supplemented with 10 µg/L biotin and 10 µM cobalt chloride (Maniatis et al. 1982). E. coli strains used for genetic manipulations were grown at 37 °C in LB. When necessary, media were supplemented with antibiotics at the following concentrations (mg/L): streptomycin, 500; spectinomycin, 100; kanamycin, 25; tetracycline, 5; and neomycin, 200. Where indicated, HU (1 mM) or methionine (0.1 mg/ml) was added to media. Sensitivity to hydrogen peroxide, cumene hydroperoxide, and menadione was assayed on LBMC plates buffered with 10 mM HEPES, pH 7.4.
Genetic and molecular techniques
The plasmid expressing E. coli metE was constructed by amplifying metE from E. coli MG1655 genomic DNA by PCR with primers 5'-GTCTCGAGTTACATATAATTAGAGGAAG and 5'-GTGGTACCCCACCCGGTTTGGATTTTACC and cloning into pMS03 (Keating et al. 2002) at the XhoI and KpnI restriction sites. The plasmid expressing A. tumefaciens nrdJ was constructed similarly by amplifying from A. tumefaciens C28 genomic DNA with the primers 5'-GTCTCGAGTGGACGAGGACACGAAACCATG and 5'-GTGGTACCTCAAGACGAACCGCTCGTCGCA. Ligation mixtures were introduced into E. coli DH5α by transformation. The plasmid expressing E. coli nrdA and nrdB was constructed by amplifying the nrdA and nrdB genes from E. coli MG1655 genomic DNA with primers 5'-GCCTCGAGACAGGTACGACATACATGAATC and 5'-GTCTCGAGCCATCAGAGCTCCAAGTTACTC and cloning into pMS03 at the XhoI site. The orientation of the insert in the resulting plasmids containing nrdAB was determined by digesting with KpnI. All plasmids were sequenced and introduced into S. meliloti by triparental mating (Leigh et al. 1985).
To construct a deletion in the chromosomal copy of nrdJ, a 790 bp fragment including the first 61 bp of the nrdJ coding sequence and the region upstream of nrdJ was amplified by PCR with primers 5'-TCATAAGCTTAGATTGCAGTCCTCCGATACGT and 5'-AATGCATGCGGAACTCAATCTCGGCATAG. A 730 bp fragment including the last 107 bp of nrdJ and the region downstream of nrdJ was amplified with primers 5'-AATGCATHGAGGGCTATACCGGCAACA and 5'-AATGGATCCCTGCCTTCGAATATGAGGGCT. Each PCR product was ligated into pCR2.1-TOPO using the TOPO-TA cloning kit (Invitrogen). The 5' fragment was digested with HindIII and SphI, the 3' fragment was digested with SphI and BamHI, and both were purified by gel extraction (Qiagen). The two products were ligated in tandem into pK18 mob sacB (Schafer et al. 1994) that was digested with HindIII and BamHI. The resulting deletion construct was introduced into S. meliloti harboring a plasmid expressing A. tumefaciens nrdJ or E. coli nrdA and nrdB. Neomycin resistant exconjugants were grown on TY plates containing 10% sucrose to select for a second crossover (Rosado and Gage 2003; Schafer et al. 1994). Neomycin sensitive colonies were screened by PCR for loss of the nrdJ gene.
Plant nodulation, acetylene reduction, and staining for β-glucuronidase activity
Seedlings of Medicago sativa cv. Iroquois were inoculated with S. meliloti strains on Jensen agar (Leigh et al. 1985). All experiments with whole plants and nodules were performed four weeks after inoculation with S. meliloti. Acetylene reduction assays on whole plants were performed as described (Oke and Long 1999a). Whole plants were imaged with an HP Photosmart scanner. Whole nodules containing bacteria harboring the gus-expressing plasmid pTH1227 (Cheng et al. 2007) were stained for β-glucuronidase activity as described (Kobayashi et al. 2008) and sectioned by hand.
