Abstract
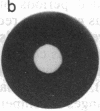
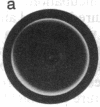
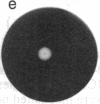
We studied the migration through semisolid agar of chemotactic and nonchemotactic cells of Escherichia coli. While swarms of nonchemotactic cells were generally smaller than those of chemotactic cells, they varied markedly in size and in structure. Cells that failed to tumble or that tumbled incessantly formed the smallest swarms. Cells that tumbled at intermediate frequencies formed much larger swarms, even when deleted for many of the genes known to be required for chemotaxis. Surprisingly, the higher the tumble frequency, the larger the swarms. Microscopic examination revealed that tumbles enable cells to back away from obstructions in the agar. Thus, not all cells that swarm effectively need be chemotactic.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemoreceptors in bacteria. Science. 1969 Dec 26;166(3913):1588–1597. doi: 10.1126/science.166.3913.1588. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Complementation of nonchemotactic mutants of Escherichia coli. Genetics. 1969 Jan;61(1):61–66. doi: 10.1093/genetics/61.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J., Dahl M. M. Nonchemotactic mutants of Escherichia coli. J Bacteriol. 1967 Jan;93(1):390–398. doi: 10.1128/jb.93.1.390-398.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Genetics of motility in Escherichia coli: complementation of paralysed mutants. Genetics. 1967 Jul;56(3):363–373. doi: 10.1093/genetics/56.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. B., Adler J. Location of genes for motility and chemotaxis on the Escherichia coli genetic map. J Bacteriol. 1969 Jan;97(1):156–161. doi: 10.1128/jb.97.1.156-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. A physicist looks at bacterial chemotaxis. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):1–9. doi: 10.1101/sqb.1988.053.01.003. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Turner L. Movement of microorganisms in viscous environments. Nature. 1979 Mar 22;278(5702):349–351. doi: 10.1038/278349a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Segall J. E., Berg H. C. Impulse responses in bacterial chemotaxis. Cell. 1982 Nov;31(1):215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- Borkovich K. A., Kaplan N., Hess J. F., Simon M. I. Transmembrane signal transduction in bacterial chemotaxis involves ligand-dependent activation of phosphate group transfer. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1208–1212. doi: 10.1073/pnas.86.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENOMOTO M., IINO T. COLONIAL DIMORPHISM IN NONMOTILE SALMONELLA. J Bacteriol. 1963 Sep;86:473–477. doi: 10.1128/jb.86.3.473-477.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto M. Genetic studies of paralyzed mutant in Salmonella. I. Genetic fine structure of the mot loci in Salmonella typhimurium. Genetics. 1966 Sep;54(3):715–726. doi: 10.1093/genetics/54.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedblom M. L., Adler J. Chemotactic response of Escherichia coli to chemically synthesized amino acids. J Bacteriol. 1983 Sep;155(3):1463–1466. doi: 10.1128/jb.155.3.1463-1466.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M., Stocker B. A. Complementation of non-flagellate Salmonella mutants. J Gen Microbiol. 1965 Oct;41(1):47–55. doi: 10.1099/00221287-41-1-47. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M., DeFranco A. L., Koshland D. E., Jr Inversion of a behavioral response in bacterial chemotaxis: explanation at the molecular level. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4150–4154. doi: 10.1073/pnas.75.9.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Koshland D. E., Jr Roles of cheY and cheZ gene products in controlling flagellar rotation in bacterial chemotaxis of Escherichia coli. J Bacteriol. 1987 Mar;169(3):1307–1314. doi: 10.1128/jb.169.3.1307-1314.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen S. H., Reader R. W., Kort E. N., Tso W. W., Adler J. Change in direction of flagellar rotation is the basis of the chemotactic response in Escherichia coli. Nature. 1974 May 3;249(452):74–77. doi: 10.1038/249074a0. [DOI] [PubMed] [Google Scholar]
- Lederberg J. Linear Inheritance in Transductional Clones. Genetics. 1956 Nov;41(6):845–871. doi: 10.1093/genetics/41.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Demethylation of methyl-accepting chemotaxis proteins in Escherichia coli induced by the repellents glycerol and ethylene glycol. J Bacteriol. 1984 Feb;157(2):576–581. doi: 10.1128/jb.157.2.576-581.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosawa K., Imae Y. Glycerol and ethylene glycol: members of a new class of repellents of Escherichia coli chemotaxis. J Bacteriol. 1983 Apr;154(1):104–112. doi: 10.1128/jb.154.1.104-112.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Complementation analysis and deletion mapping of Escherichia coli mutants defective in chemotaxis. J Bacteriol. 1978 Jul;135(1):45–53. doi: 10.1128/jb.135.1.45-53.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Houts S. E. Isolation and behavior of Escherichia coli deletion mutants lacking chemotaxis functions. J Bacteriol. 1982 Jul;151(1):106–113. doi: 10.1128/jb.151.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R. Interaction of the cheC and cheZ gene products is required for chemotactic behavior in Escherichia coli. Proc Natl Acad Sci U S A. 1979 May;76(5):2390–2394. doi: 10.1073/pnas.76.5.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Parker S. R., Talbert P. B., Houts S. E. Interactions between chemotaxis genes and flagellar genes in Escherichia coli. J Bacteriol. 1983 Jul;155(1):265–274. doi: 10.1128/jb.155.1.265-274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S. Protein phosphorylation in bacterial chemotaxis. Cell. 1988 Apr 8;53(1):1–2. doi: 10.1016/0092-8674(88)90478-3. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Abortive transduction of motility in Salmonella; a nonreplicated gene transmitted through many generations to a single descendant. J Gen Microbiol. 1956 Dec;15(3):575–598. doi: 10.1099/00221287-15-3-575. [DOI] [PubMed] [Google Scholar]
- STOCKER B. A. Transduction of flagellar characters in Salmonella. J Gen Microbiol. 1953 Dec;9(3):410–433. doi: 10.1099/00221287-9-3-410. [DOI] [PubMed] [Google Scholar]
- Segall J. E., Block S. M., Berg H. C. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherris D., Parkinson J. S. Posttranslational processing of methyl-accepting chemotaxis proteins in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6051–6055. doi: 10.1073/pnas.78.10.6051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J., Borczuk A., Chiou F., Burchenal J. E. Compensatory mutations in receptor function: a reevaluation of the role of methylation in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8364–8368. doi: 10.1073/pnas.82.24.8364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Berg H. C. Acetyladenylate plays a role in controlling the direction of flagellar rotation. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6711–6715. doi: 10.1073/pnas.85.18.6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe A. J., Conley M. P., Kramer T. J., Berg H. C. Reconstitution of signaling in bacterial chemotaxis. J Bacteriol. 1987 May;169(5):1878–1885. doi: 10.1128/jb.169.5.1878-1885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S., Fujita H., Ishihara A., Aizawa S., Macnab R. M. Subdivision of flagellar genes of Salmonella typhimurium into regions responsible for assembly, rotation, and switching. J Bacteriol. 1986 Apr;166(1):187–193. doi: 10.1128/jb.166.1.187-193.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]