Abstract
Among the proteins that mediate calcium-stimulated transmitter release, the synaptic vesicle protein 2 (SV2) stands out as a unique modulator specific to the neurons and endocrine cells of vertebrates. In synapses, SV2 regulates the expression and trafficking of the calcium sensor protein synaptotagmin, an action consistent with the reduced calcium-mediated exocytosis observed in neurons lacking SV2. Yet SV2 contains amino acid motifs consistent with it performing other actions that could regulate presynaptic functioning and that might underlie the mechanism of drug action. To test the role of these functional motifs, we performed a mutagenic analysis of SV2A and assessed the ability of mutant SV2A proteins to restore normal synaptic transmission in neurons from SV2A/B knockout mice. We report that SV2A-R231Q, harboring a mutation in a canonical transporter motif, restored normal synaptic depression (a measure of release probability and signature deficit of neurons lacking SV2). In contrast, normal synaptic depression was not restored by SV2A-W300A and SV2A-W666A, harboring mutations of conserved tryptophans in the 5th and 10th transmembrane domains. Although they did not rescue normal neurotransmission, SV2A-W300A and SV2A-W666A did restore normal levels of synaptotagmin expression and internalization. This indicates that tryptophans 300 and 666 support an essential action of SV2 that is unrelated to its role in synaptotagmin expression or trafficking. These results indicate that SV2 performs at least two actions at the synapse that contribute to neurotransmitter release.
Keywords: synapse, neurotransmission, exocytosis
the calcium-regulated secretion of neurotransmitters is a specialized form of membrane fusion that requires regulatory proteins that are unique to transmitter-containing vesicles. One of these is synaptic vesicle protein 2 (SV2), a membrane glycoprotein expressed exclusively in neurons and endocrine cells. SV2 is the binding site of a class of drugs typified by levetiracetam (5, 17, 26, 29, 31). Levetiracetam is a Food and Drug Administration-approved treatment for epilepsy (reviewed in Ref. 11) that also shows promise in the treatment of anxiety disorders (27, 28, 47), pain (12, 13, 36), dyskinesias (7, 32, 40, 43, 48), and posttraumatic stress disorder (28). Thus SV2 represents a vesicle protein whose action is likely to play an important (and targetable) regulatory action at synapses.
SV2 is essential for normal neurotransmission. Neurons lacking SV2A and SV2B demonstrate reduced neurotransmission and reduced synaptic depression (8–10, 22, 41, 44). These phenotypes reflect reduced release probability due to impaired ability of vesicles to fuse in response to elevated cytoplasmic calcium. This effect occurs after vesicle docking (10) and before formation of the SNARE (soluble N-ethylmaleimide-sensitive fusion) complex (44), suggesting that SV2 contributes to the priming of vesicles for release. Indeed, the releasable pool of vesicles is decreased in cells cultured from SV2 knockout (KO) mice (8, 10, 41, 44). SV2's action appears linked to calcium-dependent processes: in hippocampal neurons, the SV2 KO phenotype can be transiently rescued by increased calcium influx (10), whereas, in retinal bipolar neurons, decreasing elevated resting cytoplasmic calcium restores wild-type neurotransmission (41).
Loss of SV2 results in a significant decrease in the amount of the calcium sensor synaptotagmin in vesicles (46). The decrease is due to two effects of SV2, an effect on synaptotagmin expression (30, 33, 46) and an effect on synaptotagmin internalization from the plasma membrane (46). SV2's effect on synaptotagmin internalization depends on tyrosine-based endocytosis motifs in SV2 that are predicted to serve as binding sites for the clathrin adaptor AP2. Mutation of the first endocytosis motif in SV2A (SV2A-Y46A) produces a protein that does not restore normal neurotransmission or synaptotagmin internalization in neurons cultured from SV2A/B KOs. Thus SV2's role in endocytosis is essential to its function.
Although reduced synaptotagmin in vesicles from SV2 KOs is consistent with reduced calcium-evoked secretion, the neurotransmission phenotype of neurons lacking SV2 is not identical to that of neurons lacking synaptotagmin (15, 16, 34). This suggests that SV2 may perform additional functions. In considering SV2's action at the synapse, most researchers have focused on its structural similarity to transporters (3a, 14, 18) and its matrix-like glycosyl moieties (39). This focus has led to the hypotheses that SV2 is a transporter (22) or provides a lumenal matrix that concentrates neurotransmitter in the vesicle lumen (37). To test these potential actions, we generated mutations in SV2A at residues predicted to underlie these functions and assessed their ability to rescue release probability in neurons cultured from SV2A/B KO mice.
METHODS
KO mice and neuronal cultures.
Primary neuronal cultures from SV2A−/−SV2B−/− double KO mice were generated as previously described (10). The animal protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the University of Washington.
Lentiviral constructs.
Lentiviral constructs were made to encode the SV2A protein, as well as SV2A mutations: R231Q, W330A, and W666A. SV2A mutations were made in pIRES2-EGFP (internal ribosomal entry sequence 2-enhanced green fluorescent protein), using a QuikChange PCR strategy (Stratagene). The IRES sequence was removed to produce SV2A fused to EGFP, which would later act as a real-time visual reporter of infection. Constructs were sequenced and subcloned into the Lentiviral transfer vector pRRL-cPPT-CMV-X-PRE-SIN, which was graciously donated by Z. Xia, and is described in Barry et al. (4). Lentiviral helper plasmids (pLP1, pLP2, pLP/VSVG) were the Virapower packaging mix (Invitrogen).
Lentivirus production and infection.
