Abstract
Dopamine produced by renal proximal tubules increases sodium excretion via a decrease in renal sodium reabsorption. Dopamine natriuresis is impaired in obese Zucker rats; however, the mechanism is not fully understood. To test the hypothesis that renal expression of one or more of the subtypes are altered in these rats, we measured whole kidney protein levels by immunoblotting of D1-like (D1R and D5R) and D2-like (D2R, D3R, and D4R) dopamine receptors in both male and female obese and lean Zucker rats. In obese males on 1% NaCl diet, D1R, D2R, D4R, and D5R were decreased, while D3R was increased, relative to lean rats. Under a 4% NaCl diet, D2R and D3R levels in obese rats were restored to lean levels. 4% NaCl diet reduced D5R in both body types, relative to 1% NaCl diet. Female rats had higher expression of D1R and D3R than did male; however, the sex difference for D1R was markedly blunted in obese rats. In obese rats, dietary candesartan (angiotensin II type 1 receptor blocker) normalized downregulated D1R and D2R, but either decreased (D3R), did not affect (D4R), or further downregulated (D5R) the other subtypes. Candesartan also decreased D4R in lean rats. In summary, reduced renal protein levels of D1R, D2R, D4R, and D5R in obese Zucker rats could induce salt sensitivity and elevate blood pressure. Increased angiotensin II type 1 receptor activity may be mechanistically involved in the decreased expression of D1R and D2R in obese rats. Finally, reduced D1R and D3R in male rats may contribute to sex differences in blood pressure.
Keywords: hypertension, insulin resistance, kidney, sex differences, salt sensitivity, natriuresis
dopamine regulates extracellular fluid volume and blood pressure by facilitating natriuresis. Dopamine receptors are divided into two subfamilies: D1-like (D1R and D5R, or D1A and D1B, respectively) and D2-like (D2R, D3R, and D4R). All five dopamine receptors are expressed along the renal tubule, often overlapping in sites such as the proximal tubule, thick ascending limb, and collecting duct (42). Dopamine receptors inhibit sodium transport in several renal tubule segments via PKA/PKC-dependent and -independent mechanisms that are specific to each receptor subtype. Dysregulation of dopamine receptors in the kidney leads to an impairment in the ability to excrete a sodium load, which may eventually lead to the development of hypertension (42). However, the contribution of each individual subtype to this impairment is not well defined.
Renal dopamine receptor function is impaired in obese humans (27), as well as in the obese Zucker rat (31), a rodent model of human metabolic syndrome (19), a disorder that is increasing to epidemic proportions around the world (16). The impaired natriuretic effect of exogenously administered dopamine in obese Zucker rats has been reported to be caused by impaired ability of D1-like agonists to activate G proteins and inhibit renal sodium/hydrogen exchanger 3 and Na-K-ATPase activities in proximal tubule (8, 23, 31). On the other hand, increased activity through the D3R, a D2-like receptor, has been reported to be involved in renal injury in obese spontaneously hypertensive NIH corpulent rats (18).
Under conditions of positive sodium balance, at least 50% of urinary sodium excretion is caused by activation of renal D1-like receptors (10, 15, 25, 37). Furthermore, pharmacological inhibition of D1-like receptors has been demonstrated to decrease basal renal sodium excretion (10). However, because there are no commercially available drugs that can distinguish D1R from D5R, it is not known if either or both receptors are involved in the increased sodium excretion associated with positive sodium balance. Moreover, while D5R−/− mice have been shown to be salt sensitive, with regard to blood pressure changes (45), this has not yet been determined for D1R−/− mice.
In contrast to the ability of D1-like receptor antagonists to decrease basal sodium excretion, nonselective pharmacological inhibition of D2-like receptors does not decrease renal sodium excretion in volume-expanded humans (2, 14) or rats (25). However, selective D3R antagonists do decrease the ability of rats to excrete a salt load. D3R−/− mice also have an impaired ability to excrete a sodium load (3, 38). Thus it is possible that the D3R may also be important in regulating the salt sensitivity of blood pressure.
Dopamine receptors interact with the renin-angiotensin-aldosterone system to regulate renal sodium transport and blood pressure (29, 42). We have clearly shown that chronic blockade of the angiotensin II (ANG II) type 1 receptor (AT1R) reduces blood pressure in both lean and obese Zucker rats (30). However, the effect of AT1R blockade on dopamine receptor regulation in these rats has not been tested.
For these studies, we hypothesized that dopamine receptor subtypes are differentially expressed in the kidneys of obese and lean rats. To test this, we quantified whole kidney protein expression of the five renal dopamine receptors in obese and lean rats on control and high-NaCl diets and, after treatment with the AT1R antagonist, candesartan. Because our laboratory has demonstrated that high-dietary NaCl intake increased blood pressure differentially in male vs. female rats (35), we determined whether renal dopamine receptor expression is sex specific in the obese Zucker rat model.
