Abstract
The mammalian aquaporins AQP1, AQP4, and AQP5 have been shown to function not only as water channels but also as gas channels. Zebrafish have two genes encoding an AQP1 homologue, aqp1a and aqp1b. In the present study, we cloned the cDNA that encodes the zebrafish protein Aqp1a from the 72-h postfertilization (hpf) embryo of Danio rerio, as well as from the swim bladder of the adult. The deduced amino-acid sequence of aqp1a consists of 260 amino acids and is 59% identical to human AQP1. By analyzing the genomic DNA sequence, we identified four exons in the aqp1a gene. By in situ hybridization, aqp1a is expressed transiently in the developing vasculature and in erythrocytes from 16 to 48 h of development. Later, at 72 hpf, aqp1a is expressed in dermal ionocytes and in the swim bladder. Western blot analysis of adult tissues reveals that Aqp1a is most highly expressed in the eye and swim bladder. Xenopus oocytes expressing aqp1a have a channel-dependent (*) osmotic water permeability (Pf*) that is indistinguishable from that of human AQP1. On the basis of the magnitude of the transient change in surface pH (ΔpHS) that were recorded as the oocytes were exposed to either CO2 or NH3, we conclude that zebrafish Aqp1a is permeable to both CO2 and NH3. The ratio (ΔpHS*)CO2/Pf* is about half that of human AQP1, and the ratio (ΔpHS*)NH3/Pf* is about one-quarter that of human AQP1. Thus, compared with human AQP1, zebrafish Aqp1a has about twice the selectivity for CO2 over NH3.
Keywords: Xenopus oocyte, gas permeability, surface pH, intracellular pH
the zebrafish, danio rerio, is a fresh-water teleost that, for several reasons, has become a popular laboratory animal model for studying issues relevant to human physiology and disease. It is small enough to easily contain, is easy to breed, and it has a short-generation interval. The rapidly developing zebrafish embryo develops externally, and both the embryo and newly hatched larvae are transparent. The heart starts to beat 24 h postfertilization (hpf). Most other organs form and start to function within 5 days (for review, see Ref. 29).
The aquaporins exist in two major subfamilies (for review, see Ref. 1), the orthodox AQPs and the aquaglyceroporins, which also transport glycerol and other small molecules. AQP8, AQP11, and AQP12 do not appear to fall into either family. The first orthodox aquaporin to be cloned from a fish was an AQP0 homologue (54) from the lens of the killifish (Fundulus heteroclitus). The first AQP1 homologue (2) cloned from a fish was from the Japanese eel (Anguilla japonica). Northern blot analysis indicated that mRNA expression is relatively high in heart, intestine, spleen, and swim bladder. Immunohistochemistry shows that AQP1 is located in apical surface of the epithelial cells in the mucosa along the posterior intestine. Thus, this AQP1 may play an important role in water absorption and osmoregulation in the intestine of eels adapted to seawater. Two AQP1 homologues (28) have been cloned from the European eel (Anguilla anguilla), and another, SaAQP1o (12), from the ovarian tissue of a marine teleost, the gilthead sea bream (Sparus aurata). This SaAQP1o mediates oocyte hydration.
Two AQP1 homologues exist in zebrafish. We deposited the sequence of aqp1a cDNA in 2006 (accession no. DQ887675). More recently, Tingaud-Sequeira et al. (49) reported the sequence of aqp1b. Moreover, they used RT-PCR to describe the distribution of Aqp1b in adult zebrafish tissues and determined the osmotic water permeability of both Aqp1a and Aqp1b heterologously expressed in Xenopus oocytes (49).
Several aquaglyceroporins have also been cloned from fish. Homologues of mammalian AQP3 have been identified in the sea bream (43) and Mozambique tilapia (Oreochromis mossambicus) (58). A zebrafish aqp3 sequence also has been identified (NM_001004661). As is the case for the duplicated teleost homologues of mammalian AQP1 genes, the homologues of mammalian AQP3 genes are duplicated in some species of teleost fishes (7). A zebrafish AQP8 homologue (46) has been cloned, and its expression pattern determined by in situ hybridization. Finally, a homologue of mammalian aquaglyceroporins has been cloned from the European eel and named AQPe (28).
In the present study, we describe how we used RT-PCR to clone aqp1a cDNA from the total RNA of 72-hpf embryos, as well as from the swim bladder of adult zebrafish. In situ hybridization at 16–48 hpf reveals expression in developing vasculature and erythrocytes, and at 72-hpf, it shows expression in dermal ionocytes and swim bladder. Western blot analysis on tissues from adult zebrafish, using an anti-eel AQP1 antibody, indicates high levels of expression in brain, eye, gill, and swim bladder. Physiological experiments on Xenopus oocytes expressing of Aqp1a demonstrate permeability of Aqp1a to H2O, CO2, and NH3. Compared with human AQP1, which has been studied in a previous study (30), Aqp1a has about twice the selectivity for H2O over CO2, a four-fold higher selectivity for H2O over NH3, but about twice the selectivity for CO2 over NH3.
MATERIALS AND METHODS
Cloning of aqp1a cDNA
We amplified aqp1a from total-embryo (72 hpf) RNA and adult swim bladder by RT-PCR using the reverse primer 5′-GTAAATGCTACTTCCCTGCGGGGAC for first-strand cDNA synthesis, and the forward primer 5′-CACAGATTAGAGGCGTCAGTCCGTCAG, and the reverse primer 5′-GCTTTTTTTACATTTGGAATTTCCACACTGTC for PCR. The RT-PCR product was cloned into pTOPO2.1 (Invitrogen, Carlsbad, CA) for sequencing. The aqp1a cDNA was then subcloned into the Xenopus expression vector pGH19 for mRNA synthesis (pGH19-aqp1a). We attempted to amplify aqp1b from the above two tissues using the reverse primer 5′-GTGCTGCTATTAAGCATCGCCATACC for first-strand cDNA synthesis, and the forward primer 5′-GCTAACGTTTTCATTTACAAGCTCAAACTCAG and the reverse primer 5′-CGCTTCCATTGGTTCAAATTTAAGTTAGCAACAC for PCR.
