Abstract
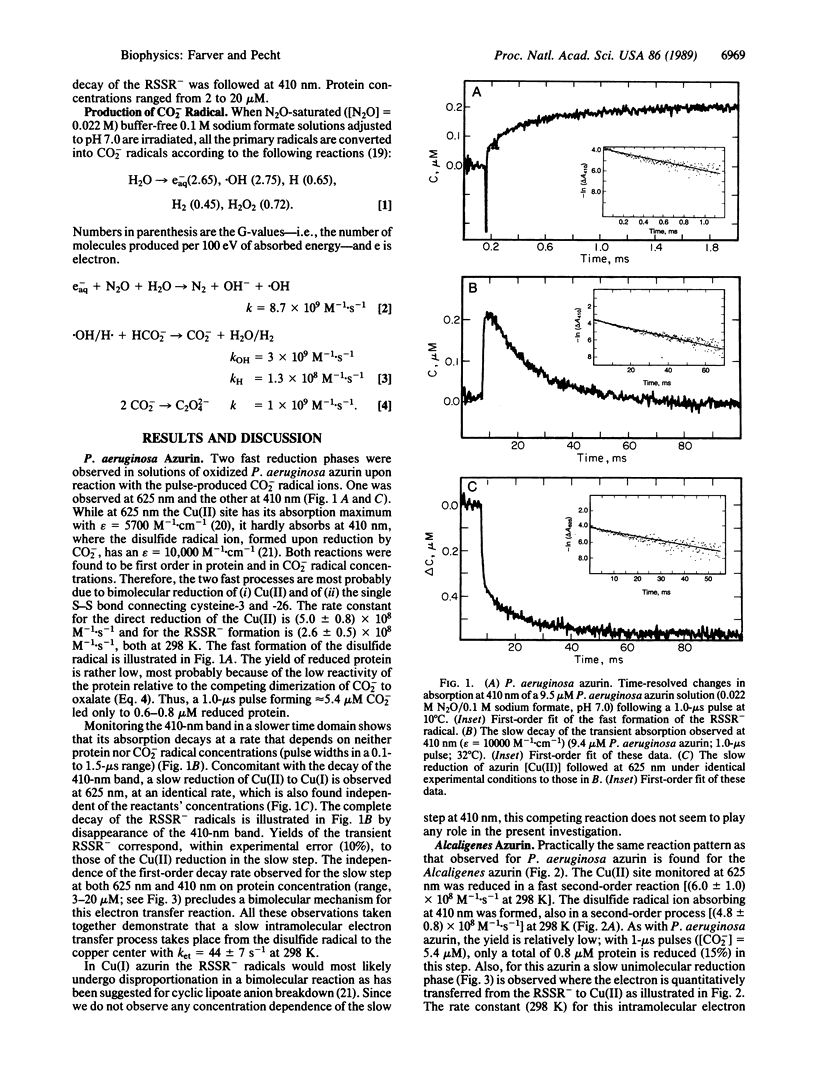
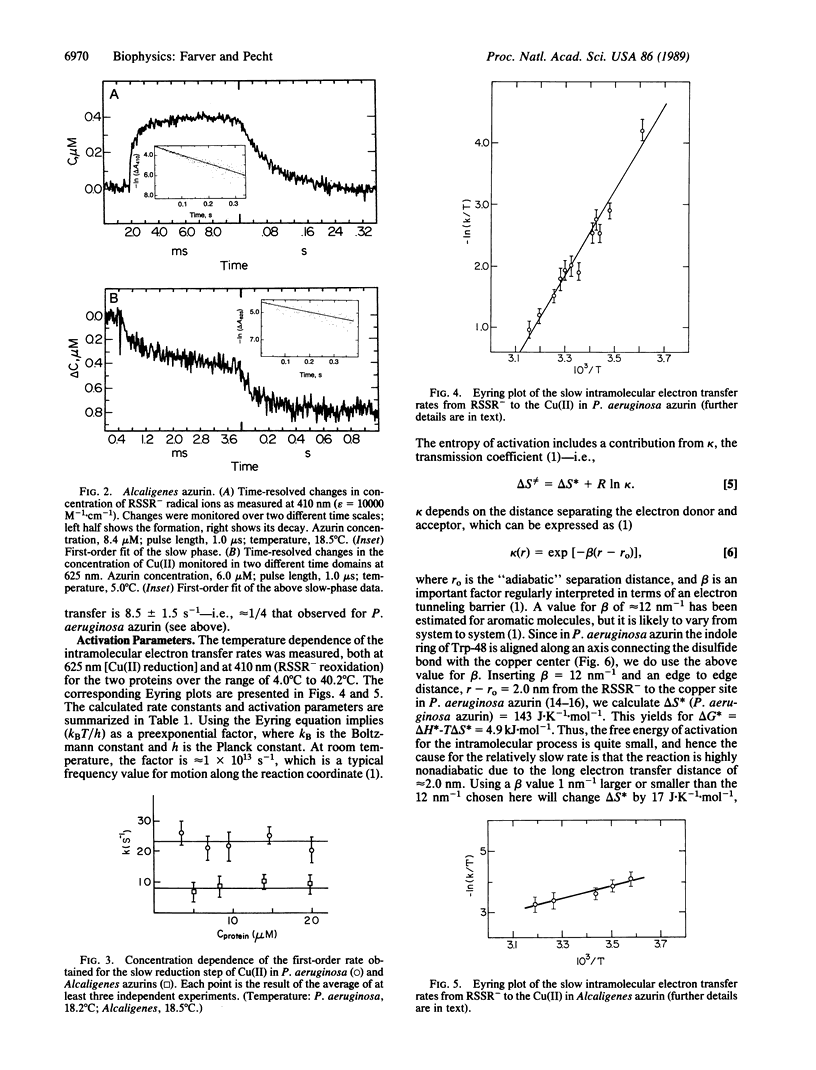
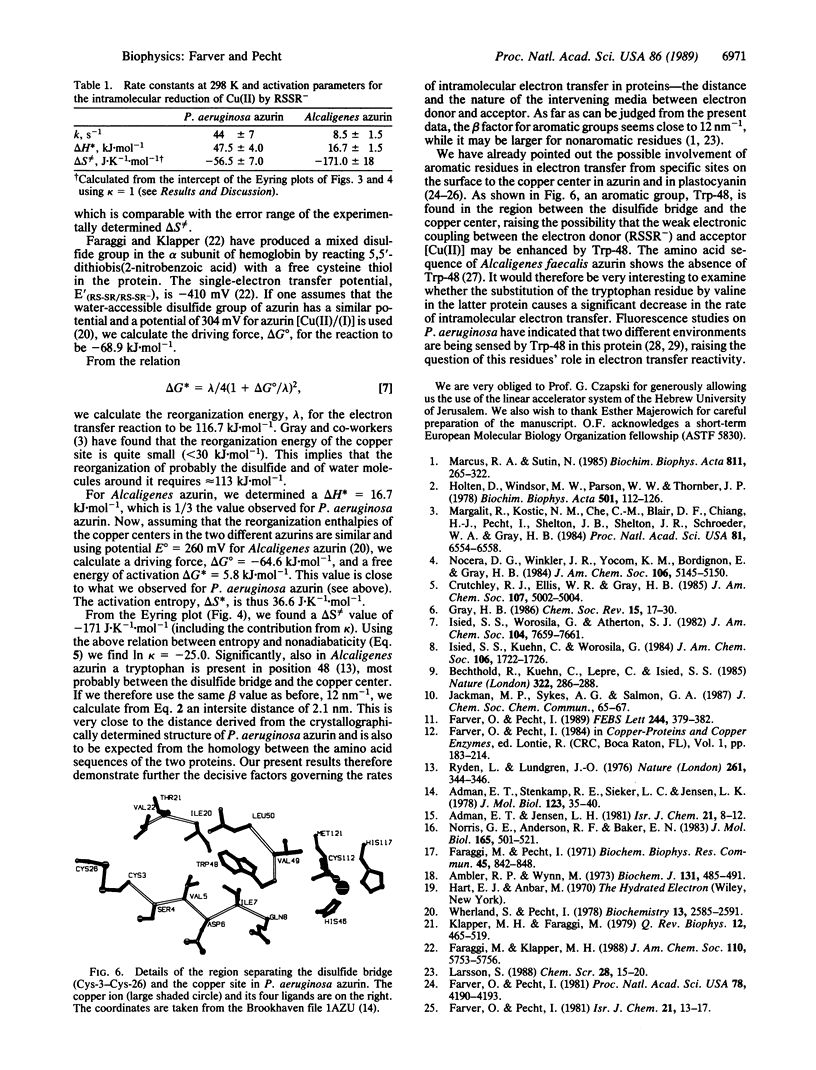
The Cu(II) sites of azurins, the blue single copper proteins, isolated from Pseudomonas aeruginosa and Alcaligenes spp. (Iwasaki) are reduced by CO2- radicals, produced by pulse radiolysis, in two distinct reaction steps: (i) a fast bimolecular phase, at the rates (5.0 +/- 0.8) x 10(8) M-1.s-1 (P. aeruginosa) and (6.0 +/- 1.0) x 10(8) M-1.s-1 (Alcaligenes); (ii) a slow unimolecular phase with specific rates of 44 +/- 7 s-1 in the former and 8.5 +/- 1.5 s-1 for the latter (all at 298 K, 0.1 M ionic strength). Concomitant with the fast reduction of Cu(II), the single disulfide bridge linking cysteine-3 to -26 in these proteins is reduced to the RSSR- radical ion as evidenced by its characteristic absorption band centered at 410 nm. This radical ion decays in a unimolecular process with a rate identical to that of the slow Cu(II) reduction phase in the respective protein, thus clearly suggesting that a long-range intramolecular electron transfer occurs between the RSSR- radicals and the Cu(II) site. The temperature dependence of the internal electron transfer process in both proteins was measured over the 4 degrees C to 42 degrees C range. The activation parameters derived are delta H* = 47.5 +/- 4.0 and 16.7 +/- 1.5 kJ.mol-1; and delta S not equal to = -56.5 +/- 7.0 and -171 +/- 18 J.K-1.mol-1, respectively. Using the Marcus theory, we found that the intramolecular electron transfer rates and their activation parameters observed for the two azurins correlate well with the distances between the reactive sites, their redox