METHODS
Animals and study designs.
Young male and female, lean (FA/?) and obese (fa/fa) Zucker rats were purchased from Charles River Laboratories (Wilmington, MA) and housed primarily in groups of three in microfilter-top cages under standardized 12:12-h light-dark cycles with controlled temperature and humidity. Rats were fed either a moderate NaCl diet (1% NaCl, LabDiet, Purina Mills, St. Louis, MO) or a 4× higher NaCl diet (4% NaCl, custom-formulated, Harlan-Teklad, Madison, WI). In an additional study, male rats were randomized within each body type to receive either ground Purina 5001 diet (1% NaCl), in a gelled agar form (19), or this same diet plus added candesartan cilexetil (AstraZeneca, Wilmington, DE), an AT1R antagonist, at 5 mg·kg·body wt−1·day−1. Diets were fed chronically from 5–14 wk before euthanizing the animals. There were five to six rats in each treatment group. All studies were preapproved and adhered to the guidelines of the Georgetown Animal Care and Use Committee, an Association for Assessment and Accreditation of Laboratory Animal Care International approved facility.
Preparation of kidney samples.
Immediately after euthanization, both kidneys were removed, and the right kidney was homogenized in 10-ml ice-cold isolation buffer (13) using a homogenizer with a saw-tooth generator. Samples of the whole kidney homogenates were prepared for immunoblotting, as previously described (13). Before immunoblotting, “loading gels” were run with equal amounts of protein, as determined by a bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL) and stained with Coomassie brilliant blue dye (BioRad) to ascertain protein loading and integrity.
Primary antibody preparation and immunoblotting.
The design and characterization of the peptide-targeted rabbit polyclonal primary antibodies against D1R, D3R, and D5R have already been reported (28, 36, 48). For D4R, we used a similar strategy to select a specific immunogenic peptide, i.e., CRRKRGAKITGRERKAMRV, which was synthesized by Lofstrand Laboratories (Gaithersburg, MD). Polyclonal antibody production proceeded at Antibodies (Davis, CA). Antibodies were affinity purified in our laboratory from whole serum using peptide-bound columns (Pierce Biotechnology, Rockford, IL). All antibodies were tested for specificity using tissues derived from their respective dopamine receptor knockout mice and in human embryonic kidney-293 cells transfected to overexpress the receptor, as well as by peptide-preadsorption experiments. The D2R antibody was purchased from Chemicon (Millipore). For loading correction, we reprobed with either a β-actin antibody, a polyclonal rabbit (Sigma), or a GAPDH antibody, a monoclonal mouse (Chemicon).
For immunoblotting, kidney protein samples were separated on 10% SDS-PAGE and transferred onto nitrocellulose membranes. The membranes were incubated with the primary antibodies overnight at 4°C, washed, and then exposed to a secondary antibody [goat anti-rabbit IgG conjugated with horseradish peroxidase at a 1:10,000 dilution (Pierce)] for 1 h at room temperature. Signals of the antibody-antigen reaction were visualized with a luminol-based, enhanced chemiluminescence substrate (LumiGLO, Kirkegaard and Perry Laboratories, Gaithersburg, MD) after exposure to X-ray film (13).
Statistical analysis.
Specific bands on the developed films were scanned using a standard flat-bed scanner, and their densities quantified using NIH Image J software (National Institutes of Health, Bethesda, MD). The protein band densities were normalized by the corresponding β-actin or GAPDH densities upon reprobe. The results are reported as percentage of the mean of the control groups. Two-sided unpaired Student's t-test was used for two-group comparisons, with P < 0.05 considered as significant. For three or more groups, one- or two-way ANOVA was used. A significant one-way ANOVA was followed by the multiple-comparisons test, Holm-Sidak or Dunns, to ascertain differences between pairs of means.
RESULTS
Obese Zucker rats have reduced renal protein levels of all dopamine receptors, except D3R.
