Abstract
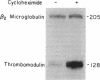
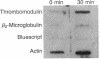
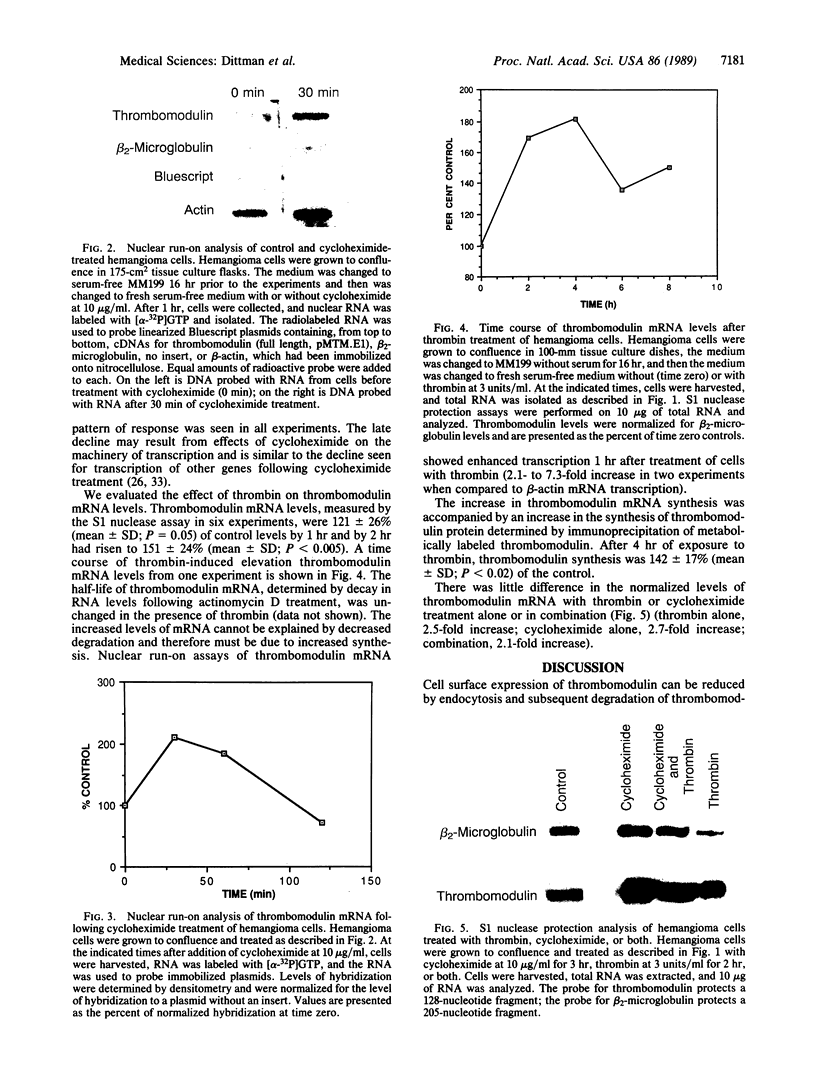
We have measured mRNA levels for thrombomodulin, an endothelial membrane cofactor for the activation of protein C by thrombin, in a mouse hemangioma cell line. Cycloheximide, an inhibitor of protein synthesis, increased levels of thrombomodulin mRNA, as measured in an S1 nuclease protection assay, to 2.5-4.0 times control levels. Thrombomodulin transcription in response to cycloheximide treatment, as determined by nuclear run-on analysis, was 3.9 +/- 1.3 (mean +/- SD) times that found in untreated cells. Thrombin also increased thrombomodulin mRNA levels to 151 +/- 21% (mean +/- SD) of control levels after 2 hr. Transcription increased in response to thrombin by 2.1- to 7.3-fold. The combination of thrombin and cycloheximide had no additive effect on thrombomodulin mRNA levels. Thrombin treatment of hemangioma cells also caused an increase in thrombomodulin protein synthesis to 142 +/- 17% (mean +/- SD) of control levels as determined by immunoprecipitation of [32S]methionine-labeled thrombomodulin. We conclude that thrombomodulin expression is determined in part by the rate of transcription and that thrombomodulin mRNA levels in hemangioma cells are increased by treatment with cycloheximide or thrombin. The increased transcription in response to cycloheximide suggests the existence of a labile protein repressor of thrombomodulin transcription.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewer G., Ross J. Poly(A) shortening and degradation of the 3' A+U-rich sequences of human c-myc mRNA in a cell-free system. Mol Cell Biol. 1988 Apr;8(4):1697–1708. doi: 10.1128/mcb.8.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Ishii S., Richert N., Merlino G. T., Pastan I. Epidermal growth factor regulates the expression of its own receptor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8374–8378. doi: 10.1073/pnas.82.24.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Conway E. M., Rosenberg R. D. Tumor necrosis factor suppresses transcription of the thrombomodulin gene in endothelial cells. Mol Cell Biol. 1988 Dec;8(12):5588–5592. doi: 10.1128/mcb.8.12.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel T. O., Gibbs V. C., Milfay D. F., Garovoy M. R., Williams L. T. Thrombin stimulates c-sis gene expression in microvascular endothelial cells. J Biol Chem. 1986 Jul 25;261(21):9579–9582. [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Depper J. M., Leonard W. J., Drogula C., Krönke M., Waldmann T. A., Greene W. C. Interleukin 2 (IL-2) augments transcription of the IL-2 receptor gene. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman W. A., Kumada T., Sadler J. E., Majerus P. W. The structure and function of mouse thrombomodulin. Phorbol myristate acetate stimulates degradation and synthesis of thrombomodulin without affecting mRNA levels in hemangioma cells. J Biol Chem. 1988 Oct 25;263(30):15815–15822. [PubMed] [Google Scholar]