Lentivirus was prepared using a modified protocol described in Horn et al. (20). Briefly, virions were prepared by a calcium-phosphate-mediated transfection of 293T cells with the transfer vector and Virapower helper plasmids. 239T cells (ATCC) were grown in DMEM, 10% FBS, 1% penicillin-streptomycin to concentration 12 × 106 in a 150-mm dish and then transfected with lentivirus plasmids. Cells were treated with 10 mM sodium butyrate during the first of three 12-h virion supernatant collections. The collected supernatant was filtered through a 0.22-um pore-size filter, concentrated 100-fold by centrifugation, and stored at −80°C. Hippocampal autaptic cultures were infected on days in vitro (DIV) 1–3, and electrophysiological recordings were made on DIV 12–19.
Semliki Forest virus production and infection.
Semliki Forest virions were generated using techniques described in Ref. 1. Briefly, a cDNA encoding SV2A and EGFP separated by an IRES was cloned into the pSFV Semliki Forest Virus plasmid (Invitrogen, Mountain View, CA). The resulting construct was linearized by restriction digest and used to generate RNA for electroporation into baby hamster kidney cells. Supernatant containing replication-deficient virions were harvested from baby hamster kidney cells 24–48 h postelectroporation and frozen for later use. DIV 12–18 cultures of autaptic hippocampal neurons were infected with unconcentrated viral supernatant ∼14–18 h before recordings.
Electrophysiology and statistical analysis.
Whole cell voltage-clamp recordings were obtained from single-neuron microislands that were selected for by visual expression of EGFP at synapses. Recordings were performed at 21°C and used methods described (10). Recording electrodes were typically 2.5–3.5 MΩ. Data are reported as the mean ± SE.
Immunocytochemistry.
Immunocytochemistry was performed 13 days after plating. Coverslips containing mouse hippocampal neurons were rinsed with PBS and fixed with 4% paraformaldehyde for 20 min, after which they were incubated in blocking solution (PBS, pH 7.4, 2% normal goat serum, 0.4% saponin, and 1% BSA), and then with a monoclonal anti-synaptophysin antibody (1:2,000 dilution; Chemicon, Temecula, CA) and a polyclonal anti-SV2A antibody (1:1,000 dilution) or anti-green fluorescent protein antibody (Santa Cruz Antibodies) in blocking solution. Antibody labeling was detected with fluorescence-conjugated secondary antibodies (goat-anti-rabbit IgG Alexa-Fluor 488 and goat-anti-mouse IgG Alexa-Fluor 568; Invitrogen, Eugene, OR). Nuclei were stained with Hoechst. Images were taken using a Nikon Eclipse E600 microscope with a ×60 oil immersion objective.
For analysis of synaptic synaptotagmin 1 levels, neurons infected with lentivirus encoding EGFP, or a COOH-terminal SV2A-EGFP fusion and its mutants, were labeled with a monoclonal anti-synaptophysin and a polyclonal anti-synaptotagmin 1 antibody. Antibody labeling was detected with fluorescent secondary antibodies (goat-anti-rabbit Alexa-Fluor 568 and goat-anti-mouse Alexa-Fluor 647; Invitrogen). Images were obtained with a confocal laser-scanning microscope (Zeiss LSM 510 Meta) with a ×100 oil-immersion objective. Images were analyzed with MetaMorph image software to determine synaptotagmin 1 immunofluorescence. Anti-synaptophysin labeling was used to define synaptic puncta.
Surface biotinylation.
Conventional cultures of hippocampal neurons were used for surface biotinylation, as previously described (46).
RESULTS
Both acute and chronic expression of SV2 restores normal neurotransmission to neurons from SV2A/B KO mice.
As with any mutation that results in total loss of protein expression, disruption of the SV2 gene may produce compensatory changes that are the direct source of the phenotype observed. To determine whether the reduced release probability observed in neurons from SV2A/B double KO mice is due to secondary or developmental effects, we examined the effect of acute expression of SV2 in autaptic hippocampal neurons cultured from SV2A/B double KOs using Semliki Forest virus-mediated expression. The expression constructs generated included cDNA encoding EGFP, separated from the SV2A cDNA by an IRES, which directed the expression of EGFP as a separate protein. This allowed identification of infected neurons. Neurons were assayed within 18 h of viral infection for synaptic depression, an indicator of release probability and the hallmark deficit of SV2 mutants. Exogenous expression of SV2A plus EGFP resulted in robust synaptic depression, indicating rescue of the SV2A/B double KO phenotype. In contrast, cells expressing EGFP alone exhibited the double KO phenotype, which consists of synaptic facilitation followed by reduced depression (Fig. 1). Thus acute expression of SV2 restores normal synaptic depression, indicating that both the phenotype and rescue are due to the absence or presence of SV2 and not to other, compensatory effects.
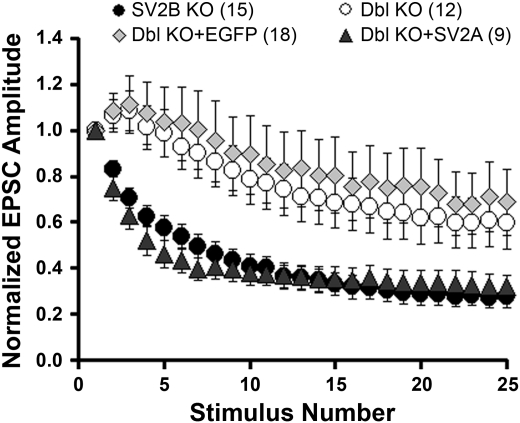
Fig. 1.