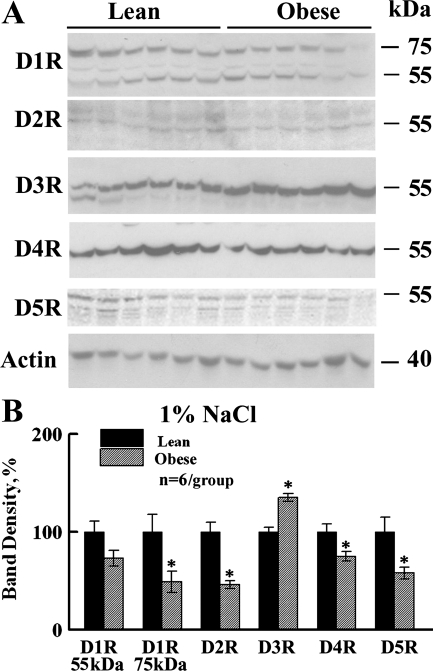
Figure 1 shows representative Western blots (A) and the densitometry summary (B) for dopamine receptor subtypes in lean and obese male rats fed a moderate 1% NaCl diet. For D1R, we detected a band at 55 kDa (immature) and an additional band at around 75 kDa (glycosylated) (47) in lean and obese rat whole-kidney homogenates. Both D1R band regions (55 and 75 kDa) were decreased in obese rat kidneys; the 75-kDa band significantly so, to a level ∼40% that of the lean rats (Fig. 1). D5R (the other D1-like receptor) was also significantly decreased (by ∼30%) in the obese rats. Among the D2-like receptors, D2R and D4R band densities were also significantly decreased; however, D3R expression was significantly increased (∼35%) in the obese rat kidneys.
Fig. 1.
Dopamine receptor protein levels in lean and obese Zucker rats under 1% NaCl diet. A: representative immunoblots of dopamine receptor subtypes (D1R, D2R, D3R, D4R, D5R) and β-actin in whole kidney homogenates from lean and obese male Zucker rats (n = 6/group) on 1% NaCl diet. Each lane was loaded with a sample from a different rat. B: bar graph of the densitometric analyses of all of the immunoblots (means ± SE, normalized by actin, shown as percentage of lean). *P < 0.05, lean vs. obese rats, Student's t-test. All dopamine receptors, except D3R, were decreased in the kidneys from obese relative to lean male rats.
A fourfold increase in dietary NaCl does not enhance these changes.
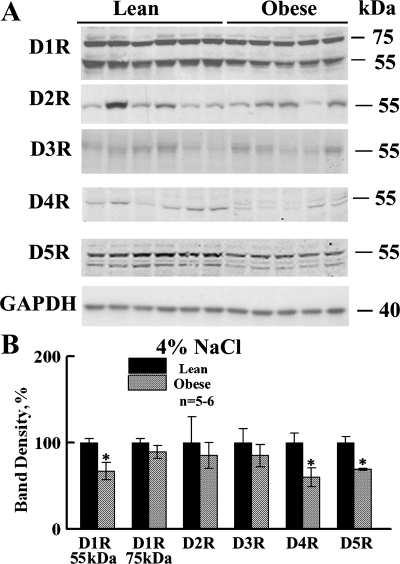
Figure 2 shows that high-NaCl diet did not exacerbate differences in dopamine receptor levels between the body types, despite greater salt sensitivity of blood pressure in the obese rats (35). In fact, there was a tendency for some differences to be attenuated or reversed. The ∼55-kDa bands associated with D1R, D5R, and D4R continued to be decreased in obese, relative to lean, male Zucker rats. However, the 75-kDa band of D1R was not decreased, nor was the band associated with D2R. Furthermore, D3R was no longer increased in the obese relative to lean rats.
Fig. 2.
Dopamine receptor protein levels in lean and obese Zucker rats under 4% NaCl diet. A: representative immunoblots of dopamine receptor subtypes and GAPDH in whole kidney homogenates from lean and obese male Zucker rats on 4% NaCl diet (n = 5 or 6/group). Each lane was loaded with a sample from a different rat. B: bar graph of the densitometric analyses of all of the immunoblots (means ± SE, normalized by GAPDH, shown as percentage of lean). *P < 0.05, lean vs. obese rats, Student's t-test. The decrease in D1R, D5R, and D4R seen on 1% NaCl diet persisted, but was not enhanced, with the increased salt diet.
Effects of high-NaCl diet, per se, on receptor expression.
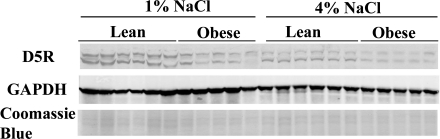
Using two-way analysis of variance, we also compared the two salt levels to each other with regard to their effects on the expression of renal dopamine receptors and their interactions with body type (Table 1). An increase in NaCl reduced renal abundance of D5R substantially (by over 50%) in both body types (Fig. 3). There was also a significant reduction in D3R with the 4% NaCl diet (Table 1). This effect was mainly apparent in obese rats.
Table 1.