Extraction of bacterial cells from nodules
Nodules from 9 plants were surface sterilized for 1 min in 100% bleach and washed three times in water. Nodules were crushed in MMS medium (Finan et al. 1991), and the liquid containing bacteroids was removed with a pipette. Samples were centrifuged twice at 2,200 g for 5 min and resuspended in MMS medium. For flow cytometry, small particles were removed by passing through a 0.2 µm filter (Millipore) prior to analysis.
Microscopy
Whole nodules excised from roots and hand-sectioned nodules stained for β-glucuronidase activity were examined with a Nikon Eclipse microscope equipped with a CCD camera. Free-living bacteria grown in M9 and bacterial cells extracted from nodules were stained with DAPI (Molecular Probes) and visualized by microscopy on slides prepared as described (Lemon and Grossman 1998). Images were processed with Adobe Photoshop. Tissue preparation, sectioning, staining, and electron microscopy were performed as described (Hirsch et al. 1983).
Flow cytometry
Samples of free-living bacteria and nodule extracts were fixed by mixing with ethanol to a final concentration of 85%. Following overnight incubation at 4 °C, cells were harvested by centrifugation at 4,000 g for 4 min and resuspended in 1 volume 50 mM sodium citrate buffer containing 3.3 mg/ml RNase. Samples were incubated overnight at 50 °C and passed through a cell strainer cap (BD Falcon) to remove large particles. The samples were then stained with the nucleic acid binding dye Sytox Green (Molecular Probes) at a final concentration of 0.83 µM and analyzed on a Becton Dickinson FACScan flow cytometer. Data were analyzed with FlowJo software (Tree Star, Inc.).
Supplementary Material
ACKNOWLEDGMENTS
We are indebted to Nicki Watson and the W. M. Keck Foundation Biological Imaging Facility for preparing nodule sections and performing electron microscopy; Mary Lou Pardue, Melanie Barker Berkmen, and Alan Grossman for assistance with microscopy; and Glenn Paradis, Michele Griffin, Andy Fan, Celeste Peterson, and the MIT Flow Cytometry Core Facility for assistance with flow cytometry. We thank Ann Hirsch for her insights about the electron micrographs, Kathryn Jones for critical reading of the manuscript, and members of the Walker laboratory for helpful discussions. This work was funded by NIH grant GM31010 to G.C.W. and NIH grant K99 GM083343 and a postdoctoral fellowship from the Jane Coffin Childs Memorial Fund for Medical Research to M.E.T. G.C.W. is an American Cancer Society Research Professor.
LITERATURE CITED
- Abbas BA, Vineetha KE, Prasad CK, Vij N, Hassani R, Randhawa GS. Symbiotic characteristics of cysteine and methionine auxotrophs of Sinorhizobium meliloti. Indian J Exp Biol. 2002;40:1121–1130. [PubMed] [Google Scholar]
- Ampe F, Kiss E, Sabourdy F, Batut J. Transcriptome analysis of Sinorhizobium meliloti during symbiosis. Genome Biol. 2003;4:R15. doi: 10.1186/gb-2003-4-2-r15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Chowdhury S. Methylmalonyl-CoA mutase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc; 1999. pp. 707–729. [Google Scholar]
- Barra L, Fontenelle C, Ermel G, Trautwetter A, Walker GC, Blanco C. Interrelations between glycine betaine catabolism and methionine biosynthesis in Sinorhizobium meliloti strain 102F34. J Bacteriol. 2006;188:7195–7204. doi: 10.1128/JB.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistoni F, Platero R, Noya F, Fabiano E. Intracellular Fe content influences nodulation competitiveness of Sinohizobium meliloti strains as inocula of alfalfa. Soil Biol. Biochem. 2002;34:593–597. [Google Scholar]
- Baudouin E, Pieuchot L, Engler G, Pauly N, Puppo A. Nitric oxide is formed in Medicago truncatula-Sinorhizobium meliloti functional nodules. Mol Plant Microbe Interact. 2006;19:970–975. doi: 10.1094/MPMI-19-0970. [DOI] [PubMed] [Google Scholar]