In Situ Hybridization
Whole-mount in-situ hybridization was performed as previously described (48). All procedures involving housing and use of zebrafish have been approved by the Massachusetts General Hospital Subcommittee on Research Animal Care. A 700-bp fragment of aqp1a (nucleotides −94 to +606 relative to the ATG initiation codon) was subcloned in pCRII-TOPO (Invitrogen, Carlsbad, CA). For antisense probe synthesis, pCRII-aqp1a was linearized with NotI and incubated with SP6 RNA polymerase. Whole-mount stained embryos cleared in benzyl benzoate:benzyl alcohol and photographed on a Leica MZ12 stereomicroscope equipped with a Spot digital camera (Diagnostic Instruments). For histological sections, whole mount in situ embryos were embedded in JB-4 glycolomethacrylate (Polysciences), sectioned with glass knives, and photographed on a Nikon E800 microscope.
Antibodies
Affinity-purified anti-European eel AQP1 antibody (28) was kindly provided by Dr. Gordon Cramb (University of St. Andrews, Scotland, UK). Anti-Japanese eel AQP1 antibody (2) was kindly provided by Dr. Toyoji Kaneko (University of Tokyo, Japan). Mouse anti-actin monoclonal antibody was purchased from Chemicon (Chemicon International, Temecula, CA).
Protein Preparation and Western Blot Analysis
Adult zebrafish.
All procedures involving tissue collections have been approved by the Institutional Animal Care and Use Committees at Yale University and Case Western Reserve University. Tissues of brain, eye, gill, swim bladder, intestine, oocytes, and muscle were isolated from adult zebrafish and placed in K-HEPES buffer containing 1% of protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The tissues were then homogenized. The whole homogenate was stored in −80°C until use.
Xenopus oocytes.
All procedures involving oocyte isolation have been approved by the Institutional Animal Care and Use Committees at Yale University and Case Western Reserve University. About 15 oocytes were pooled in 1 ml of Na-phosphate buffer containing 7.5 mM NaH2PO4, 250 mM sucrose, 5 mM EDTA, 5 mM EGTA, pH 7.0, and 1% protease inhibitor cocktail for mammalian tissues (Sigma-Aldrich). Within ∼1 min, the oocytes were homogenized and then centrifuged at 3,000 g (6,000 rpm) on a desktop centrifuge at 4°C for 15 min. The supernatant was ultracentrifuged at 100,000 g at 4°C for 1 h. The pellet was resuspended in a protein suspension buffer containing 5% SDS, 20 mM Tris-HCl, 5 mM EDTA, pH 8.0. The concentration of membrane protein preparation was measured using Pierce BCA reagent (Pierce, Rockford, IL). The membrane protein preparations were stored in aliquots at −80°C until use.
Western blot analysis.
Proteins were separated on 4–20% SDS gel and transferred onto a PVDF membrane. The blot was blocked overnight in 5% milk in Tris-buffered saline-Tween (TBST) buffer at 4°C and then incubated with primary antibody at a dilution of 1:4,000 in TBST containing 1% milk at room temperature for 2 h. The blot was then washed in TBST ×5 for 6 min, followed by incubation with secondary antibody in TBST containing 1% milk at room temperature for 1 h, followed by washing in TBST ×5 for 6 min. For detection, we exposed the blot to ECL plus Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ) for 5 min prior to exposure to X-ray film.
Expression of aqp1a Protein in Xenopus Oocytes
Oocytes were isolated from female Xenopus laevis frogs according to the methods described previously (15). Briefly, frogs were anesthetized in 0.2% MS-222 (ethyl 3-aminobenzoate methanesulfonate, Sigma-Aldrich). Ovarian lobes were removed and placed in 0-Ca solution (98 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.5). After being washed in 0-Ca solution ×5, the oocytes were dissociated and defolliculated by enzymatic digestion using 2 mg/ml type IA collagenase (Sigma-Aldrich) in 0-Ca solution. Stage V-VI oocytes were selected and kept at 18°C in sterile filtered OR3 medium that contained (per 2 liters) one pack of powdered Leibovitz L-15 media with l-glutamine (GIBCO-BRL, Carlsbad, CA), 100 ml of penicillin (10,000 U/ml) and streptomycin (10,000 U/ml) solution (Sigma-Aldrich), and 5 mM HEPES titrated to pH 7.5, with an osmolality 195 mOsm.
Capped cRNA was transcribed from the expression vector pGH19-aqp1a using Message Machine kit (Ambion, Austin, TX). Oocytes were injected with 50 nl of 0.2 μg/μl of cRNA-encoding Aqp1a.
Solutions for Physiological Assays
For ND96, the solution consisted of (in mM): 96 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, with a pH of 7.50, osmolality 195 mOsm. The hypotonic solution [for osmotic water permeability coefficient (Pf) assays] consisted of (in mM) 43 NaCl, 2 KCl, 1 MgCl2, 1.8 CaCl2, 5 HEPES, with a pH of 7.50 and an osmolality of 100 mOsm. For 5% CO2/33 mM HCO3− (for CO2 permeability), 33 mM NaHCO3 replaced 33 mM NaCl of the ND96 solution, and the solution was bubbled with 5% CO2. For 0.5 mM NH3/NH4+ in ND96 (for NH3 permeability), we first made 5.0 mM NH3/NH4+ ND96 by replacing 5 mM NaCl of ND96 with 5 mM NH4Cl and then diluted this solution 1:10 with ND96 solution.
Measurement of Oocyte Pf
A volumetric assay to measure oocyte water permeability was performed as previously described (40, 55). Oocytes were illuminated from below. In some experiments, oocytes were placed in a perfusion chamber, and the extracellular solution was switched from isotonic solution ND96 to the hypotonic solution to induce cell swelling. In other experiments, the oocytes were dropped into a petri dish containing the hypotonic solution. Photographs were obtained every 1 or 2 s. The volume and idealized surface area of the oocyte were calculated from the projection area of the oocyte, using an adjacent ball bearing for calibration, assuming the oocyte to be a sphere. The water permeability of the oocyte was calculated according to Eq. 1:
| (1) |
where d(V/Vo) is the rate of the volume increase, Δosm is the osmotic gradient across the oocyte membrane, and Vw is the molar volume of water. S is the area of the oocyte membrane, which was obtained by multiplying the idealized surface area by a factor of 9 (5).