Acute expression of synaptic vesicle protein 2A (SV2A) restores normal synaptic depression in hippocampal neurons from SV2A/B double (Dbl) knockout (KO) mice. Hippocampal neurons from SV2A/B double KO mice were infected with Semliki Forest virus containing cDNA encoding SV2A + enhanced green fluorescent protein (EGFP) (solid triangles), or EGFP alone (shaded diamonds). Synaptic responses to depolarizing trains were assayed within 18 h of infection. Shown are average excitatory postsynaptic current (EPSC) amplitudes in response to a 10-Hz train of depolarizing pulses, normalized to the amplitude of the first response. Values are means ± SE. Neurons expressing SV2A displayed robust synaptic depression, whereas those expressing EGFP alone did not. Also shown for comparison are responses from uninfected SV2B KO, which have a wild-type (WT) phenotype (solid circles) and SV2A/B double KO neurons (open circles).
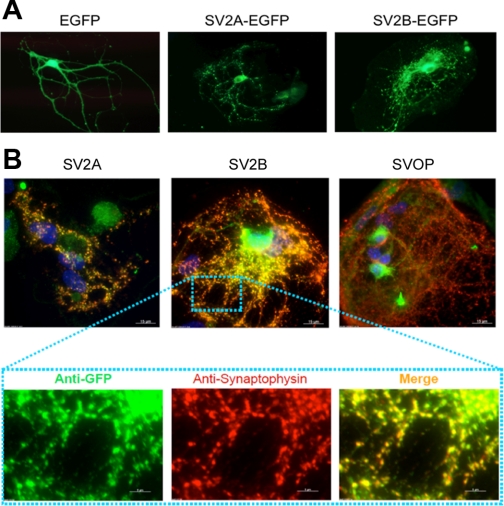
We also examined the effects of chronic expression of SV2 using a Lenti virus expression system. For these experiments, we utilized viral vectors encoding SV2-EGFP fusion proteins rather than bicistronic cassettes, as attempts to generate lentivirions containing SV2 and EGFP separated by an IRES sequence resulted in consistently poor infection rates. Moreover, they allowed us to easily monitor the trafficking of the expressed protein. Neurons were infected within 4 days of plating and assayed 10–12 days later. Expression of either SV2A-EGFP or SV2B-EGFP produced punctate localization of the fusion protein (Fig. 2A), consistent with a synaptic localization. To verify this, we compared the location of SV2-EGFP fusion proteins with that of the synaptic vesicle protein synaptophysin. In both cases, we observed colocalization (Fig. 2B), indicating that the fusion proteins were trafficked to synapses, and thus that the presence of EGFP on the carboxy terminus of SV2A does not disrupt its expression and localization in neurons. In contrast, an EGFP fusion of a related protein, SVOP (synaptic vesicle 2-related protein) (23), did not traffic to synapses.
Fig. 2.
Location of EGFP fusion proteins in hippocampal neurons. A: fluorescent images of live SV2A/B double KO hippocampal neurons infected with lentivirions encoding EGFP, SV2A-EGFP, and SV2B-EGFP. B: immunolabeling of SV2A/B double KO neurons infected with SV2A-EGFP, SV2B-EGFP, and SVOP (synaptic vesicle 2-related protein)-EGFP constructs. Virally delivered SV2A-EGFP and SV2B-EGFP (visualized with anti-green fluorescent protein, green channel) colocalized with the synaptic marker synaptophysin (anti-p38, red channel). In contrast, an SVOP-EGFP fusion protein did not localize to synapses.
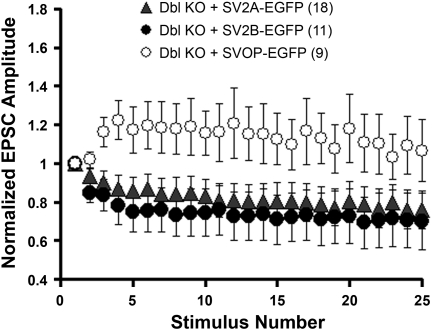
Expression of SV2A-EGFP in neurons from SV2A/B KO mice resulted in synaptic depression similar to that seen in wild-type neurons (Fig. 3). This indicates that the addition of EGFP at the carboxy terminus of SV2A does not disrupt its function. In contrast, expression of SVOP-EGFP did not restore synaptic depression. We also assayed the ability of SV2B-EGFP to rescue synaptic depression. Of the three SV2 isoforms, SV2B is the most divergent (3, 23). It is almost universally coexpressed with SV2A and displays pronounced changes in expression during development (2, 33). In addition, SV2B lacks a synaptotagmin binding site that is present at the amino terminus of SV2A and SV2C (38). Together, these observations suggested that SV2B acts differently than other SV2 isoforms. We found, however, that SV2B-EGFP restored synaptic depression as efficiently as SV2A. This indicates that SV2B can provide full SV2 action at the synapse. It also indicates that the synaptotagmin-binding domain in the amino terminus of SV2A/C is not crucial to SV2's effects on synaptic transmission. Therefore, the more severe phenotype in SV2A mutants likely reflects its higher expression levels in the central nervous system.
Fig. 3.
Chronic expression of SV2A-EGFP or SV2B-EGFP, but not SVOP-EGFP, restores synaptic depression in autaptic hippocampal neurons. Autaptic hippocampal neurons were infected with Lenti virions encoding the indicated protein 1–2 days after plating. Synaptic responses to stimulus trains were measured 11–16 days later. Neurons expressing SV2A-EGFP or SV2B-EGFP showed synaptic depression, whereas neurons expressing the related protein SVOP-EGFP did not.