- Dittman W. A., Majerus P. W. Sequence of a cDNA for mouse thrombomodulin and comparison of the predicted mouse and human amino acid sequences. Nucleic Acids Res. 1989 Jan 25;17(2):802–802. doi: 10.1093/nar/17.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp H. S., Austin K. S., Blaisdell J., Rubin R. A., Nelson K. G., Lee L. W., Grisham J. W. Epidermal growth factor (EGF) stimulates EGF receptor synthesis. J Biol Chem. 1986 Apr 15;261(11):4777–4780. [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T., Owen W. G. Identification of an endothelial cell cofactor for thrombin-catalyzed activation of protein C. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2249–2252. doi: 10.1073/pnas.78.4.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon C. T. The regulation of natural anticoagulant pathways. Science. 1987 Mar 13;235(4794):1348–1352. doi: 10.1126/science.3029867. [DOI] [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A., Griffin J. H., Harker L. A., Hanson S. R. Inhibition of platelet-dependent thrombus formation by human activated protein C in a primate model. Blood. 1989 Feb 15;73(3):639–642. [PubMed] [Google Scholar]
- Kavanaugh W. M., Harsh G. R., 4th, Starksen N. F., Rocco C. M., Williams L. T. Transcriptional regulation of the A and B chain genes of platelet-derived growth factor in microvascular endothelial cells. J Biol Chem. 1988 Jun 15;263(17):8470–8472. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Canfield W. M., Ericsson L. H., Davie E. W. Anticoagulant properties of bovine plasma protein C following activation by thrombin. Biochemistry. 1977 Dec 27;16(26):5824–5831. doi: 10.1021/bi00645a029. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Kudlow J. E., Cheung C. Y., Bjorge J. D. Epidermal growth factor stimulates the synthesis of its own receptor in a human breast cancer cell line. J Biol Chem. 1986 Mar 25;261(9):4134–4138. [PubMed] [Google Scholar]
- Kumada T., Dittman W. A., Majerus P. W. A role for thrombomodulin in the pathogenesis of thrombin-induced thromboembolism in mice. Blood. 1988 Mar;71(3):728–733. [PubMed] [Google Scholar]
- Ley T. J., Connolly N. L., Katamine S., Cheah M. S., Senior R. M., Robbins K. C. Tissue-specific expression and developmental regulation of the human fgr proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):92–99. doi: 10.1128/mcb.9.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Maruyama I., Majerus P. W. Protein C inhibits endocytosis of thrombin-thrombomodulin complexes in A549 lung cancer cells and human umbilical vein endothelial cells. Blood. 1987 May;69(5):1481–1484. [PubMed] [Google Scholar]
- Maruyama I., Majerus P. W. The turnover of thrombin-thrombomodulin complex in cultured human umbilical vein endothelial cells and A549 lung cancer cells. Endocytosis and degradation of thrombin. J Biol Chem. 1985 Dec 15;260(29):15432–15438. [PubMed] [Google Scholar]