To examine the inhibitory effect of p-chloromercuribenzene sulfonate (pCMBS) on the water permeability of Xenopus oocytes expressing Aqp1a, oocytes were preincubated for 20 min in ND96 solution containing 1 mM pCMBS (Sigma, St. Louis, MO) before switching to hypotonic solution containing 1 mM pCMBS. To examine the reversibility of the pCMBS inhibition, oocytes were incubated in ND96 solution containing 5 mM β-mercaptoethanol for 5 min following the treatment with pCMBS before switching to hypotonic solution.
pHi and pHS Measurements
Measurement of intracellular pH.
As described previously (27, 34, 51), pHi was measured using liquid-membrane pH-sensitive microelectrodes with tip diameters ∼1 μm. Briefly, the oocyte was impaled with two microelectrodes, one for measuring membrane potential (Vm amplified by a model 725 two-electrode oocyte voltage-clamp amplifier; Warner Instruments, Hamden, CT) and the other for measuring pHi (amplified by a model FD223 high-impedance electrometer; World Precision Instruments, Sarasota, FL). Intracellular pH (pHi) was obtained by subtracting the signal of the Vm electrode from that of the pH electrode. Data were acquired and analyzed using software written in-house. The system was calibrated with buffered pH standards at pH 6.0 and 8.0. An additional single-point calibration was performed with the standard ND96 solution of pH 7.50 in the bath before the oocyte was impaled. All oocytes had a spontaneous Vm at least as negative as −40 mV.
Measurement of surface pH. As described previously (11, 30, 31), we measured surface pH (pHS), using a liquid-membrane microelectrode with a tip diameter of 15–20 μm (amplified by a FD223 electrometer; World Precision Instruments, Sarasota, FL). The external reference electrode was a calomel half cell, bridged to the chamber via a glass micropipette filled with 3 M KCl (amplified by a model 750 electrometer; World Precision Instruments). pHS was obtained by subtracting the calomel signal from the pH signal. Virtual ground was established with an Ag/AgCl half cell (connected to the ISense input of the Warner voltage-clamp amplifier) bridged to the chamber by a second glass microelectrode filled with 3 M KCl, and positioned with its tip close to the oocyte. An ultrafine computer-controlled micromanipulator (model MPC-200 system, Sutter Instrument, Novato, CA) positioned the flat tip of the pHS electrode until it just touched the surface of the oocyte, and then to further advance it until a slight dimple was observed in the membrane (∼50 μm). Periodically, during the experiment, the electrode was withdrawn 200–300 μm from the oocyte to recalibrate it at the pH of the bulk extracellular fluid (pH 7.50). The bath surrounding the oocyte flowed at 3 ml/min, and the microelectrode tip, with respect to the flow of solution, was in the “shadow” of the oocyte.
Statistics and Data Analysis
Initial dpHi/dt.
The initial rate of change of pHi (dpHi/dt) was determined from the slope of a linear regression line fitted to the steepest portion of the pHi vs. time data, obtained soon after switching to the CO2-HCO3− solution.
Intrinsic intracellular buffering power.
We computed the intrinsic intracellular buffering power (βI, mM/[pH unit]) from the magnitude of the change in steady-state pHi produced by exposing the cells to a solution containing 5% CO2/33 mM HCO3−, as described previously (4):
| (2) |
Here, Δ[HCO3−]i is the steady-state increase in [HCO3−]i produced by the influx of CO2. The initial [HCO3−]i is assumed to be zero, and the final [HCO3−]i is computed from the steady-state pHi and the Henderson-Hasselbalch equation. ΔpHi is the magnitude of the fall in steady-state pHi caused by the exposure to the CO2-HCO3− solution.
Maximum pHS spike heights.
As described previously (30, 31), we determined the maximum magnitude (i.e., the “spike height” or ΔpHS) of the pHS transient that occurred after we applied extracellular CO2/HCO3− or 0.5 mM extracellular NH3/NH4+. The initial pHS, before the application of CO2/HCO3− or NH3/NH4+ (i.e., in HEPES buffer), was computed by comparing the voltage reading obtained with the electrode tip at the oocyte surface with the voltage reading obtained with the electrode tip in the bulk extracellular fluid (assumed to have a pH of 7.50). The maximum pHS during exposure to CO2/HCO3−, or the minimum pHS during exposure to NH3/NH4+ was computed by comparing the voltage reading (at a time corresponding to the highest or to the lowest pHS) obtained with the electrode tip at the oocyte surface with the voltage reading obtained a few minutes later (after pHS returned to near 7.50) when the electrode tip was again in the bulk extracellular fluid (assumed to have a pH of 7.50). The ΔpHS was the difference between the two pHS values, one obtained from the calibration in HEPES buffer and the other obtained from a separate calibration in the CO2-HCO3− or NH3-NH4+ solution.
Statistics.
Data are presented as means ± SE. To compare the difference between two means, Student's t-tests (two tails) were performed. To compare more than two means, one-way ANOVA and a Student-Newman-Keuls multiple-comparison test were performed by using KaleidaGraph (ver. 4, Synergy Software, Essex Junction, VT). P < 0.05 was considered significant.
