Abstract
We have isolated two recombinant cDNAs whose corresponding RNAs have an increased abundance in scrapie-infected hamster brain. DNA sequence analysis has shown that these two recombinants represent the genes for sulfated glycoprotein 2 and transferrin. The abundance of sulfated glycoprotein 2 RNA is increased in hippocampus from patients with Alzheimer disease and Pick disease, whereas transferrin RNA is not strongly modulated in these conditions. Expression of two previously identified scrapie-modulated genes, encoding glial fibrillary acidic protein and metallothionein, is also increased in both of these neurodegenerative diseases.
Full text
PDF


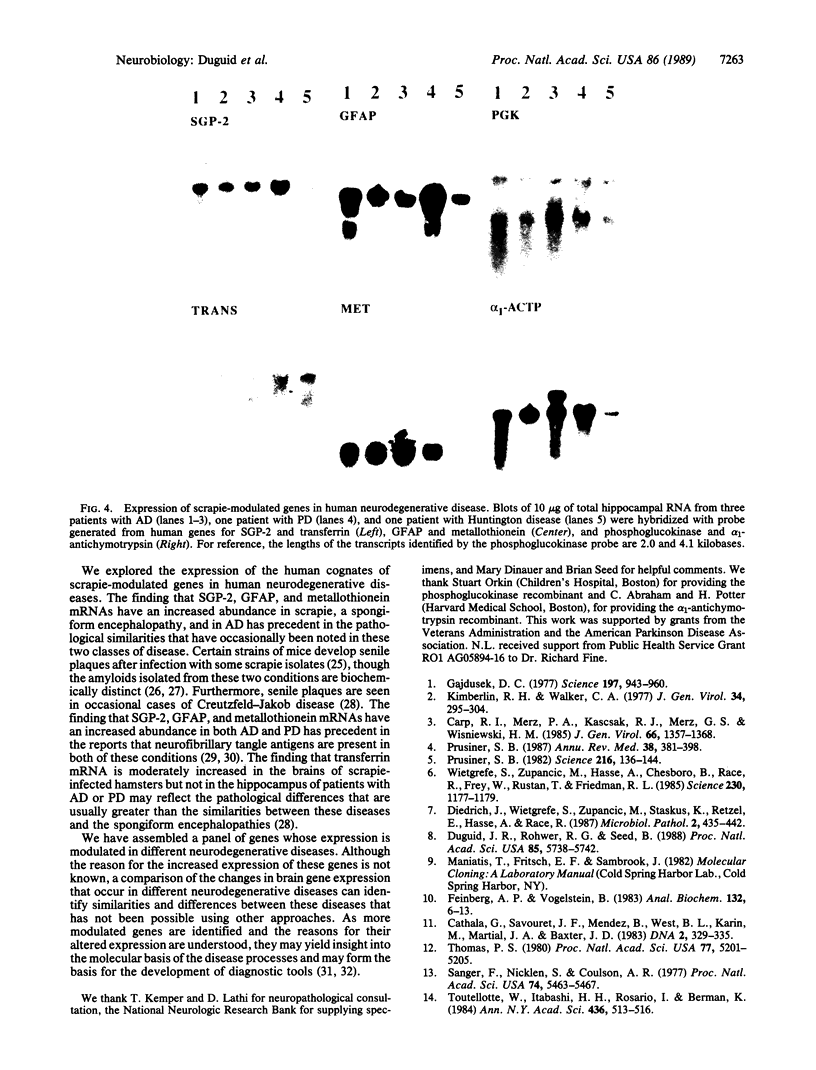

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham C. R., Selkoe D. J., Potter H. Immunochemical identification of the serine protease inhibitor alpha 1-antichymotrypsin in the brain amyloid deposits of Alzheimer's disease. Cell. 1988 Feb 26;52(4):487–501. doi: 10.1016/0092-8674(88)90462-x. [DOI] [PubMed] [Google Scholar]
- Albrechtsen M., Sørensen P. S., Gjerris F., Bock E. High cerebrospinal fluid concentration of glial fibrillary acidic protein (GFAP) in patients with normal pressure hydrocephalus. J Neurol Sci. 1985 Oct;70(3):269–274. doi: 10.1016/0022-510x(85)90168-6. [DOI] [PubMed] [Google Scholar]
- Bloch B., Popovici T., Levin M. J., Tuil D., Kahn A. Transferrin gene expression visualized in oligodendrocytes of the rat brain by using in situ hybridization and immunohistochemistry. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6706–6710. doi: 10.1073/pnas.82.19.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp R. I., Merz P. A., Kascsak R. J., Merz G. S., Wisniewski H. M. Nature of the scrapie agent: current status of facts and hypotheses. J Gen Virol. 1985 Jul;66(Pt 7):1357–1368. doi: 10.1099/0022-1317-66-7-1357. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Collard M. W., Griswold M. D. Biosynthesis and molecular cloning of sulfated glycoprotein 2 secreted by rat Sertoli cells. Biochemistry. 1987 Jun 16;26(12):3297–3303. doi: 10.1021/bi00386a008. [DOI] [PubMed] [Google Scholar]
- Crols R., Saerens J., Noppe M., Lowenthal A. Increased GFAp levels in CSF as a marker of organicity in patients with Alzheimer's disease and other types of irreversible chronic organic brain syndrome. J Neurol. 1986 Jun;233(3):157–160. doi: 10.1007/BF00314423. [DOI] [PubMed] [Google Scholar]
- Diedrich J., Wietgrefe S., Zupancic M., Staskus K., Retzel E., Haase A. T., Race R. The molecular pathogenesis of astrogliosis in scrapie and Alzheimer's disease. Microb Pathog. 1987 Jun;2(6):435–442. doi: 10.1016/0882-4010(87)90050-7. [DOI] [PubMed] [Google Scholar]
- Duguid J. R., Rohwer R. G., Seed B. Isolation of cDNAs of scrapie-modulated RNAs by subtractive hybridization of a cDNA library. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5738–5742. doi: 10.1073/pnas.85.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gajdusek D. C. Unconventional viruses and the origin and disappearance of kuru. Science. 1977 Sep 2;197(4307):943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- Huggenvik J. I., Idzerda R. L., Haywood L., Lee D. C., McKnight G. S., Griswold M. D. Transferrin messenger ribonucleic acid: molecular cloning and hormonal regulation in rat Sertoli cells. Endocrinology. 1987 Jan;120(1):332–340. doi: 10.1210/endo-120-1-332. [DOI] [PubMed] [Google Scholar]