- Miletich J. P., Broze G. J., Jr, Majerus P. W. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980 Jul 1;105(2):304–310. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- Moore K. L., Andreoli S. P., Esmon N. L., Esmon C. T., Bang N. U. Endotoxin enhances tissue factor and suppresses thrombomodulin expression of human vascular endothelium in vitro. J Clin Invest. 1987 Jan;79(1):124–130. doi: 10.1172/JCI112772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Esmon C. T., Esmon N. L. Tumor necrosis factor leads to the internalization and degradation of thrombomodulin from the surface of bovine aortic endothelial cells in culture. Blood. 1989 Jan;73(1):159–165. [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nawroth P. P., Handley D. A., Esmon C. T., Stern D. M. Interleukin 1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci U S A. 1986 May;83(10):3460–3464. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Stern D. M. Modulation of endothelial cell hemostatic properties by tumor necrosis factor. J Exp Med. 1986 Mar 1;163(3):740–745. doi: 10.1084/jem.163.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Seidman J. G. Structure of wild-type and mutant mouse beta 2-microglobulin genes. Cell. 1982 Jun;29(2):661–669. doi: 10.1016/0092-8674(82)90182-9. [DOI] [PubMed] [Google Scholar]
- Ponte P., Ng S. Y., Engel J., Gunning P., Kedes L. Evolutionary conservation in the untranslated regions of actin mRNAs: DNA sequence of a human beta-actin cDNA. Nucleic Acids Res. 1984 Feb 10;12(3):1687–1696. doi: 10.1093/nar/12.3.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Kelly P. A., Friesen H. G. Prolactin receptors in rat liver: possible induction by prolactin. Science. 1975 Apr 4;188(4183):57–59. doi: 10.1126/science.163493. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed B. C., Kaufmann S. H., Mackall J. C., Student A. K., Lane M. D. Alterations in insulin binding accompanying differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4876–4880. doi: 10.1073/pnas.74.11.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reem G. H., Yeh N. H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science. 1984 Jul 27;225(4660):429–430. doi: 10.1126/science.6429853. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Cantrell D. A. Interleukin 2 regulates its own receptors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):864–868. doi: 10.1073/pnas.82.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Stenflo J., Dahlbäck B., Teodorsson B. Inactivation of human coagulation factor V by activated protein C. J Biol Chem. 1983 Feb 10;258(3):1914–1920. [PubMed] [Google Scholar]
- Taylor F. B., Jr, Chang A., Esmon C. T., D'Angelo A., Vigano-D'Angelo S., Blick K. E. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987 Mar;79(3):918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. L., Rosner M. R. Regulation of epidermal growth factor receptor gene expression by retinoic acid and epidermal growth factor. J Biol Chem. 1989 Feb 25;264(6):3230–3234. [PubMed] [Google Scholar]
- Treisman R., Maniatis T. Simian virus 40 enhancer increases number of RNA polymerase II molecules on linked DNA. Nature. 1985 May 2;315(6014):73–75. doi: 10.1038/315072a0. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]