RESULTS
Cloning of Zebrafish Aqp1a cDNA by RT-PCR
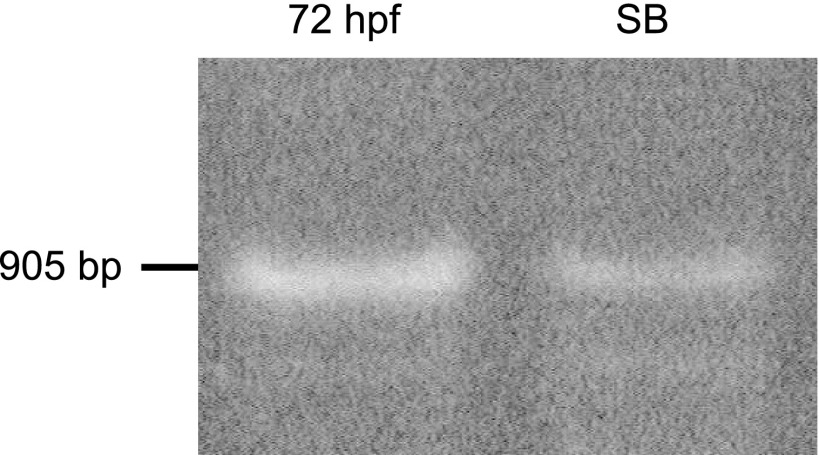
We obtained a 905-bp fragment by RT-PCR from 72 hpf embryo RNA (Fig. 1, lane 1). Because in situ hybridization studies (Expression of aqp1a During Embryogenesis) demonstrated aqp1a in the swim bladder, we likewise performed RT-PCR on the swim bladder of adult fish, also obtaining a 905-bp product (Fig. 1, lane 2). We cloned the two RT-PCR products (corresponding to lanes 1 and 2) into a TOPO vector and confirmed by DNA sequencing analysis. We failed to obtain the aqp1b cDNA by RT-PCR from either of the two RNA sources, even after attempting to optimize the experimental conditions. The sequence of aqp1a cDNA, which agrees with the predicted sequence from genomic DNA, has been deposited in GenBank (accession no. DQ887675).
Fig. 1.
Nested RT-PCR of zebrafish aqp1a. Lane 1 shows RT-PCR result from total RNA of 72-h postfertilization (hpf) embryo. Lane 2 shows RT-PCR result from total RNA of swim bladder of adult fish.
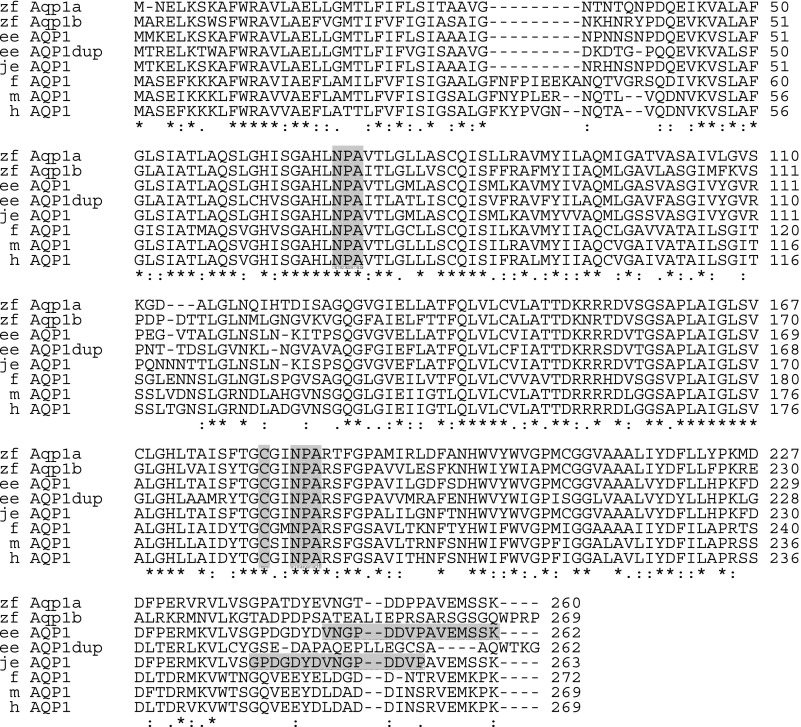
The top row in Fig. 2 shows the deduced sequence of the 260 amino acids of Aqp1a. The second row similarly shows the sequence for Aqp1b. Fig. 2 also shows the alignment of the Aqp1 sequences with AQP1 sequences from several other species. The deduced amino-acid sequence of zebrafish Aqp1a is 80% identical to European eel AQP1, 66% identical to zebrafish Aqp1b, 58% identical to frog AQP1, and 59% identical to human AQP1. Both zebrafish Aqp1a and Aqp1b have two NPA motifs, which is characteristic of aquaporins. Note that compared to Aqp1a (or European eel AQP1), Aqp1b (like the European eel AQP1dup) has an Ile-to-Val substitution after the first NPA motif (i.e., NPAITL instead of NPAVTL). Aqp1a has a Thr-to-Ser substitution after the second NPA motif (NPARTF instead of NPARSF). Both Aqp1a and Aqp1b have a Cys residue at a position that corresponds to the Cys189 in human AQP1, which is required for inhibition of human AQP1's water permeability by mercury (41).
Fig. 2.
Alignment of aquaporin 1 (AQP1) sequences from zebrafish and other selected species. Sequence alignment was performed using the online program ClustalW of European Bioinformatics Institute (EBI; http://www.ebi.ac.uk/clustalw/) with default gap parameters (GapOpen = 10.0, EndGap = −1, GapExtension = 0.2, and GapDistance = 4). GenBank accession numbers of the sequences analyzed are given in parentheses: zebrafish (zf, Danio rerio) aqp1a (DQ887675) and aqp1b (EU327345); European eel (ee, Anguilla anguilla) AQP1 (AJ564420) and AQP1dup (AJ564421); Japanese eel (je, Anguilla japonica) AQP1 (AB094501); frog (f, Rana esculenta) AQP1 (L24754); mouse (m, Mus musculus) AQP1 (BC007125); and human (h, homo sapiens) AQP1 (NP_000376). Gray columns indicate the two NPA motifs and the cysteine residue that are responsible for mercury inhibition of water transport. The two horizontal gray bars indicate the residues against which the two antibodies used in the present study were raised (2, 28). An asterisk (*) indicates that the amino acid residues are identical in all sequences in the alignment: a colon (:) indicates conserved substitutions: and a period (.) indicates semiconserved substitutions.