Effect of dietary NaCl level on dopamine receptor protein levels in lean and obese rats
| Group | D1R, 55 kDa | D1R, 75 kDa | D2R | D3R | D4R | D5R |
|---|---|---|---|---|---|---|
| 1% NaCl-lean | 100 ± 11 | 100 ± 18a | 100 ± 10a | 100 ± 5a,b | 100 ± 8 | 100 ± 8a |
| 1% NaCl-obese | 73 ± 8 | 49 ± 11b | 46 ± 4b | 135 ± 4a | 51 ± 7 | 45 ± 5b |
| 4% NaCl-lean | 110 ± 7 | 100 ± 5a | 118 ± 36a,b | 96 ± 15a,b | 124 ± 15 | 52 ± 1b |
| 4% NaCl-obese | 78 ± 11 | 93 ± 6a,b | 108 ± 20a | 88 ± 14b | 79 ± 15 | 37 ± 3b |
| Two-way ANOVA (P values) | ||||||
| Diet | 0.438 | 0.077 | 0.081 | 0.025* | 0.244 | <0.001* |
| Body type | 0.005* | 0.025* | 0.159 | 0.211 | 0.006* | <0.001* |
| Interaction | 0.771 | 0.072 | 0.325 | 0.058 | 0.388 | 0.001* |
Values are means ± SE for each group. D1R–D5R, dopamine receptors 1–5. Densitometry of bands for all groups was normalized to the mean of 1% NaCl-lean rats. For each protein, results of two-way ANOVA are shown below densitometry.
a,b,c After multiple comparisons tests, the letter “a” was assigned to the highest mean and all means not different from it, followed by “b” then “c”, etc. Overlapping letter designations indicate means are not different from each other, P < 0.05.
Significant P values.
Fig. 3.
Dopamine receptor D5 protein levels in lean and obese rats fed 1 vs. 4% NaCl diets. Representative immunoblots are shown of dopamine receptor D5R and GAPDH in whole kidney homogenates from lean and obese male Zucker rats (n = 5 or 6/group) on a 1 or 4% NaCl diet. Each lane was loaded with a sample from a different rat. Protein loading was similar in all samples confirmed by Coomassie blue staining (bottom).
Sex affects dopamine receptor expression levels.
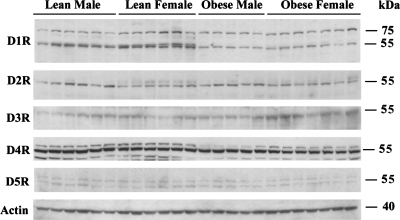
In Fig. 4 we show representative Western blots of dopamine receptors in obese and lean male and female rats on the 4% NaCl diet. Densitometry and statistics are shown in Table 2. Previously, our laboratory showed that blood pressure was increased by 20–30 mmHg with a switch from a 0.4% to a 4% NaCl diet in obese rats (20). This increase was observed in both sexes, although slightly attenuated in obese females. In the lean rats, males showed a small, but still significant, rise in blood pressure (mean approximately +5 mmHg), while lean females showed a slight, nonsignificant dip (mean approximately −4 mmHg) (20). We determined whether differential dopamine receptor expression might play a role in these sex differences in salt sensitivity of blood pressure. In agreement with potentially greater natriuretic capability, lean females had the highest expression of both the 55- and 75-kDa bands of D1R, relative to other groups. No sex differences in D1R were seen in the obese rats. The lean female rats also had a doublet 55-kDa D1R band that was not observed in the other groups; the significance of this band is unknown. There was no difference in D5R expression between female and male rats of either body type. Among D2-like receptors, D3R was also significantly higher in female rats, with a trend for this effect to be pronounced in obese rats (interactive term P = 0.075).
Fig. 4.
Effect of sex on renal dopamine receptor subtypes. Representative immunoblots are shown of dopamine receptor subtypes in whole kidney homogenates from lean and obese male and female rats on a 4% NaCl diet. Statistics for the densitometric analyses are shown in Table 2 (n = 5–7/group).
Table 2.
Effect of sex on dopamine receptor protein levels in lean and obese rats
| Group | D1R, 55 kDa | D1R, 75 kDa | D2R | D3R | D4R | D5R |
|---|---|---|---|---|---|---|
| Lean male | 100 ± 9b | 100 ± 12b | 100 ± 16 | 100 ± 19b | 100 ± 3a,b | 100 ± 9a,b |
| Lean female | 136 ± 6a | 192 ± 32a | 124 ± 9 | 130 ± 17b | 108 ± 8a | 108 ± 11a |
| Obese male | 45 ± 4c | 107 ± 8b | 132 ± 11 | 142 ± 15b | 72 ± 1b | 74 ± 11a,b |
| Obese female | 48 ± 5c | 133 ± 10a,b | 134 ± 6 | 241 ± 19a | 74 ± 4b | 69 ± 6b |
| Two-way ANOVA (P values) | ||||||
| Sex | 0.009* | 0.004* | 0.237 | 0.002* | 0.059 | 0.918 |
| Body type | <0.0001* | 0.16 | 0.063 | <0.001* | <0.001* | 0.002* |
| Interaction | 0.023* | 0.077 | 0.337 | 0.075 | 0.583 | 0.433 |
Values are means ± SE for each group. Densitometry of bands for all groups was normalized to the mean of lean male rats. For each protein, results of two-way ANOVA are shown below densitometry.
a,b,c After multiple comparisons tests, the letter “a” was assigned to the highest mean and all means not different from it; followed by “b” then “c”, etc. Overlapping letter designations indicate means are not different from each other, P < 0.05.
