Abstract
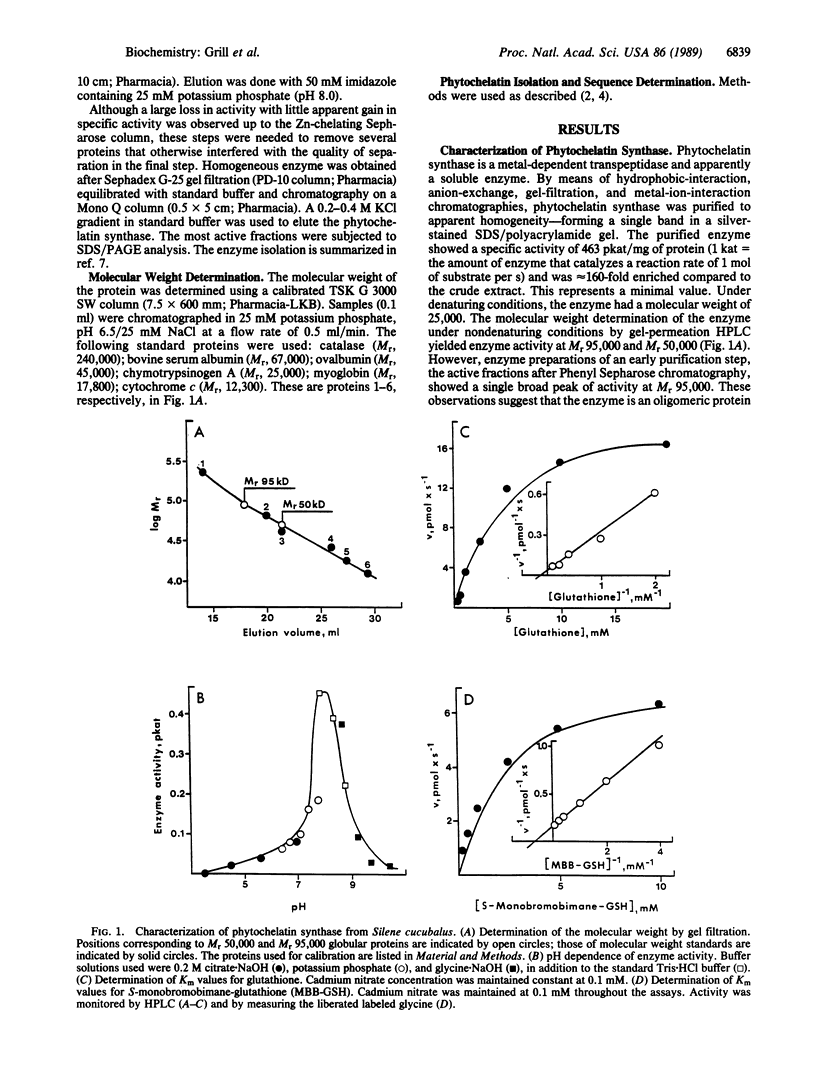
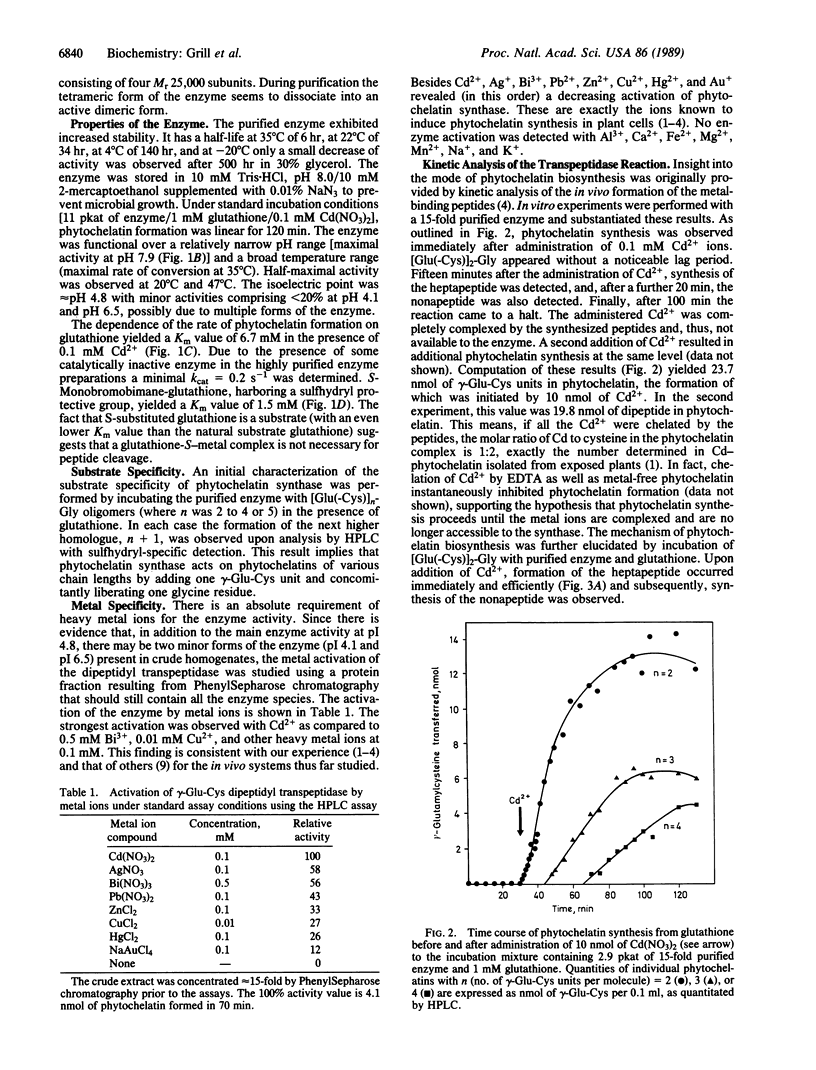
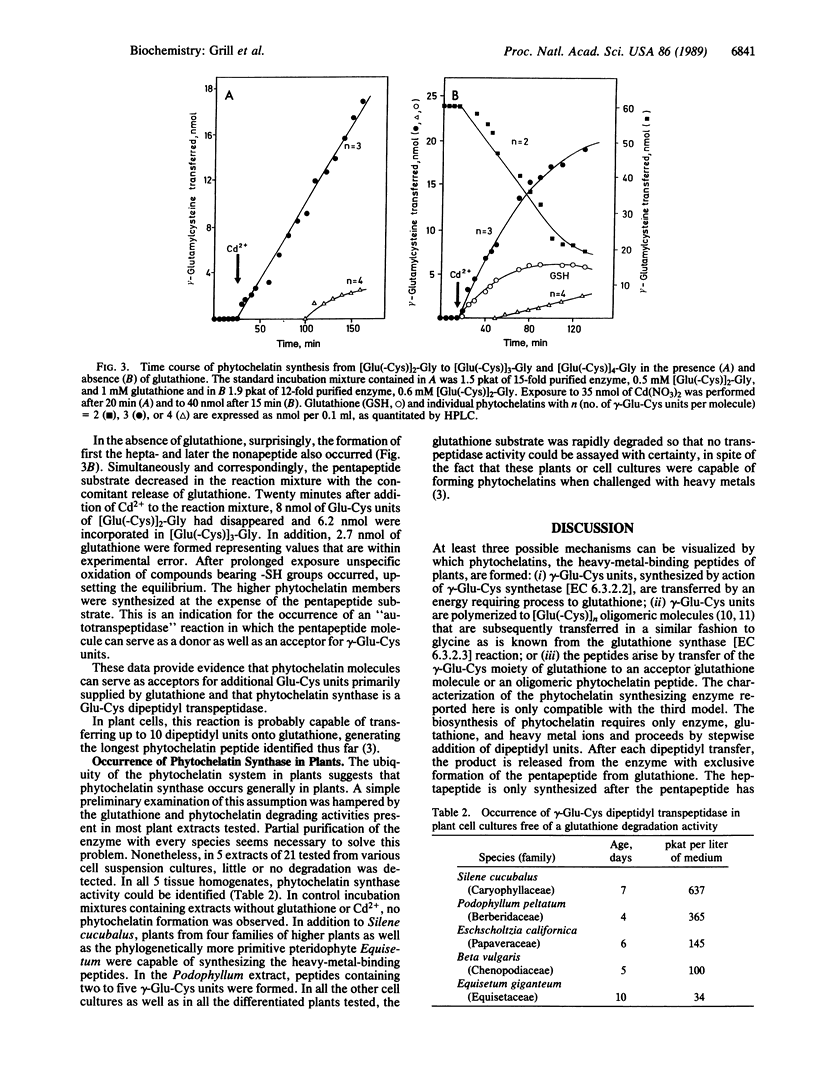
An enzyme has been discovered and characterized from Silene cucubalus cell suspension cultures that catalyzes the transfer of the γ-glutamylcysteine dipeptide moiety of glutathione to an acceptor glutathione molecule or a growing chain of [Glu(-Cys)]n-Gly oligomers, thus synthesizing phytochelatins, the metal-binding peptides of higher plants and select fungi. The enzyme was named γ-glutamylcysteine dipeptidyl transpeptidase and given the trivial name phytochelatin synthase. The primary reaction catalyzed is [Glu(-Cys)]-Gly + [Glu(-Cys)]n-Gly → [Glu(-Cys)]n+1-Gly + Gly. The enzyme is isoelectric near pH 4.8 and has temperature and pH optima at 35°C and 7.9, respectively. Phytochelatin synthase is constitutively present in cell cultures of various plant species and its formation is not noticeably induced by heavy metal ions in the growth medium. The enzyme (Mr95,000) seems to be composed of four subunits, the dimer (Mr50,000) being also catalytically active. Cd2+ is by far the best metal activator of the enzyme followed by Ag+, Bi3+, Pb2+, Zn2+, Cu2+, Hg2+, and Au+. The Km for glutathione is 6.7 mM. The enzyme activity seems to be self-regulated in that the product of the reaction (the phytochelatins) chelates the enzyme-activating metal, thus terminating the enzyme reaction. The molar ratio of the γ-glutamylcysteine dipeptide in phytochelatin to Cd2+ in the newly formed complex was 2:1.