Testing hypotheses of SV2 action by mutational analysis.
In addition to its role in the trafficking of synaptotagmin, SV2 has been proposed to function as a transporter and to serve as the anchor of a vesicular glycosyl matrix. To test these hypotheses, we generated a series of SV2 mutants, targeting residues predicted to support each of these proposed actions (Table 1).
Table 1.
Mutant SV2A proteins
| Mutation | Type | Synaptic Location | Rescue |
|---|---|---|---|
| D179N E182Q | Acidic residues in TM1 hypothesized to contribute to cation transport | No | |
| D227N | Acidic residue in loop between TM domains 2-3 that is essential to action of many MF transporters | No | |
| D227A | Acidic residue in loop between TM domains 2-3 that is essential to action of many MF transporters | No | |
| R231Q | Basic residue in loop between TM domains 2-3 that is essential to action of a subset of MF transporters | Yes | Yes |
| W300A | Hydrophobic residue conserved in TM domain 5 of all SV2 s and in SVOP | Yes | No |
| G303A | Glycine in TM domain 5 conserved in all SV2s, SVOP, and some MFS transporters | No | |
| W666A | Transmembrane residue conserved in all SV2 and in SVOP isoforms | Yes | No |
| K694A | Basic residue in TM domain 11 conserved in all SV2 isoforms and SVOP (the corresponding residue is acidic residue in a related cation transporter) | No | |
| N498D N548D N573D | Sites of N-linked glycosylation, predicted to anchor vesicular matrix. These mutants were generated singly and in combination. None trafficked to synapses | No |
TM, transmembrane; MF, major facilitator; SV2, synaptic vesicle protein 2; SVOP, synaptic vesicle 2-related protein.
To disrupt SV2's putative transport function, we mutated residues shown to be essential to the transport activity of other major facilitator (MF) proteins. MF transporters contain a signature sequence, DXXGRR/K, in the cytoplasmic loop between transmembrane domains 2 and 3. Within this motif, the aspartate and first arginine are reported to be crucial to transport activity in one or more MF transporters (24, 45). In SV2A, these residues are D227 and R231. Other residues reported to be essential to MF transporter action include aromatic residues, especially tryptophans, in the 10th transmembrane domain (25, 35). In SV2A W666, a tryptophan in the 10th transmembrane domain is conserved across SV2 isoforms. We also targeted a conserved tryptophan in the fifth transmembrane domain, W300, which is the analog of W666 in the first half of the protein and is also conserved across isoforms. Since charged residues in membrane domains often contribute to transport activity, we also mutated conserved charged and polar residues predicted to be in transmembrane domains. These included two acidic residues predicted to be in the first transmembrane domain, D179 and E182, a basic residue in transmembrane domain 11, K694.
To test the hypothesis that the sugar side chains of SV2 constitute a matrix that concentrates neurotransmitter (37), we mutated the three consensus sites for N-linked glycosylation in the predicted loop between membrane domains 7 and 8. Finally, we examined the sequences of SV2A, SV2B, and SV2C for conserved residues. Based on this analysis, we mutated a highly conserved glycine in the fifth transmembrane domain, G303.
All mutant proteins were expressed in cultured hippocampal neurons at significant levels; however, only three of them were localized to synapses, with all others demonstrating diffuse localization concentrated in the cell body (Table 1). This precluded our testing the requirement for several residues implicated in transporter function and SV2's potential role as the source of the vesicular matrix. There was no correlation between the location of a mutation and aberrant trafficking. Mutation of residues in transmembrane domains and residues predicted to be in cytoplasmic loops both produced proteins that trafficked and proteins that did not. One of the mutants, D227N (also D227A), deserves special mention because it was identified in analyses of bacterial MF transporters to be universally essential to function (24). Our results suggest that it may play a crucial role in protein folding. Two chemical chaperones, glycerol (1.25 M) and trimethylamine N-oxide (100 mM), which rescue trafficking of cystic fibrosis transmembrane conductance regulator mutants (6), did not improve the trafficking of the D227N mutant when added to the medium at the same time as the virions (data not shown). Therefore, SV2 folding and/or trafficking appears to be very sensitive to changes in protein composition in a manner that cannot be rescued with chemical chaperones that support proper folding of cystic fibrosis transmembrane conductance regulator.
Arginine 231, a canonical residue in the MF transporter signature motif, is not required for SV2 function.
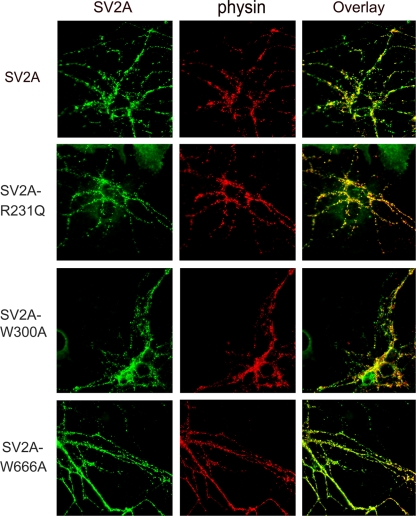
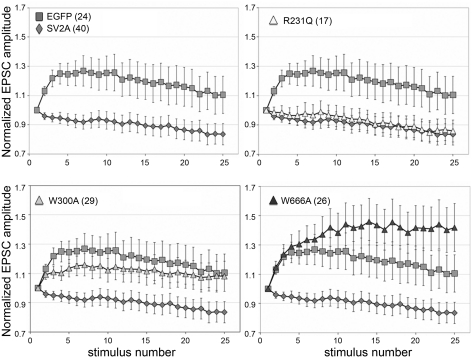
Mutation of either the aspartate (D) or the first arginine (R) in the MF signature sequence DXXGRR/K is associated with loss of transport activity in sugar and antibiotic transporters (19, 24, 45). Thus we targeted these residues in SV2A to test the hypothesis that SV2 is a transporter. Of the mutants we generated, only the R231Q mutant was properly trafficked to synapses (Fig. 4). Neither of two aspartate 227 mutants was found at synapses (Table 1). Expression of SV2A-R231Q restored synaptic depression to autaptic hippocampal neurons from SV2A/B KO mice. The level of synaptic depression was the same as seen when wild-type SV2A was expressed (Fig. 5). Thus this residue is not crucial to SV2's action at the synapse.