Significant P values.
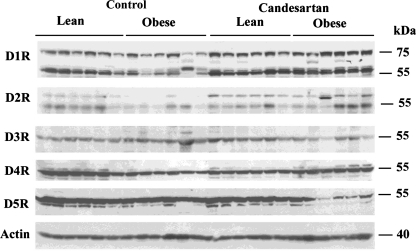
Candesartan mainly affects expression in obese rats.
To determine whether blockade of AT1R would normalize altered renal D1-like and D2-like receptors in the male obese rats, the dopamine receptor subtypes in the kidney were quantified in rats treated with control or candesartan-supplemented diet (Fig. 5). Density summary and statistics are shown in Table 3. Candesartan had only one significant effect in lean rats in that it reduced D4R levels. In obese rats, it basically normalized the expression of D1R (both bands) and D2R, so that densities were no longer significantly different from those of lean controls. It caused a reduction in D3R, which was originally increased in the obese rats. It had no effect on the existing lower level of D4R, and caused a further reduction in D5R.
Fig. 5.
Effect of candesartan treatment on renal dopamine receptor subtypes in male rats. Representative immunoblots of dopamine receptor subtypes are shown in whole kidney homogenates from lean and obese male rats treated with 1% NaCl diet plus or minus candesartan. Statistics for the densitometric analyses are shown in Table 3 (n = 5–7/group).
Table 3.
Effect of candesartan on dopamine receptor protein levels in lean and obese rats
| Group | D1R, 55 kDa | D1R, 75 kDa | D2R | D3R | D4R | D5R |
|---|---|---|---|---|---|---|
| Lean control | 100 ± 10a | 100 ± 10 | 100 ± 10a | 100 ± 6a | 100 ± 8a | 100 ± 5a |
| Obese control | 56 ± 7b | 62 ± 11 | 32 ± 5b | 115 ± 7a | 38 ± 6b | 68 ± 6b |
| Lean candesartan | 109 ± 6a | 102 ± 10 | 79 ± 9a,b | 116 ± 12a | 52 ± 4b | 85 ± 7a,b |
| Obese candesartan | 90 ± 6a | 105 ± 13 | 102 ± 19a | 53 ± 11b | 56 ± 4b | 27 ± 7c |
| Two-way ANOVA (P values) | ||||||
| Treatment | 0.011* | 0.056 | 0.062 | 0.025* | 0.022* | <0.001* |
| Body type | <0.001* | 0.127 | 0.086 | 0.021* | <0.001* | <0.001* |
| Interaction | 0.126 | 0.083 | 0.001* | <0.001* | <0.001* | 0.06 |
Values are means ± SE for each group. Densitometry of bands for all groups was normalized to the mean of lean control rats. For each protein, results of two-way ANOVA are shown below densitometry.
a,b,c After multiple comparisons tests, the letter “a” was assigned to the highest mean and all means not different from it; followed by “b” then “c”, etc. Overlapping letter designations indicate means are not different from each other, P < 0.05.
Significant P values.
DISCUSSION
The renal dopaminergic system is important in the regulation of renal sodium handling, especially during states of positive sodium balance (10, 15, 25, 37). A decreased renal production of dopamine, dopamine receptor density, and/or an uncoupling of the dopamine receptors from their G protein effector complexes has been described in essential hypertension (6, 42) and in the metabolic syndrome (11, 33). However, endogenous renal dopamine production is normal or even increased in obese humans (46), monkeys (43), and rats (39). Therefore, the dopaminergic defect in the obese male rat has been suggested to be at the receptor or postreceptor level (4, 5, 7, 23, 24, 40). Our finding of decreased renal expression of D1R in obese male rats provides a potential explanation for the reduced D1-like dopaminergic signaling previously reported in the proximal tubule of these rats (5, 31). Furthermore, obese Zucker rats also had reduced expression of D5R. Our laboratory has shown in several reports that deletion of the D5R gene in mice, regardless of sex, is similarly associated with an elevation in blood pressure (1, 21, 29, 45).
Among the D2-like receptors, renal D2R and D4R expression were also decreased in the obese, relative to lean, rats. D2R binding in various areas of the brain has been reported to be decreased in morbidly obese male and female humans (41), obese male Zucker rats (12), male Otsuka Long Evans Tokushima Fatty rats (20), and obesity-prone female rats (17). Disruption of D2R or D4R in mice generates mice with normal plasma renin levels, but AT1R-related hypertension (41, 42). Thus the decreased protein expression of those two receptors may also have contributed to the pathogenesis of hypertension in the obese rats. In contrast to the decreased expression of the other D2-like receptors, renal D3R expression was increased in obese relative to lean rats. Because a high-fat diet increased body fat in D3−/− mice (32), the increase in D3R expression could represent a compensatory mechanism.