The human aquaporins include at least 13 members (AQP0-AQP12). These group into two large subfamilies (the orthodox aquaporins and aquaglyceroporins), a smaller subfamily (AQP11 and AQP12), and AQP8 (see supplemental data and Supplemental Fig. S1 in the online version of this article). Phylogenetic analysis indicates that both aqp1a and aqp1b group with human AQP1 in the orthodox aquaporin subfamily. Relative to other fish species, zebrafish aqp1a is the homologue of the European eel AQP1, whereas zebrafish aqp1b is the homologue of the eel's AQP1dup. The existence of two genes encoding human AQP1 homologues in some fish species could be the result of a whole-genome duplication that occurred in teleost evolution after divergence from higher vertebrate lineages (for reviews, see Refs. 22 and 53). Alternatively, the existence of aqp1 paralogs in fish could have arisen by local tandem chromosome duplication events. The zebrafish aqp1a gene has been mapped to chromosome 2 by radiation hybrid analysis (from the Zebrafish Information Network, University of Oregon, Eugene, OR; www.zfin.org). At this locus, both aqp1a and aqp1b are linked to a single scaffold (Zv6_scaffold172) in the same orientation and separated by ∼16 Kb. This observation is consistent with the hypothesis that zebrafish aqp1 paralogs arose by a local tandem duplication event as opposed to whole genome duplication.
Expression of aqp1a During Embryogenesis
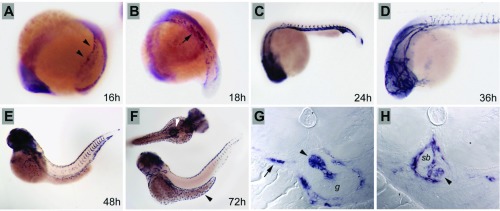
Zebrafish aqp1a is first expressed in the lateral mesoderm during somitogenesis (Fig. 3A), in a pattern that is similar to the known gene expression patterns of vasculogenic and hematopoietic markers (8). By 18 hpf (Fig. 3B), aqp1a-positive cells are observed converging at the embryo midline, where the first major blood vessels will form. By 24 hpf, aqp1a is strongly expressed in the dorsal aorta and axial vein (Fig. 3C); aqp1a expression is also seen at this stage in forming intersomitic vessels (Fig. 3C) and persists in cranial blood vessels at 36 hpf (Fig. 3D). By 48 hpf, aqp1a expression is downregulated in the major vessels of the trunk, and only the most recently formed, caudal intersomitic vessels continue to express aqp1a (Fig. 3E). At the same stage, individual aqp1a-positive cells are observed within the vasculature, suggesting that blood cells also express aqp1a (Fig. 3, E and H). By 72 hpf, aqp1a expression in the vasculature is nearly extinguished, while blood cells continue to express aqp1a (Fig. 3F). In addition, new sites of aqp1a expression are seen in the skin ionocytes and in cells associated with the forming swim bladder (Fig. 3F). Sections of 72 hpf embryos show strong expression of aqp1a in the pneumatic duct that links the gut to the swim bladder (Fig. 3G) and in blood vessels surrounding the swim bladder (Fig. 3H).
Fig. 3.
Expression of Aqp1a during embryogenesis. A: during somitogenesis, Aqp1a is expressed in the lateral mesoderm (arrowheads) and later is observed in cells converging at the midline to form the major trunk vessels (B; arrow). C: strong expression is observed at 24 hpf in the vasculature, including the forming intersomitic vessels. D: cranial vessels at 36 hpf are clearly delineated by Aqp1a expression. E: by 48 hpf, Aqp1a expression is downregulated in the vasculature but persists in single erythrocytes, here in the dorsal vasculature. F: ionocytes in the dermis over the yolk express aqp1a at 72 hpf (black arrowhead), while cells around the forming swim bladder also strongly express aqp1a (white arrowhead). G: histological sections of the forming swim bladder at 72 hpf show strong expression of aqp1a in the pneumatic duct (arrowhead) linking the gut (g) and the swim bladder and in erythrocytes in vessels (arrow). H: sections of the 72-hpf swim bladder (sb) show expression in the pneumatic duct where it joins the swim bladder (arrowhead) and in the vasculature surrounding the swim bladder.
Western Blot Analysis of Aqp1a in Adult Zebrafish Tissues
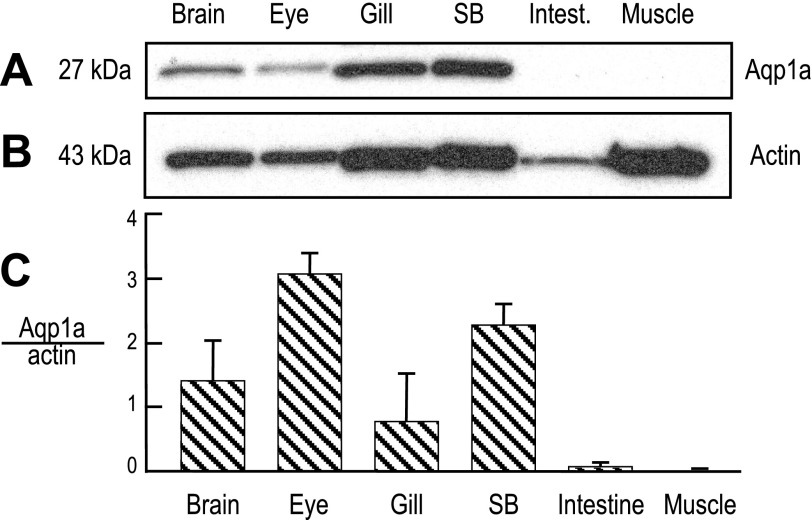
Figure 4A shows Western blots of tissues isolated from adult zebrafish, probed with an antibody generated against a peptide consisting of the last 15 amino acids (VNGPDDVPAVEMSSK) of the European eel AQP1 (28). Because this eel AQP1 sequence is virtually identical to that of Aqp1a (13 of 15 amino acids, Fig. 2), but very different from that of Aqp1b, we would expect the antibody to recognize Aqp1a, but not Aqp1b. Lanes 1–4 of Fig. 4A show that the antibody recognizes a band at ∼27 kDa, close to the predicted molecular weight (MW) of the Aqp1a monomer (i.e., 27.4 kDa). Although not shown, the antibody also recognizes a band at ∼96 kDa in lanes 1 (brain) and 6 (muscle) (see supplemental data and Supplemental Figs. S2 and S3 in the online version of this article). Densitometry of the 27-kDa band, normalized to that of actin (Fig. 4B), reflects the relative abundance of Aqp1a protein in zebrafish tissues. Note that, although we loaded equal amounts of protein in each lane, the actin level was not uniform among the tissues and was particularly low in the intestine. As summarized in Fig. 4C, Aqp1a protein is most abundant in eye and swim bladder but less so in brain and gill. We observed no signal for Aqp1a in intestine or muscle.