- Buckel W, Kratky C, Golding BT. Stabilisation of methylene radicals by cob(II)alamin in coenzyme B12 dependent mutases. Chemistry. 2005;12:352–362. doi: 10.1002/chem.200501074. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Reuhs BL, Walker GC. Chronic intracellular infection of alfalfa nodules by Sinorhizobium meliloti requires correct lipopolysaccharide core. Proc Natl Acad Sci U S A. 2002;99:3938–3943. doi: 10.1073/pnas.062425699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell GRO, et al. Sinorhizobium meliloti bluB is necessary for production of 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc. Natl. Acad. Sci. USA. 2006;103:4634–4639. doi: 10.1073/pnas.0509384103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J, Fuchs JA, Messing J. Primary structure of the Escherichia coli ribonucleoside diphosphate reductase operon. Proc Natl Acad Sci U S A. 1984;81:4294–4297. doi: 10.1073/pnas.81.14.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles TC, Aneja P. Methylmalonyl-CoA mutase encoding gene of Sinorhizobium meliloti. Gene. 1999;226:121–127. doi: 10.1016/s0378-1119(98)00555-1. [DOI] [PubMed] [Google Scholar]
- Cheng J, Sibley CD, Zaheer R, Finan TM. A Sinorhizobium meliloti minE mutant has an altered morphology and exhibits defects in legume symbiosis. Microbiology. 2007;153:375–387. doi: 10.1099/mic.0.2006/001362-0. [DOI] [PubMed] [Google Scholar]
- Chu J, et al. Cloning and expression of the metE gene in Escherichia coli. Arch Biochem Biophys. 1985;239:467–474. doi: 10.1016/0003-9861(85)90713-1. [DOI] [PubMed] [Google Scholar]
- Cowles JR, Evans HJ. Some properties of the ribonucleotide reductase from Rhizobium meliloti. Arch Biochem Biophys. 1968;127:770–778. doi: 10.1016/0003-9861(68)90288-9. [DOI] [PubMed] [Google Scholar]
- Davies BW, Kohanski MA, Simmons LA, Winkler JA, Collins JJ, Walker GC. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell. 2009;36:845–860. doi: 10.1016/j.molcel.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BW, Walker GC. Disruption of sitA compromises Sinorhizobium meliloti for manganese uptake required for protection against oxidative stress. J Bacteriol. 2007;189:2101–2109. doi: 10.1128/JB.01377-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes SS, et al. Ribonucleotide reduction in Mycobacterium tuberculosis: function and expression of genes encoding class Ib and class II ribonucleotide reductases. Infect Immun. 2003;71:6124–6131. doi: 10.1128/IAI.71.11.6124-6131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day WA, Jr, Sajecki JL, Pitts TM, Joens LA. Role of catalase in Campylobacter jejuni intracellular survival. Infect Immun. 2000;68:6337–6345. doi: 10.1128/iai.68.11.6337-6345.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote MA, et al. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci U S A. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehal PS, et al. MicrobesOnline: an integrated portal for comparative and functional genomics. Nucleic Acids Res. 2010;38:D396–D400. doi: 10.1093/nar/gkp919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MA. Sinorhizobium meliloti metabolism in the root nodule: a proteomic perspective. Proteomics. 2004;4:1859–1872. doi: 10.1002/pmic.200300802. [DOI] [PubMed] [Google Scholar]
- Elford HL. Effect of hydroxyurea on ribonucleotide reductase. Biochem Biophys Res Commun. 1968;33:129–135. doi: 10.1016/0006-291x(68)90266-0. [DOI] [PubMed] [Google Scholar]
- Evans HJ, Kliewer M. Vitamin B12 Compounds In Relation To The Requirement Of Cobalt For Higher Plants And Nitrogen-Fixing Organisms. Ann N Y Acad Sci. 1964;112:735–755. doi: 10.1111/j.1749-6632.1964.tb45052.x. [DOI] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. Epub 2006 Oct 2028. [DOI] [PubMed] [Google Scholar]
- Finan TM, McWhinnie E, Driscoll B, Watson RJ. Complex Symbiotic Phenotypes Result from Gluconeogenic Mutations in Rhizobium meliloti. Mol Plant Microbe Interact. 1991;4:386–392. [Google Scholar]