- Jamada M., Mehraein P. Verteilungsmuster der senilen Veränderungen im Gehirn. Die Beteiligung des limbischen Systems bei hirnatrophischen Prozessen des Seniums und bei Morbus Alzheimer. Arch Psychiatr Nervenkr (1970) 1968;211(3):308–324. doi: 10.1007/BF00340827. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes: molecular cloning and sequence analysis of the mRNA. Nucleic Acids Res. 1982 May 25;10(10):3165–3173. doi: 10.1093/nar/10.10.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin R. H., Walker C. Characteristics of a short incubation model of scrapie in the golden hamster. J Gen Virol. 1977 Feb;34(2):295–304. doi: 10.1099/0022-1317-34-2-295. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. L., Gajdusek D. C., Gibbs C. J., Jr Creutzfeldt-Jakob disease virus isolations from the Gerstmann-Sträussler syndrome with an analysis of the various forms of amyloid plaque deposition in the virus-induced spongiform encephalopathies. Brain. 1981 Sep;104(3):559–588. doi: 10.1093/brain/104.3.559. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Prions causing degenerative neurological diseases. Annu Rev Med. 1987;38:381–398. doi: 10.1146/annurev.me.38.020187.002121. [DOI] [PubMed] [Google Scholar]
- Rasool C. G., Selkoe D. J. Sharing of specific antigens by degenerating neurons in Pick's disease and Alzheimer's disease. N Engl J Med. 1985 Mar 14;312(11):700–705. doi: 10.1056/NEJM198503143121107. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos C., Squicciarini J., Fine R. E. Chick embryo spinal cord neurons synthesize a transferrin-like myotrophic protein. FEBS Lett. 1983 Mar 21;153(2):387–390. doi: 10.1016/0014-5793(83)80649-8. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tourtellotte W. W., Itabashi H. H., Rosario I., Berman K. The National Neurological Research Bank. A collection of cryopreserved human neurological specimens for neuroscientists. Ann N Y Acad Sci. 1984;436:513–516. doi: 10.1111/j.1749-6632.1984.tb14835.x. [DOI] [PubMed] [Google Scholar]
- Uzan G., Frain M., Park I., Besmond C., Maessen G., Trépat J. S., Zakin M. M., Kahn A. Molecular cloning and sequence analysis of cDNA for human transferrin. Biochem Biophys Res Commun. 1984 Feb 29;119(1):273–281. doi: 10.1016/0006-291x(84)91648-6. [DOI] [PubMed] [Google Scholar]
- Wietgrefe S., Zupancic M., Haase A., Chesebro B., Race R., Frey W., 2nd, Rustan T., Friedman R. L. Cloning of a gene whose expression is increased in scrapie and in senile plaques in human brain. Science. 1985 Dec 6;230(4730):1177–1179. doi: 10.1126/science.3840915. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Bruce M. E., Fraser H. Infectious etiology of neuritic (senile) plaques in mice. Science. 1975 Dec 12;190(4219):1108–1110. doi: 10.1126/science.1237933. [DOI] [PubMed] [Google Scholar]
- Wong C. W., Quaranta V., Glenner G. G. Neuritic plaques and cerebrovascular amyloid in Alzheimer disease are antigenically related. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8729–8732. doi: 10.1073/pnas.82.24.8729. [DOI] [PMC free article] [PubMed] [Google Scholar]