potential, and the nature of the separating medium. Thus, azurins with distinct structural and reactivity characteristics isolated from different bacteria or modified by site-directed mutagenesis can be used in comparing long-range electron transfer process between their conserved disulfide bridge and the Cu(II) sites.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Stenkamp R. E., Sieker L. C., Jensen L. H. A crystallographic model for azurin a 3 A resolution. J Mol Biol. 1978 Jul 25;123(1):35–47. doi: 10.1016/0022-2836(78)90375-3. [DOI] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold R., Kuehn C., Lepre C., Isied S. S. Directional electron transfer in ruthenium-modified horse heart cytochrome c. Nature. 1986 Jul 17;322(6076):286–288. doi: 10.1038/322286a0. [DOI] [PubMed] [Google Scholar]
- Faraggi M., Pecht I. The reaction of Pseudomonas azurin with hydrated electrons. Biochem Biophys Res Commun. 1971 Nov;45(4):842–848. doi: 10.1016/0006-291x(71)90415-3. [DOI] [PubMed] [Google Scholar]
- Farver O., Pecht I. Identification of an electron transfer locus in plastocyanin by chromium(II) affinity labeling. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4190–4193. doi: 10.1073/pnas.78.7.4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinvald A., Schlessinger J., Pecht I., Steinberg I. Z. Homogeneity and variability in the structure of azurin molecules studied by fluorescence decay and circular polarization. Biochemistry. 1975 May 6;14(9):1921–1929. doi: 10.1021/bi00680a018. [DOI] [PubMed] [Google Scholar]