Fig. 4.
Expression of Aqp1a in adult zebrafish tissues. Proteins (10 μg per lane) from whole homogenates of zebrafish tissues were separated by SDS-PAGE and prepared for Western blot analysis. The blot was double-stained with an antibody directed against European eel AQP1 and an anti-actin antibody. A: Western blot analysis of Aqp1a. B: Western blot analysis of actin, from the same blot and for the same exposure as in A. C: summary of the densitometry data of Aqp1a, normalized to actin (n = 4).
Aqp1a Heterologously Expressed in Xenopus Oocytes
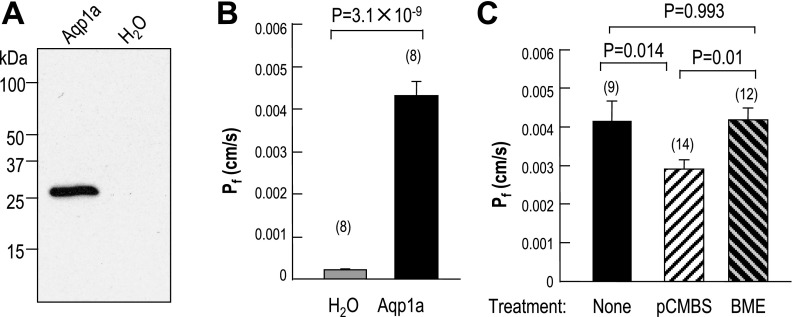
Western blot analysis.
Figure 5A shows a Western blot of oocytes expressing zebrafish Aqp1a vs. control oocytes injected with water. The antibody directed against the European-eel AQP1 recognizes a band at ∼27 kDa in oocytes expressing Aqp1a (lane 1) but not control oocytes (lane 2). These results confirm that the antibody (28) directed against a portion of the COOH terminus of European-eel AQP1 recognizes Aqp1a. A second polyclonal antibody directed against a portion of the COOH terminus of Japanese-eel AQP1 (2) recognizes several higher-MW bands even in H2O-injected oocytes (not shown). However, this antibody recognizes a single Aqp1a-dependent band (∼27 kDa) in oocytes.
Fig. 5.
Functional expression of Aqp1a in Xenopus oocytes. A: Western blot. Oocytes injected with aqp1a cRNA or H2O were incubated at 18°C for 4–5 days. Membrane proteins (10 μg per lane) from these oocytes were separated by SDS-PAGE and prepared for Western blot analysis. The expression of Aqp1a protein was examined with an antibody directed against European eel AQP1. B: water permeability of oocytes injected with H2O or aqp1a cRNA. Values are expressed as means ± SE, with numbers of oocytes in parentheses. The P value is indicated. C: inhibition of water permeability of Xenopus oocytes expressing Aqp1a by pCMBS, and reversibility of the inhibitory effect of p-chloromercuribenzene sulfonate (pCMBS) by β-mercaptoethanol (BME). The three bars represent, untreated oocytes expressing aqp1a (solid bar), aqp1a-expressing oocytes treated with 1 mM pCMBS 20 min (open hatched bar), and aqp1a-expressing oocytes treated with pCMBS and then with 5 mM β-mercaptoethanol for 5 min (solid hatched bar).
Osmotic water permeability.
As summarized in Fig. 5B, the Pf of oocytes expressing Aqp1a is (4.3 ± 0.3) × 10−3 cm/s, more than 21-fold higher than that of H2O-injected controls (0.21 ± 0.03) × 10−3 cm/s. The osmotic water permeability of oocytes expressing Aqp1a is about the same as the value recently reported by Musa-Aziz et al. (30) for human AQP1, but more than twice that of the novel AQP from Xenopus laevis oocytes (55).
We measured the inhibitory effect of the organic mercurial pCMBS on the water permeability of Aqp1a-expressing oocytes. As summarized in Fig. 5C, pretreatment of oocytes with pCMBS caused a ∼30% reduction of Pf, and treatment with β-mercaptoethanol completely reversed this effect.
Permeability of Aqp1a to CO2 and NH3
Principles.
In 2006, Endeward et al. (11) introduced an approach—later extended in 2009 by Musa-Aziz et al. (30)—for obtaining a semiquantitative estimate of CO2 permeability based on the measurement of surface pH. In brief, the introduction of CO2 into the extracellular fluid leads to the entry of CO2 into the oocyte, and thus a transient depletion of CO2 at the extracellular cell surface, promoting the reaction HCO3− + H+ → H2O + CO2, and thereby producing a transient rise in pHS. The maximal rise in pHS, ΔpHS, reflects the maximal CO2 influx. Musa-Aziz et al. (30, 31) recently introduced a similar pHS approach for assessing NH3 permeability. As NH3 enters the oocyte and [NH3]S falls, the reaction NH4+ → NH3 + H+ on the oocyte surface causes a fall in pHS. Here, the magnitude of the maximal fall in pHS, −ΔpHS, reflects the maximal NH3 influx.