Fig. 4.
SV2A-R231Q, W300A, and W666A are trafficked to synapses. Autaptic hippocampal neurons from SV2A/B double KO mice were infected with Lenti virions encoding the indicated SV2A-EGFP fusion protein. Cultures were fixed and processed for immunolabeling with monoclonal anti-synaptophysin and polyclonal anti-SV2A antibodies. Antibody binding was visualized with goat-anti-rabbit Alexa-Fluor 488 (green) and goat-anti-mouse Alexa-Fluor 568 (red). Shown are images collected with a Nikon upright microscope. SV2A-EGFP, SVA-R231Q, W300A, and W666A trafficked properly to the synapses and colocalized with synaptophysin.
Fig. 5.
SV2A-R231Q, but not W300A or W666A, restores synaptic depression in neurons from SV2 double KO mice. Recordings are from autaptic hippocampal neurons cultured from SV2A/B double KO mice expressing the indicated SV2A-EGFP fusion protein. EPSC amplitudes in response to a 10-Hz stimulus train were normalized to the amplitude of the first response. Average normalized values are graphed with error bars representing the SE. The number of cells recorded from (nos. in parentheses) is shown for each protein. Expression of SV2A-R231Q restored synaptic depression of the same magnitude as observed when WT SV2A was expressed. Neurons expressing SV2A-W300A or SV2A-W666A showed synaptic facilitation, indicative of a reduced release probability.
Tryptophans in the 5th and 10th transmembrane domains are essential to SV2 function.
The SV2s contain tryptophan residues in the 5th and 10th transmembrane domains that are conserved across isoforms. Hydrophobic residues in these membrane domains are crucial to the action of the glucose transporter GLUT-1 (25) and to human organic anion transporter activity (35), where they are proposed to line the pore of the transport channel. When expressed in neurons cultured from SV2A/B KO mice, both SV2A-W300A and SV2A W666A trafficked to synaptic terminals (Fig. 4). Neurons expressing SV2A-W300A demonstrated reduced synaptic depression (Fig. 5), suggesting that W300 contributes to SV2 action. An even greater effect was seen when W666 was mutated. Neurons expressing SV2A-W666A had a more severe phenotype than neurons expressing EGFP, indicating that this residue is essential for SV2-mediated modulation of synaptic transmission.
Mutation of tryptophans 300 or 666 does not affect synaptotagmin expression or trafficking.
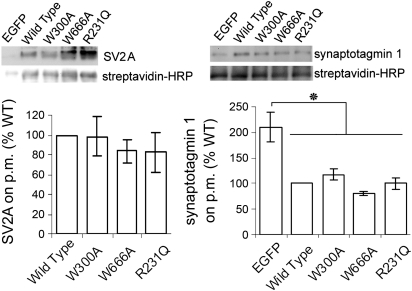
The crucial role of SV2 in maintaining the vesicular content synaptotagmin can explain the reduced calcium-stimulated exocytosis in SV2 KOs. Thus the question arises whether this is the only action of SV2. If so, we would expect that the W300A or W666A SV2A mutants would not rescue synaptotagmin expression and/or trafficking. To test this, we compared expression levels and internalization of SV2 and synaptotagmin in neurons expressing SV2A-WT, SV2A-R231Q, SV2A-W300A, and SV2A-W666A. The expression of SV2 and synaptotagmin was measured by Western blot analyses of hippocampal neurons grown in conventional cultures (Fig. 6A). The results revealed that the SV2A mutants were expressed at ∼80%, the level of wild-type SV2A, although the difference was not significant (P > 0.05, one-way ANOVA). Synaptotagmin I levels were not decreased in neurons expressing either wild-type or mutant SV2A. In cultures expressing EGFP, however, synaptotagmin was significantly decreased (P < 0.05).
Fig. 6.
SV2A-R231Q, W300A, and W666A restore expression of synaptotagmin 1. A, top: a representative Western blot analysis of conventional cultures of hippocampal neurons expressing the indicated SV2A-EGFP fusion construct or EGFP alone. Blots were probed for SV2A (left) and synaptotagmin 1 (right). Anti-actin labeling was used as loading control. Band net intensity was normalized to the net intensity of actin in the same lane. Bottom: average values normalized to WT in the same blot. The graphs represent data from four to seven independent experiments. Error bars represent SE. All three mutants restored synaptotagmin 1 expression levels to those seen in neurons expressing WT SV2A. B: comparison of synaptotagmin 1 levels at synapses expressing EGFP, SV2A, SV2A-R231Q, SV2AW300A, or SV2A-W666A. Synapses, detected with an anti-synaptophysin antibody, were labeled with a polyclonal antibody directed against synaptophysin 1. Normalized fluorescent labeling intensity was normalized to WT within each experiment. The table lists the normalized averages from three independent cultures. Data were assessed for significant differences by one-way ANOVA. N indicates the total number of images analyzed.