High-salt intake increased blood pressure to a greater extent in obese than in lean Zucker rats (35). On the high-salt diet, differences between the body types for the expression of most receptor subtypes were maintained, but were not exacerbated. Thus a greater “decrease” in dopamine receptor subtypes in the obese rats did not account for the difference in blood pressure between genotypes with high NaCl. In other words, the NaCl in the diet did not cause the fall in dopamine receptor subtype expression, except D5R, which is decreased to a similar extend in lean or obese rats by the salt loading (Table 1). However, the marginal dopamine-mediated natriuresis in the obese rats may have become “physiologically” more relevant with a 4× higher NaCl load that needed to be excreted. Among the D2-like receptors, only the expression of D4R continued to be decreased in obese male rats on 4% NaCl diet (compared with the 1% NaCl diet, Table 1). Because the D3R interacts with D1-like receptors to increase sodium excretion (26), it is possible that the increase in the expression of D3R and D2R with high-NaCl diet in obese rats is a compensatory response to the persistent reduction in D1-like receptor expression.
We also found that renal dopamine receptor expression was influenced by sex. Lean males had reduced levels of both the 55- and 75-kDa bands associated with D1R, compared with the lean females. This reduction in D1R in these males may have accounted for the modest salt sensitivity of blood pressure observed in these rats, compared with their lean female counterparts (35). This modest salt sensitivity of lean male Zucker rats confirmed other reports (9). The absence of a sex difference in D1R protein levels in obese rats could not be explained by a reduction in circulating estradiol levels in the obese females, since we showed these levels were not significantly different between lean and obese female rats (34). On the other hand, both obese and lean females had greater levels of D3R than their male counterparts. Mechanisms underlying these sex differences remain to be determined.
AT1R expression is increased in the kidney of obese male Zucker rats relative to lean (22, 44). AT1R blockade in the obese rats increased to lean level expression of D1R and D2R. In contrast, candesartan did not correct, but led to a further reduction in D5R expression (Table 3). It also decreased the expression of D3R, which was originally elevated. These results are surprising, because, in nonobese mice, AT1R and D5R and AT1R and D3R counterregulate each other's expression (42). It is possible that the interactions between dopamine receptors and AT1R are altered by the obese state (see below) or that a decrease in D5R expression is innate to obese Zucker rats, while decreased expression of the D1R is secondary, as has been reported (5).
Except for a decrease in D4R, candesartan did not affect renal dopamine receptor expression in lean rats. D4R negatively regulates AT1R, but whether the converse occurs is not known. It is possible that, in lean rats, AT1R increases D4R expression that can serve as a brake for a further increase in its own expression.
In summary, our studies show a reduction in the renal expression of the dopamine receptor subtypes, except for D3R, in Zucker obese rats fed a moderate NaCl diet. This downregulation could contribute to the salt sensitivity of blood pressure in these rats. AT1R blockade increased the expression of D1R and D2R and reduced D3R and D5R expression; however, the reduced levels of D4R in the obese rats were resistant to candesartan, suggesting that increased renal AT1R activity in obese rats may have a role in some, but not all, of the observed dopamine receptor alterations. Finally, female Zucker rats had higher D1R and D3R than did male rats. This may have contributed to reduced salt sensitivity of blood pressure in female rats.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grants HL073193 and HL074142 and the American Heart Association Established Investigator Award (C. M. Ecelbarger). X. Wang received salary support from Georgetown University and National Institute of Diabetes and Digestive and Kidney Diseases grant DK39308 (P. A. Jose).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Albrecht FE, Drago J, Felder RA, Printz MP, Eisner GM, Robillard JE, Sibley DR, Westphal HJ, Jose PA. Role of the D1A dopamine receptor in the pathogenesis of genetic hypertension. J Clin Invest 97: 2283–2288, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen MJ, Ang VT, Bennett ED. Domperidone, a DA2-specific dopamine antagonist, has no effect on the renal or haemodynamic response to atrial natriuretic peptide in man. Clin Sci (Lond) 75: 569–575, 1988 [DOI] [PubMed] [Google Scholar]