- Fontecave M. Ribonucleotide reductases and radical reactions. Cell Mol Life Sci. 1998;54:684–695. doi: 10.1007/s000180050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontecave M, Gerez C. Tyrosyl radicals and ribonucleotide reductase. Methods Enzymol. 2002;348:21–30. doi: 10.1016/s0076-6879(02)48621-1. [DOI] [PubMed] [Google Scholar]
- Gage DJ. Analysis of infection thread development using Gfp- and DsRed-expressing Sinorhizobium meliloti. J Bacteriol. 2002;184:7042–7046. doi: 10.1128/JB.184.24.7042-7046.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage DJ, Margolin W. Hanging by a thread: invasion of legume plants by rhizobia. Curr Opin Microbiol. 2000;3:613–617. doi: 10.1016/s1369-5274(00)00149-1. [DOI] [PubMed] [Google Scholar]
- Garriga X, et al. nrdD and nrdG genes are essential for strict anaerobic growth of Escherichia coli. Biochem Biophys Res Commun. 1996;229:189–192. doi: 10.1006/bbrc.1996.1778. [DOI] [PubMed] [Google Scholar]
- Gibson KE, Kobayashi H, Walker GC. Molecular determinants of a symbiotic chronic infection. Annu Rev Genet. 2008;42:413–441. doi: 10.1146/annurev.genet.42.110807.091427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J, Ichige A, Walker GC. A Rhizobium meliloti homolog of the Escherichia coli peptide-antibiotic transport protein SbmA is essential for bacteroid development. Genes Dev. 1993;7:1485–1497. doi: 10.1101/gad.7.8.1485. [DOI] [PubMed] [Google Scholar]
- Gonzalez JC, Banerjee RV, Huang S, Sumner JS, Matthews RG. Comparison of cobalamin-independent and cobalamin-dependent methionine synthases from Escherichia coli: two solutions to the same chemical problem. Biochemistry. 1992;31:6045–6056. doi: 10.1021/bi00141a013. [DOI] [PubMed] [Google Scholar]
- Hirsch AM, Bang M, Ausubel FM. Ultrastructural analysis of ineffective alfalfa nodules formed by nif∷Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983;155:367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogbom M, Stenmark P, Voevodskaya N, McClarty G, Graslund A, Nordlund P. The radical site in chlamydial ribonucleotide reductase defines a new R2 subclass. Science. 2004;305:245–248. doi: 10.1126/science.1098419. [DOI] [PubMed] [Google Scholar]
- Hondorp ER, Matthews RG. Oxidative stress inactivates cobalamin-independent methionine synthase (MetE) in Escherichia coli. PLoS Biol. 2004;2:e336. doi: 10.1371/journal.pbio.0020336. Epub 2004 Oct 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet A, Mandon K, Puppo A, Herouart D. H2O2 is required for optimal establishment of the Medicago sativa/Sinorhizobium meliloti symbiosis. J Bacteriol. 2007;189:8741–8745. doi: 10.1128/JB.01130-07. Epub 2007 Oct 8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamet A, Sigaud S, Van de Sype G, Puppo A, Herouart D. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol Plant Microbe Interact. 2003;16:217–225. doi: 10.1094/MPMI.2003.16.3.217. [DOI] [PubMed] [Google Scholar]
- Jiang W, Xie J, Norgaard H, Bollinger JM, Jr, Krebs C. Rapid and quantitative activation of Chlamydia trachomatis ribonucleotide reductase by hydrogen peroxide. Biochemistry. 2008;47:4477–4483. doi: 10.1021/bi702085z. Epub 2008 Mar 4422. [DOI] [PubMed] [Google Scholar]
- Jones KM, Kobayashi H, Davies BW, Taga ME, Walker GC. How rhizobial symbionts invade plants: the Sinorhizobium-Medicago model. Nat Rev Microbiol. 2007;5:619–633. doi: 10.1038/nrmicro1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KM, Sharopova N, Lohar DP, Zhang JQ, VandenBosch KA, Walker GC. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc Natl Acad Sci U S A. 2008;105:704–709. doi: 10.1073/pnas.0709338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating DH, Willits MG, Long SR. A Sinorhizobium meliloti lipopolysaccharide mutant altered in cell surface sulfation. J Bacteriol. 