Because Western blots measure total protein expression in both neurons and astrocytes, we also measured synaptotagmin levels at synapses using immunocytochemistry. Synapses were identified with an antibody against the vesicle protein synaptophysin (Fig. 6B), and the intensity of anti-synaptotagmin labeling quantified in those regions. When assessed in this way, all SV2A constructs significantly increased synaptotagmin 1 expression compared with neurons expressing just EGFP (P < 0.00001, one-way ANOVA). Synaptotagmin expression in neurons expressing mutant SV2As had ∼20% less synaptotagmin at synapses than neurons expressing wild-type SV2A, a difference that was significant (P < 0.01). There was, however, no difference in the amount of synaptotagmin expressed in mutants that did rescue synaptic depression (SV2A-R231Q) compared with those that did not (W300A and W666A). Thus the ∼20% decrease in synaptotagmin cannot account for the failure of SV2A-W300A and SV2A-W666A to rescue synaptic depression. Thus we have identified mutations that fail to rescue synaptic release probability that do not alter synaptotagmin expression or turnover.
To determine whether W300 and W666 play a role in SV2 or synaptotagmin trafficking, we assessed protein internalization by measuring the proportion of total protein that was biotinylated after treatment with surface biotinylating reagent. As reported previously (46), neurons expressing EGFP had significantly more surface synaptotagmin. Expression of all SV2s, both wild-type and mutant, resulted in a significant decrease in the proportion of biotinylated synaptotagmin (Fig. 7). Therefore, the two SV2A mutants that did not restore synaptic depression did restore internalization of synaptotagmin. These findings are consistent with W300 and W666 contributing to an action other than regulation of vesicle synaptotagmin levels.
Fig. 7.
SV2A-R231Q, W300A, and W666A do not affect internalization of synaptotagmin. To assay for effects on protein trafficking, we examined the proportion of SV2A and synaptotagmin 1 on the plasma membrane (p.m.). This was measured by surface biotinylation followed by immunoprecipitation (with anti-SV2A or synaptotagmin1 antibody) and quantification of immunoprecipitated protein and biotin content. The ratio of biotin to SV2 or synaptotagmin 1 was calculated for each lane and normalized to the ratio obtained from neurons expressing WT SV2A in the same blot. Top: representative blot series. The proportion of biotinylated SV2A did not differ significantly between constructs (P > 0.05, one-way ANOVA), indicating that none of the mutations affected SV2A internalization. Compared with neurons expressing EGFP, neurons expressing any form of SV2A had significant less surface labeling of synaptotagmin 1 (*P < 0.001). The proportion of biotinylated synaptotagmin did not differ between neurons expressing all forms of SV2A (P > 0.05). This indicates that the mutations did not affect synaptotagmin 1 internalization at the synapse. The error bars represent SE (n = 3–5). HRP, horseradish peroxidase.
DISCUSSION
Of the synapse-specific proteins, SV2 is unique in having no clear homolog in invertebrates. It is also, at present, the only vesicle protein known to be a drug target. Because of these features, understanding SV2's contribution to the unique features of synaptic transmission is of special importance to developing therapies targeted at synaptic functioning. The data presented here indicate that SV2 is likely to perform at least two actions at the synapse, both of which directly impact synaptic release probability.
Previously published studies of mice lacking SV2 revealed that it is essential for normal levels of calcium-stimulated neurotransmission. A potential confound in interpreting analyses of mouse mutants is the possibility that phenotypic changes are due to indirect developmental effects or compensatory changes. Our finding that acute, Semliki Forest virus-mediated expression of SV2 rescues synaptic depression in neurons lacking SV2 indicates that the KO phenotype reflects the loss of SV2, and not ancillary changes that occur in its absence. Thus SV2 performs an action that contributes directly to synaptic release probability.
In previously published work, we showed that SV2 interacts with clathrin adaptor proteins and regulates the expression and trafficking of the calcium sensor protein synaptotagmin. Significantly reduced vesicular synaptotagmin is consistent with the reduced ability of calcium to trigger transmitter release. Thus it was possible that this constituted the sole action of SV2. In these studies, we undertook further mutational analysis of SV2 to test other proposed actions, specifically to test the hypotheses that SV2 acts as a transporter or the scaffold for a vesicular matrix. Mutations of residues implicated in these functions were made in SV2A, and the ability of the mutant to rescue synaptic depression in neurons from SV2A/B KOs tested. We found that the majority of single amino acid changes we made disrupted SV2 trafficking to synapses. Mutation of any of the glycosylation consensus sites in SV2's large lumenal domain resulted in a protein that did not traffic to synapses, indicating that glycosylation plays an essential role in SV2 trafficking. SV2's sensitivity to other single amino acid substitutions suggests that precise folding or protein interactions are also required for SV2's exit from the endoplasmic reticulum/Golgi. Of particular interest is our finding that mutation of aspartate 227 results in a protein that is not trafficked to synapses. This residue is part of the major facilitator transporter motif in SV2 and has been proposed to be the essential residue in this motif (19). Our findings suggest that a primary function of this residue may be establishment of protein topology in the membrane. Similarly, acidic residues in the first transmembrane domain that were hypothesized to support SV2 transport of cations (23) appear to be essential for SV2 folding, as mutation of them resulted in a protein that appeared trapped in the endoplasmic reticulum.