- 3.Asico LD, Ladines C, Fuchs S, Accili D, Carey RM, Semeraro C, Pocchiari F, Felder RA, Eisner GM, Jose PA. Disruption of the dopamine D3 receptor gene produces renin-dependent hypertension. J Clin Invest 102: 493–498, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine-mediated inhibition of renal Na,K-ATPase is reduced by insulin. Hypertension 41: 1353–1358, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D(1) receptor dysfunction is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol 287: F109–F116, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep 10: 268–275, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor-G-protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Beheray SA, Hussain T, Lokhandwala MF. Dopamine inhibits Na,H-exchanger via D1-like receptor-mediated stimulation of protein kinase a in renal proximal tubules. Clin Exp Hypertens 22: 635–644, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Carlson SH, Shelton J, White CR, Wyss JM. Elevated sympathetic activity contributes to hypertension and salt sensitivity in diabetic obese Zucker rats. Hypertension 35: 403–408, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chen CJ, Lokhandwala MF. An impairment of renal tubular DA-1 receptor function as the causative factor for diminished natriuresis to volume expansion in spontaneously hypertensive rats. Clin Exp Hypertens A 14: 615–628, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Contreras F, Fouillioux C, Bolivar A, Simonovis N, Hernandez-Hernandez R, Armas-Hernandez MJ, Velasco M. Dopamine, hypertension and obesity. J Hum Hypertens 16, Suppl 1: S13–S17, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Davis LM, Michaelides M, Cheskin LJ, Moran TH, Aja S, Watkins PA, Pei Z, Contoreggi C, McCullough K, Hope B, Wang GJ, Volkow ND, Thanos PK. Bromocriptine administration reduces hyperphagia and adiposity and differentially affects dopamine D2 receptor and transporter binding in leptin-receptor-deficient Zucker rats and rats with diet-induced obesity. Neuroendocrinology 89: 152–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Farrow S, Mahayni A, Banta G, Sowers J, Lockette W. Metoclopramide does not inhibit atrial natriuretic factor-stimulated diuresis in man. Aviat Space Environ Med 67: 248–252, 1996 [PubMed] [Google Scholar]
- 15.Felder RA, Seikaly MG, Cody P, Eisner GM, Jose PA. Attenuated renal response to dopaminergic drugs in spontaneously hypertensive rats. Hypertension 15: 560–569, 1990 [DOI] [PubMed] [Google Scholar]
- 16.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287: 356–359, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J 22: 2740–2746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross ML, Koch A, Muhlbauer B, Adamczak M, Ziebart H, Drescher K, Gross G, Berger I, Amann KU, Ritz E. Renoprotective effect of a dopamine D3 receptor antagonist in experimental type II diabetes. Lab Invest 86: 262–274, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM. Metabolic syndrome: therapeutic considerations. Handb Exp Pharmacol 170: 107–133, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hajnal A, Margas WM, Covasa M. Altered dopamine D2 receptor function and binding in obese OLETF rat. Brain Res Bull 75: 70–76, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hollon TR, Bek MJ, Lachowicz JE, Ariano MA, Mezey E, Ramachandran R, Wersinger SR, Soares-da-Silva P, Liu ZF, Grinberg A, Drago J, Young WS, 3rd, Westphal H, Jose PA, Sibley DR. Mice lacking D5 dopamine receptors have increased sympathetic tone and are hypertensive. J Neurosci 22: 10801–10810, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Tiwari S, Riazi S, Hu X, Wang X, Ecelbarger CM. Regulation of angiotensin II type I receptor (AT1R) protein levels in the obese Zucker rat kidney and urine. Clin Exp Hypertens 31: 49–63, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Hussain T, Becker M, Beheray S, Lokhandwala MF. Dopamine fails to inhibit Na,H-exchanger in proximal tubules of obese Zucker rats. Clin Exp Hypertens 23: 591–601, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hussain T, Beheray SA, Lokhandwala MF. Defective dopamine receptor function in proximal tubules of obese Zucker rats. Hypertension 34: 1091–1096, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Jose PA, Asico LD, Eisner GM, Pocchiari F, Semeraro C, Felder RA. Effects of costimulation of dopamine D1- and D2-like receptors on renal function. Am J Physiol Regul Integr Comp Physiol 275: R986–R994, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Kaneko S, Albrecht F, Asico LD, Eisner GM, Robillard JE, Jose PA. Ontogeny of DA1 receptor-mediated natriuresis in the rat: in vivo and in vitro correlations. Am J Physiol Regul Integr Comp Physiol 263: R631–R638, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Kikuchi K, Iimura O, Yamaji I, Shibata S, Nishimura M, Aoki K, Nozawa A, Hasegawa T, Honma C, Kobayakawa H, et al. The pathophysiological role of water-sodium balance and renal dopaminergic activity in overweight patients with essential hypertension. Am