2002;184:6681–6689. doi: 10.1128/JB.184.23.6681-6689.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Simmons LA, Yuan DS, Broughton WJ, Walker GC. Multiple Ku orthologues mediate DNA non-homologous end-joining in the free-living form and during chronic infection of Sinorhizobium meliloti. Mol Microbiol. 2008;67:350–363. doi: 10.1111/j.1365-2958.2007.06036.x. Epub 2007 Dec 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sunako M, Hayashi M, Murooka Y. DNA synthesis and fragmentation in bacteroids during Astragalus sinicus root nodule development. Biosci Biotechnol Biochem. 2001;65:510–515. doi: 10.1271/bbb.65.510. [DOI] [PubMed] [Google Scholar]
- Kolberg M, Strand KR, Graff P, Andersson KK. Structure, function, and mechanism of ribonucleotide reductases. Biochim Biophys Acta. 2004;1699:1–34. doi: 10.1016/j.bbapap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Latch JN, Margolin W. Generation of buds, swellings, and branches instead of filaments after blocking the cell cycle of Rhizobium meliloti. J Bacteriol. 1997;179:2373–2381. doi: 10.1128/jb.179.7.2373-2381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh JA, Signer ER, Walker GC. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc Natl Acad Sci U S A. 1985;82:6231–6235. doi: 10.1073/pnas.82.18.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon KP, Grossman AD. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science. 1998;282:1516–1519. doi: 10.1126/science.282.5393.1516. [DOI] [PubMed] [Google Scholar]
- Lindgren H, Shen H, Zingmark C, Golovliov I, Conlan W, Sjostedt A. Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect Immun. 2007;75:1303–1309. doi: 10.1128/IAI.01717-06. Epub 2007 Jan 1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodwig EM, Poole PS. Metabolism of Rhizobium bacteroids. Critical Reviews in Plant Sciences. 2003;22:37–78. [Google Scholar]
- Lundin D, Torrents E, Poole AM, Sjoberg BM. RNRdb, a curated database of the universal enzyme family ribonucleotide reductase, reveals a high level of misannotation in sequences deposited to Genbank. BMC Genomics. 2009;10:589. doi: 10.1186/1471-2164-10-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Publications; 1982. [Google Scholar]
- Matthews RG. Cobalamin-dependent methionine synthase. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York: John Wiley & Sons, Inc; 1999. pp. 681–706. [Google Scholar]
- Maunoury N, et al. Differentiation of symbiotic cells and endosymbionts in Medicago truncatula nodulation are coupled to two transcriptome-switches. PLoS One. 2010;5:e9519. doi: 10.1371/journal.pone.0009519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JP, McDonald BR, Moran NA. Convergent evolution of metabolic roles in bacterial co-symbionts of insects. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0906424106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade HM, Long SR, Ruvkun GB, Brown SE, Ausubel FM. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982;149:114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert P, et al. Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci U S A. 2006;103:5230–5235. doi: 10.1073/pnas.0600912103. Epub 2006 Mar 5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RW, McRae DG, Al-Jobore A, Berndt WB. Respiration supported nitrogenase activity of isolated Rhizobium meliloti bacteroids. J Cell Biochem. 1988;38:35–49. doi: 10.1002/jcb.240380105. [DOI] [PubMed] [Google Scholar]
- Nogales J, Munoz S, Olivares J, Sanjuan J. Sinorhizobium meliloti genes involved in tolerance to the antimicrobial peptide protamine. FEMS Microbiol Lett. 2006;264:160–167. doi: 10.1111/j.1574-6968.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Oke V, Long SR. Bacterial genes induced within the nodule during the Rhizobium-legume symbiosis. Mol Microbiol. 1999a;32:837–849. doi: 10.1046/j.1365-2958.1999.01402.x. [DOI] [PubMed] [Google Scholar]