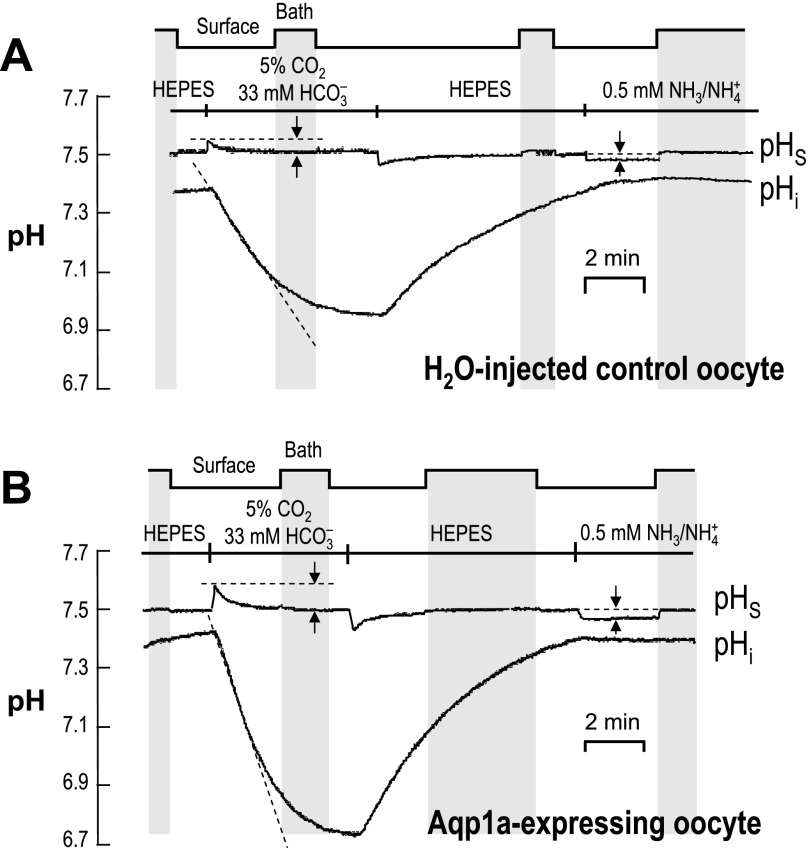
CO2 permeation in control oocytes.
Figure 6A shows a typical recording of pHS and pHi for a control, water-injected oocyte. The “step-chart” at the top of the figure indicates whether the extracellular pH microelectrode was in the “Bath” (i.e., bulk extracellular fluid; also indicated by vertical gray stripes, at pH 7.50) or against the oocyte “Surface.” As shown in the left half of the figure, exposing the extracellular surface of the oocyte to CO2/HCO3− caused the familiar fall in pHi and a small, transient rise in pHS. Both changes are reversible.
Fig. 6.
Recordings of surface and intracellular pH. A: typical pH records from a control Xenopus oocyte injected with H2O. The step chart at the top and the vertical gray bars indicate periods during which the extracellular pH electrode was moved away from the cell surface for calibration. Extracellular solutions were changed, as indicated, in the order HEPES → CO2/HCO3− → HEPES → NH3/NH4+. The upper trace represents surface pH (pHS), with the pairs of vertical arrows indicating the maximal pHS changes elicited by exposure to CO2/HCO3− or NH3/NH4+. The lower trace indicates intracellular pH (pHi), with the sloped dashed line indicating the initial rate of pHi decline. B: typical pH records for pHS (upper trace) and pHi (lower trace) from a Xenopus oocyte injected with 25 ng of aqp1a cRNA.
CO2 permeation in Aqp1a-expressing oocytes.
The left half of Fig. 6B shows a typical recording of pHS and pHi for an Aqp1a oocyte. Compared with the water-injected oocyte in Fig. 6A, switching to CO2/HCO3− caused a more rapid fall in pHi and a larger transient rise in pHS.
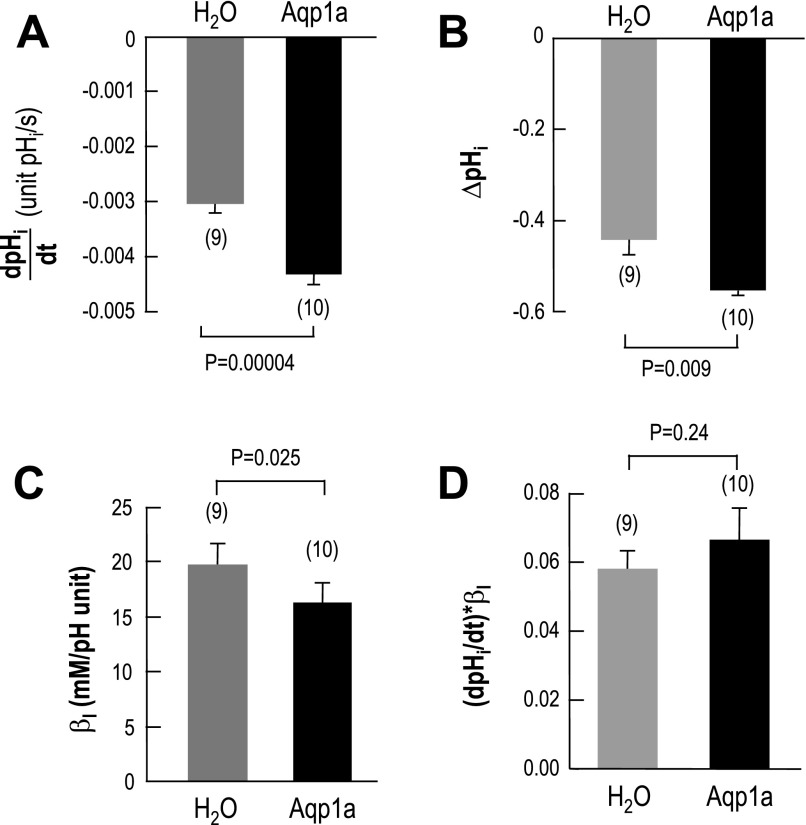
Summary of CO2-HCO3− data.