Yet despite the sensitivity to single amino acid changes, attachment of EGFP to SV2's carboxy terminus did not block its trafficking or function at the synapse. We note, however, that addition of EGFP does affect trafficking of some mutants. One of the mutants we generated (K694A) did not traffic when expressed with EGFP at the carboxy terminus (Table 1), but was properly trafficked and rescued normal neurotransmission, when expressed as a fusion protein with EGFP at the amino terminus (8).
A major finding of this work is that typtophans in membrane domains 5 and 10 are essential to SV2 action, but not for the expression or trafficking of synaptotagmin. Neither synaptotagmin expression nor internalization was affected in neurons expressing SV2A-W300A and SV2A-666A. Yet neither of these mutant SV2 proteins rescued normal synaptic depression. Thus SV2 appears to have at least two actions in the synapse.
The finding that disruption of a canonical MF transporter motif in SV2 does not impair its ability to support neurotransmission suggests that it does not act as a transporter. On the other hand, two conserved tryptophans that contribute to transport activity in some MF transporters are required for SV2's ability to function properly in the synapse. Given the variable effects of mutating residues implicated in transporter function, it still remains unclear whether SV2 acts as a transporter. If it does, W300 and W666 are likely to be crucial to transport activity.
Because SV2A is the binding site of a promising class of new drugs, determining how it acts and how it is regulated at the synapse will have important implications for further drug development. Both decreasing (22, 42) and increasing (10) cytoplasmic calcium can reverse the neurotransmission deficit in neurons from SV2 KO mice. Thus SV2 has been hypothesized to regulate cytoplasmic calcium (22, 42), or to stabilize a priming step produced by calcium (10). On the other hand, loss of SV2 results in reduced exocytosis in the absence of changes in cytoplasmic calcium (21, 44), consistent with an action that is independent of calcium. The mutations reported here provide the basis for testing these hypotheses of SV2 function as well as for future structural analyses of SV2 and drugs that modify its action.
GRANTS
This work was supported by the National Institute of Mental Health, Grant R01 MH 059842 to S. M. Bajjalieh.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Drs. Jane Sullivan and Richard Gardner for comments on the manuscript, and Lisa Baldwin for animal husbandry. We thank Drs. John Scott and Catherine Pawson for help with confocal microscopy and Dr. Ruimao Zheng for help with statistical analyses.
REFERENCES
- 1.Ahlquist RM, Sullivan JM. Overexpression of proteins in neurons using replication-deficient virus. Methods Mol Biol 337: 15–26, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bajjalieh SM, Franz G, Weimann JM, McConnell SK, Scheller RH. Differential expression of Synaptic Vesicle Protein 2 (SV2) isoforms. J Neurosci 14: 5223–5235, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajjalieh SM, Peterson K, Linial M, Scheller RH. Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A 90: 2150–2154, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Bajjalieh SM, Peterson K, Shinghal R, Scheller RH. SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257: 1271–1273, 1992 [DOI] [PubMed] [Google Scholar]
- 4.Barry SC, Harder B, Brzezinski M, Flint LY, Seppen J, Osborne WR. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum Gene Ther 12: 1103–1108, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Bennett B, Matagne A, Michel P, Leonard M, Cornet M, Meeus MA, Toublanc N. Seletracetam (UCB 44212). Neurotherapeutics 4: 117–122, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones 1: 117–125, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bushara KO, Malik T, Exconde RE. The effect of levetiracetam on essential tremor. Neurology 64: 1078–1080, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2+-induced exocytosis. J Neurosci 29: 883–897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowder KM, Gunther JM, Jones TA, Hale BD, Zhang HZ, Peterson MR, Scheller RH, Chavkin C, Bajjalieh SM. Abnormal neurotransmission in mice lacking synaptic vesicle protein 2A (SV2A). Proc Natl Acad Sci U S A 96: 115268–115273, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Custer KL, Austin NS, Sullivan JM, Bajjalieh SM. Synaptic vesicle protein 2 enhances release probability at quiescent synapses. J Neurosci 26: 1303–1313, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Smedt T, Raedt R, Vonck K, Boon P. Levetiracetam: part II, the clinical profile of a novel anticonvulsant drug. CNS Drug Rev 13: 57–78, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunteman ED. Levetiracetam as an adjunctive analgesic in neoplastic plexopathies: case series and commentary. J Pain Palliat Care Pharmacother 19: 35–43, 2005 [PubMed] [Google Scholar]
- 13.Enggaard TP, Klitgaard NA, Sindrup SH. Specific effect of levetiracetam in experimental human pain models. Eur J Pain 10: 193–198, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Feany MB, Lee S, Edwards RH, Buckley KM. The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 70: 861–867, 1992 [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major calcium sensor for transmitter release at a central synapse. Cell 79: 717–727, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Gillard M, Chatelain P, Fuks B. Binding characteristics of levetiracetam to synaptic vesicle protein 2A (SV2A) in human brain and in CHO cells expressing the human recombinant protein. Eur J Pharmacol 536: 102–108, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Gingrich JA, Anderson PH, Tiberi M, Mestikawy S, Jorgensen PN, Fremeau RT, Caron MG. Identification, characterization, and molecular cloning of a novel transporter-like protein localized to the central nervous system. FEBS Lett 312: 115–122, 1992 [DOI] [PubMed] [Google Scholar]