Keywords: detoxification, dipeptidyl transpeptidase, phytochelatin biosynthesis
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fahey R. C., Newton G. L., Dorian R., Kosower E. M. Analysis of biological thiols: quantitative determination of thiols at the picomole level based upon derivatization with monobromobimanes and separation by cation-exchange chromatography. Anal Biochem. 1981 Mar 1;111(2):357–365. doi: 10.1016/0003-2697(81)90573-x. [DOI] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci U S A. 1987 Jan;84(2):439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill E., Winnacker E. L., Zenk M. H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985 Nov 8;230(4726):674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Jackson P. J., Unkefer C. J., Doolen J. A., Watt K., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine: its role in cadmium resistance in plant cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6619–6623. doi: 10.1073/pnas.84.19.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Tarbet E. B., Gray W. R., Winge D. R. Metal-specific synthesis of two metallothioneins and gamma-glutamyl peptides in Candida glabrata. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8815–8819. doi: 10.1073/pnas.85.23.8815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh N., Hayashi Y. Isolation of mutants of Schizosaccharomyces pombe unable to synthesize cadystin, small cadmium-binding peptides. Biochem Biophys Res Commun. 1988 Feb 29;151(1):32–39. doi: 10.1016/0006-291x(88)90555-4. [DOI] [PubMed] [Google Scholar]
- NILSSON K. K., FRUTON J. S. POLYMERIZATION REACTIONS CATALYZED BY INTRACELLULAR PROTEINASES. IV. FACTORS INFLUENCING THE POLYMERIZATION OF DIPEPTIDE AMIDES BY CATHEPSIN C. Biochemistry. 1964 Sep;3:1220–1224. doi: 10.1021/bi00897a006. [DOI] [PubMed] [Google Scholar]
- Reese R. N., Mehra R. K., Tarbet E. B., Winge D. R. Studies on the gamma-glutamyl Cu-binding peptide from Schizosaccharomyces pombe. J Biol Chem. 1988 Mar 25;263(9):4186–4192. [PubMed] [Google Scholar]
- Reese R. N., Wagner G. J. Properties of tobacco (Nicotiana tabacum) cadmium-binding peptide(s). Unique non-metallothionein cadmium ligands. Biochem J. 1987 Feb 1;241(3):641–647. doi: 10.1042/bj2410641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller H. V., Huang B., Hatch E., Goldsbrough P. B. Phytochelatin synthesis and glutathione levels in response to heavy metals in tomato cells. Plant Physiol. 1987 Dec;85(4):1031–1035. doi: 10.1104/pp.85.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]