J Hypertens 1: 31–37, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D(1)-like and D(2)-like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol 281: R1071–R1078, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Li H, Armando I, Yu P, Escano C, Mueller SC, Asico L, Pascua A, Lu Q, Wang X, Villar VA, Jones JE, Wang Z, Periasamy A, Lau YS, Soares-da-Silva P, Creswell K, Guillemette G, Sibley DR, Eisner G, Gildea JJ, Felder RA, Jose PA. Dopamine 5 receptor mediates Ang II type 1 receptor degradation via a ubiquitin-proteasome pathway in mice and human cells. J Clin Invest 118: 2180–2189, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madala Halagappa VK, Tiwari S, Riazi S, Hu X, Ecelbarger CM. Chronic candesartan alters expression and activity of NKCC2, NCC, and ENaC in the obese Zucker rat. Am J Physiol Renal Physiol 294: F1222–F1231, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Marwaha A, Lokhandwala MF. Diminished natriuretic response to dopamine D1 receptor agonist, SKF-38393 in obese Zucker rats. Clin Exp Hypertens 25: 509–515, 2003 [DOI] [PubMed] [Google Scholar]
- 32.McQuade JA, Benoit SC, Xu M, Woods SC, Seeley RJ. High-fat diet induced adiposity in mice with targeted disruption of the dopamine-3 receptor gene. Behav Brain Res 151: 313–319, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol 480: 125–131, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Riazi S, Hu X, Madala Halagappa VK, Ecelbarger CA. Sex differences in blood pressure and kidney sodium transporters and channels in response to high salt diet in lean and obese Zucker rats (Abstract). FASEB J 20: A338, 2006 [Google Scholar]
- 35.Riazi S, Madala Halagappa VK, Dantas AP, Hu X, Ecelbarger CA. Sex differences in renal nitric oxide synthase (NOS), NAD(P)H oxidase, and blood pressure in obese Zucker rats. Gend Med 4: 1–16, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sanada H, Jose PA, Hazen-Martin D, Yu PY, Xu J, Bruns DE, Phipps J, Carey RM, Felder RA. Dopamine-1 receptor coupling defect in renal proximal tubule cells in hypertension. Hypertension 33: 1036–1042, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Siragy HM, Felder RA, Howell NL, Chevalier RL, Peach MJ, Carey RM. Evidence that intrarenal dopamine acts as a paracrine substance at the renal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 257: F469–F477, 1989 [DOI] [PubMed] [Google Scholar]
- 38.Staudacher T, Pech B, Tappe M, Gross G, Muhlbauer B, Luippold G. Arterial blood pressure and renal sodium excretion in dopamine D3 receptor knockout mice. Hypertens Res 30: 93–101, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Svec F, Thompson H, Corll C, Porter J. Levels of hypothalamic neurotransmitters in lean and obese Zucker rats. Nutr Neurosci 5: 321–326, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Umrani DN, Banday AA, Hussain T, Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese Zucker rats. Hypertension 40: 880–885, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42: 1537–1543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Villar VA, Armando I, Eisner GM, Felder RA, Jose PA. Dopamine, kidney, and hypertension: studies in dopamine receptor knockout mice. Pediatr Nephrol 23: 2131–2146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolden-Hanson T, Davis GA, Baum ST, Kemnitz JW. Insulin levels, physical activity, and urinary catecholamine excretion of obese and non-obese rhesus monkeys. Obes Res 1: 5–17, 1993 [DOI] [PubMed] [Google Scholar]
- 44.Xu ZG, Lanting L, Vaziri ND, Li Z, Sepassi L, Rodriguez-Iturbe B, Natarajan R. Upregulation of angiotensin II type 1 receptor, inflammatory mediators, and enzymes of arachidonate metabolism in obese Zucker rat kidney: reversal by angiotensin II type 1 receptor blockade. Circulation 111: 1962–1969, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Yang Z, Asico LD, Yu P, Wang Z, Jones JE, Escano CS, Wang X, Quinn MT, Sibley DR, Romero GG, Felder RA, Jose PA. D5 dopamine receptor regulation of reactive oxygen species production, NADPH oxidase, and blood pressure. Am J Physiol Regul Integr Comp Physiol 290: R96–R104, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Young JB, Troisi RJ, Weiss ST, Parker DR, Sparrow D, Landsberg L. Relationship of catecholamine excretion to body size, obesity, and nutrient intake in middle-aged and elderly men. Am J Clin Nutr 56: 827–834, 1992 [DOI] [PubMed] [Google Scholar]
- 47.Yu P, Yang Z, Jones JE, Wang Z, Owens SA, Mueller SC, Felder RA, Jose PA. D1 dopamine receptor signaling involves caveolin-2 in HEK-293 cells. Kidney Int 66: 2167–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 48.Zeng C, Yang Z, Wang Z, Jones J, Wang X, Altea J, Mangrum AJ, Hopfer U, Sibley DR, Eisner GM, Felder RA, Jose PA. Interaction of angiotensin II type 1 and D5 dopamine receptors in renal proximal tubule cells. Hypertension 45: 804–810, 2005 [DOI] [PubMed] [Google Scholar]