- 19.Hirai T, Heymann JA, Maloney PC, Subramaniam S. Structural model for 12-helix transporters belonging to the major facilitator superfamily. J Bacteriol 185: 1712–1718, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horn PA, Morris JC, Bukovsky AA, Andrews RG, Naldini L, Kurre P, Kiem HP. Lentivirus-mediated gene transfer into hematopoietic repopulating cells in baboons. Gene Ther 9: 1464–1471, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Iezzi M, Theander S, Janz R, Loze C, Wollheim CB. SV2A and SV2C are not vesicular Ca2+ transporters but control glucose-evoked granule recruitment. J Cell Sci 118: 5647–5660, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24: 1003–1016, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Janz R, Hofmann K, Sudhof TC. SVOP, an evolutionarily conserved synaptic vesicle protein, suggests novel transport functions of synaptic vesicles. J Neurosci 15: 9269–9281, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jessen-Marshall AE, Paul NJ, Brooker RJ. The conserved motif, GXXX(D/E)(R/K)XG[X](R/K)(R/K), in hydrophilic loop 2/3 of the lactose permease. J Biol Chem 270: 16251–16257, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Kasahara T, Kasahara M. Tryptophan 388 in putative transmembrane segment 10 of the rat glucose transporter Glut1 is essential for glucose transport. J Biol Chem 273: 29113–29117, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kenda BM, Matagne AC, Talaga PE, Pasau PM, Differding E, Lallemand BI, Frycia AM, Moureau FG, Klitgaard HV, Gillard MR, Fuks B, Michel P. Discovery of 4-substituted pyrrolidone butanamides as new agents with significant antiepileptic activity. J Med Chem 47: 530–549, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Kinrys G, Worthington JJ, Wygant L, Nery F, Reese H, Pollack MH. Levetiracetam as adjunctive therapy for refractory anxiety disorders. J Clin Psychiatry 68: 1010–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kinrys G, Wygant LE, Pardo TB, Melo M. Levetiracetam for treatment-refractory posttraumatic stress disorder. J Clin Psychiatry 67: 211–214, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Lambeng N, Grossmann M, Chatelain P, Fuks B. Solubilization and immunopurification of rat brain synaptic vesicle protein 2A with maintained binding properties. Neurosci Lett 398: 107–112, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lazzell DR, Belizaire R, Thakur P, Sherry DM, Janz R. SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem 279: 52124–52131, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Lynch BA, Lambeng N, Nocka K, Kensel-Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci USA 101: 9861–9866, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGavin CL, John V, Musser WS. Levetiracetam as a treatment for tardive dyskinesia: a case report. Neurology 61: 419, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Morgans CW, Kensel-Hammes P, Hurley JB, Burton K, Idzerda R, McKnight GS, Bajjalieh SM. Loss of the synaptic vesicle protein SV2B results in reduced neurotransmission and altered synaptic vesicle protein expression in the retina. PLoS ONE 4: e5230, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci 24: 6127–6132, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perry JL, Dembla-Rajpal N, Hall LA, Pritchard JB. A three-dimensional model of human organic anion transporter 1: aromatic amino acids required for substrate transport. J Biol Chem 281: 38071–38079, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price MJ. Levetiracetam in the treatment of neuropathic pain: three case studies. Clin J Pain 20: 33–36, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Reigada D, Diez-Perez I, Gorostiza P, Verdaguer A, Gomez de Aranda I, Pineda O, Vilarrasa J, Marsal J, Blasi J, Aleu J, Solsona C. Control of neurotransmitter release by an internal gel matrix in synaptic vesicles. Proc Natl Acad Sci USA 100: 3485–3490, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schivell AE, Mochida S, Kensel-Hammes P, Custer KL, Bajjalieh SM. SV2A and SV2C contain a unique synaptotagmin-binding site. Mol Cell Neurosci 29: 56–64, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Scranton TW, Iwata M, Carlson SS. The SV2 protein of synaptic vesicles is a keratan sulfate proteoglycan. J Neurochem 61: 29–44, 1993 [DOI] [PubMed] [Google Scholar]
- 40.Striano P, Elefante A, Coppola A, Tortora F, Zara F, Minetti C, Striano S. Dramatic response to levetiracetam in post-ischaemic Holmes' tremor. J Neurol Neurosurg Psychiatry 78: 438–439, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 66: 884–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wan QF, Zhou ZY, Thakur P, Vila A, Sherry DM, Janz R, Heidelberger R. SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 66: 884–895, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods SW, Saksa JR, Baker CB, Cohen SJ, Tek C. Effects of levetiracetam on tardive dyskinesia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 69: 546–554, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Xu T, Bajjalieh SM. SV2 modulates the size of the readily releasable pool of secretory vesicles. Nat Cell Biol 3: 691–698, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Yamaguchi A, Someya Y, Sawai T. Metal-tetracycline/H+ antiporter of Escherichia coli encoded by transposon Tn10. The role of a conserved sequence motif, GXXXXRXGRR, in a putative cytoplasmic loop between helices 2 and 3. J Biol Chem 267: 19155–19162, 1992 [PubMed] [Google Scholar]
- 46.Yao J, Nowack A, Kensel-Hammes P, Gardner RG, Bajjalieh SM. Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci 30: 5569–5578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Connor KM, Davidson JR. Levetiracetam in social phobia: a placebo controlled pilot study. J Psychopharmacol 19: 551–553, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Zivkovic SA, Costa G, Bond G, Abu-Elmagd KM. Treatment of tardive dyskinesia with levetiracetam in a transplant patient. Acta Neurol Scand 117: 351–353, 2008 [DOI] [PubMed] [Google Scholar]