- Holten D., Windsor M. W., Parson W. W., Thornber J. P. Primary photochemical processes in isolated reaction centers of Rhodopseudomonas viridis. Biochim Biophys Acta. 1978 Jan 11;501(1):112–126. doi: 10.1016/0005-2728(78)90100-7. [DOI] [PubMed] [Google Scholar]
- Klapper M. H., Faraggi M. Applications of pulse radiolysis to protein chemistry. Q Rev Biophys. 1979 Nov;12(4):465–519. doi: 10.1017/s0033583500002791. [DOI] [PubMed] [Google Scholar]
- Margalit R., Kostić N. M., Che C. M., Blair D. F., Chiang H. J., Pecht I., Shelton J. B., Shelton J. R., Schroeder W. A., Gray H. B. Preparation and characterization of pentaammineruthenium-(histidine-83)azurin: thermodynamics of intramolecular electron transfer from ruthenium to copper. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6554–6558. doi: 10.1073/pnas.81.20.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris G. E., Anderson B. F., Baker E. N. Structure of azurin from Alcaligenes denitrificans at 2.5 A resolution. J Mol Biol. 1983 Apr 15;165(3):501–521. doi: 10.1016/s0022-2836(83)80216-2. [DOI] [PubMed] [Google Scholar]
- Ryden L., Lundgren J. Homology relationships among the small blue proteins. Nature. 1976 May 27;261(5558):344–346. doi: 10.1038/261344a0. [DOI] [PubMed] [Google Scholar]
- Szabo A. G., Stepanik T. M., Wayner D. M., Young N. M. Conformational heterogeneity of the copper binding site in azurin. A time-resolved fluorescence study. Biophys J. 1983 Mar;41(3):233–244. doi: 10.1016/S0006-3495(83)84433-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherland S., Pecht I. Protein-protein electron transfer. A Marcus theory analysis of reactions between c type cytochromes and blue copper proteins. Biochemistry. 1978 Jun 27;17(13):2585–2591. doi: 10.1021/bi00606a020. [DOI] [PubMed] [Google Scholar]