- Oke V, Long SR. Bacteroid formation in the Rhizobium-legume symbiosis. Curr Opin Microbiol. 1999b;2:641–646. doi: 10.1016/s1369-5274(99)00035-1. [DOI] [PubMed] [Google Scholar]
- Paau AS, Cowles JR. Development of Bacteroids in Alfalfa (Medicago sativa) Nodules. Plant Physiol. 1978a;62:526–530. doi: 10.1104/pp.62.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau AS, Cowles JR. Studies on bacteroid size and nucleic acid content of alfalfa bacteroids fractionated by velocity sedimentation. Can J Microbiol. 1978b;24:1283–1287. doi: 10.1139/m78-207. [DOI] [PubMed] [Google Scholar]
- Paau AS, Oro J, Cowles JR. DNA Content of Free Living Rhizobia and Bacteroids of Various Rhizobium-Legume Associations. Plant Physiol. 1979;63:402–405. doi: 10.1104/pp.63.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzolo-Ballance AM, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180:500–509. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- Peleg-Grossman S, Volpin H, Levine A. Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide-regulated endocytosis and reactive oxygen species. J Exp Bot. 2007;58:1637–1649. doi: 10.1093/jxb/erm013. [DOI] [PubMed] [Google Scholar]
- Raux E, Schubert HL, Warren MJ. Biosynthesis of cobalamin (vitamin B12): a bacterial conundrum. Cell Mol Life Sci. 2000;57:1880–1893. doi: 10.1007/PL00000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodionov DA, Vitreschak AG, Mironov AA, Gelfand MS. Comparative genomics of the vitamin B12 metabolism and regulation in prokaryotes. J Biol Chem. 2003;278:41148–41159. doi: 10.1074/jbc.M305837200. [DOI] [PubMed] [Google Scholar]
- Roop RM, 2nd, Gaines JM, Anderson ES, Caswell CC, Martin DW. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med Microbiol Immunol. 2009;198:221–238. doi: 10.1007/s00430-009-0123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado M, Gage DJ. Transcriptional control of a rRNA promoter of the nodulating symbiont Sinorhizobium meliloti. FEMS Microbiol Lett. 2003;226:15–22. doi: 10.1016/S0378-1097(03)00603-7. [DOI] [PubMed] [Google Scholar]
- Roth JR, Lawrence JG, Bobik TA. Cobalamin (coenzyme B12): synthesis and biological significance. Annu Rev Microbiol. 1996;50:137–181. doi: 10.1146/annurev.micro.50.1.137. [DOI] [PubMed] [Google Scholar]
- Rubio MC, et al. Localization of superoxide dismutases and hydrogen peroxide in legume root nodules. Mol Plant Microbe Interact. 2004;17:1294–1305. doi: 10.1094/MPMI.2004.17.12.1294. [DOI] [PubMed] [Google Scholar]
- Ryzhkova EP. Multiple functions of corrinoids in prokaryote biology. Appl Biochem Microbiol. 2003;39:133–159. [PubMed] [Google Scholar]
- Santos R, Herouart D, Puppo A, Touati D. Critical protective role of bacterial superoxide dismutase in rhizobium-legume symbiosis. Mol Microbiol. 2000;38:750–759. doi: 10.1046/j.1365-2958.2000.02178.x. [DOI] [PubMed] [Google Scholar]
- Sato K, Inukai S, Shimizu S. Vitamin B12-dependent methionine synthesis in Rhizobium meliloti. Biochem Biophys Res Commun. 1974;60:723–728. doi: 10.1016/0006-291x(74)90300-3. [DOI] [PubMed] [Google Scholar]
- Schafer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, Walker GC. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature. 2007;446:449–453. doi: 10.1038/nature05611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH, Valbuena GA, Olano JP. Pathogenic mechanisms of diseases caused by Rickettsia. Ann N Y Acad Sci. 2003;990:1–11. doi: 10.1111/j.1749-6632.2003.tb07331.x. [DOI] [PubMed] [Google Scholar]
- Wang D, et al. A nodule-specific protein secretory pathway required for nitrogen-fixing symbiosis. Science. 2010;327:1126–1129. doi: 10.1126/science.1184096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF, Skot L, Revsbech NP. Direct evidence for changes in the resistance of legume root nodules to O2 diffusion. J. Exp. Botany. 1987;38:1129–1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.