Figure 7A summarizes the mean initial rate of the CO2-induced pHi decrease (dpHi/dt) for larger groups of water-injected and Aqp1a-expressing oocytes. The magnitude of d(pHi)/dt was 43% greater for Aqp1a Fig. 7A than for H2O. Note that the difference in initial pHi values for the H2O-injected oocytes (7.38 ± 0.05, n = 7) vs. the Aqp1a oocytes (7.45 ± 0.03, n = 8) did not reach statistical significance (P = 0.2). Fig. 7B summarizes the magnitude of the CO2-induced change in steady-state pHi (ΔpHi). In the presence of zebrafish Aqp1a, CO2 caused a pHi change (Fig. 7B) that was 25% higher than that of the control, H2O oocyte. This larger ΔpHi for the Aqp1a oocytes reflects a ∼18% decrease in intrinsic intracellular buffering power (βI), as summarized in Fig. 7C. It is not clear why the expression of Aqp1a should reduce βI. On the other hand, recent work shows that expression of an AE1-based construct (35) also reduces oocyte βI. If we multiply the dpHi/dt values of individual H2O-injected or Aqp1a-expressing oocytes by their respective βI values, we obtain the initial rates of H+ production caused by exposing oocytes to CO2. As summarized in Fig. 7D, the rates in H2O vs. Aqp1a oocytes are not significantly different. Thus, the maximal net influx of CO2 was probably the same in the two groups of oocytes.
Fig. 7.
Summary of intracellular-pH data. A: summary of the maximal rate of pHi decline (dpHi/dt) produced by the switch from HEPES to 5% CO2-33 mM HCO3− in H2O-injected or Aqp1a-expressing oocytes. B: summary of changes in steady-state pHi produced by the application of 5% CO2/33 mM HCO3−. C: summary of the intrinsic intracellular buffering power (βI) of H2O-injected or Aqp1a-expressing oocytes, computed from ΔpHi data (see B) of individual oocytes. D: initial rate of cytosolic H+ production from CO2. For each oocyte, the dpHi/dt value was multiplied by the respective βI value computed for that oocyte.
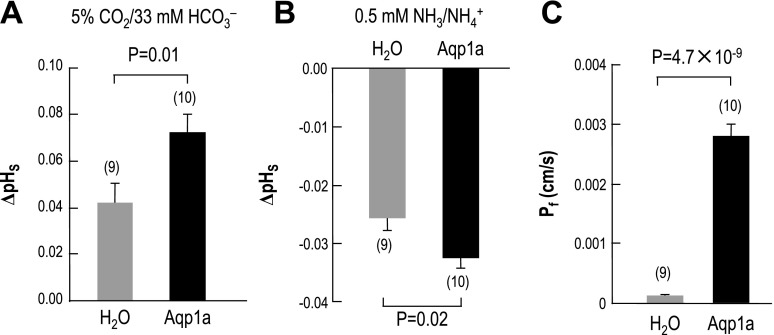
Summary of the mean maximal change in pHS caused by the application of CO2/HCO3− in the two groups of oocytes.
When exposed to 5% CO2/33 mM HCO3−, oocytes expressing Aqp1a exhibit a maximal ΔpHS that is 71% higher than the control H2O oocytes. This increase in maximal ΔpHS in oocytes expressing Aqp1a—as well as in those expressing human AQP1, rat AQP4, and rat AQP5 in previous studies (11, 30)—indicates that Aqp1a oocytes have a higher CO2 permeability than H2O-injected controls.
NH3 permeation in control oocytes.
We return now to Fig. 6A, the rightmost portion of which shows a typical recording of pHS and pHi for a control water-injected oocyte exposed for ∼3 min to 0.5 mM NH4Cl. Exposing the extracellular surface of the oocyte to NH3/NH4+ caused no discernable change in pHi and a very small, reversible decrease in pHS. As noted in a recent study, the small fall in pHS indicates that NH3 influx makes a greater contribution to pHS than does NH4+ influx, and the stability of pHi reflects the unusual NH3 handing of oocytes (30).
NH3 permeation in Aqp1a-expressing oocytes.
The rightmost portion of Fig. 6B shows a typical recording of pHS and pHi for an Aqp1a oocyte exposed to 0.5 mM NH3/NH4+. Compared with the water-injected oocyte in Fig. 6A, switching to NH3/NH4+ caused a slightly larger decrease in pHS.
Summary of NH3/NH4+ data.
Figure 8B summarizes the mean maximal change in pHS caused by the application of NH3/NH4+ in the two groups of oocytes. Note that, because we always performed these experiments in a paired fashion, the oocytes summarized in Fig. 8B for NH3 are the same oocytes summarized in Fig. 8A for CO2. When exposed to 0.5 mM NH3/NH4+, oocytes expressing Aqp1a exhibited a maximal ΔpHS that was 27% higher than the control oocytes. This small increase in the magnitude of the maximal ΔpHS indicates that Aqp1a oocytes have a somewhat higher NH3 permeability than H2O-injected controls. A previous study showed that the expression of human AQP1 causes the magnitude of the maximal ΔpHS to increase by 50% (30).
Fig. 8.
Summary of surface-pH and osmotic-permeability data. A: mean maximal changes in pHS (ΔpHS) caused by the application of 5% CO2-33 mM HCO3−. B: mean maximal changes in pHS (ΔpHS) caused by the application of 0.5 mM NH3-NH4+. C: mean osmotic water permeability, computed from rate of cell-volume increase caused by lowering the osmolality to 100 mOsm. The same oocytes were sequentially subjected to the CO2, NH3, and Pf assays.
Matched Pf values.
After exposing an oocyte to CO2/HCO3− (Fig. 6, A and B, left) and then to NH3/NH4+ (Fig. 6, A and B, right), we routinely determined the osmotic water permeability of the same oocyte. These Pf data, summarized in Fig. 8C, show that the expression of Aqp1a increased Pf by 23-fold, nearly the same as the value noted above for Fig. 5B. Note that the oocytes summarized in Fig. 8C represent a different population from that summarized in Fig. 5B.
DISCUSSION
Phylogenetic Analysis of Zebrafish Aquaporins
As described in this paper, in 2006, we first cloned the aqp1a, both from the 72-hpf zebrafish embryo and the swim bladder of adult zebrafish, and we deposited the sequence in GenBank (accession number DQ887675). In 2008, Tingaud-Sequeira et al. (49) reported the cloning of aqp1b.
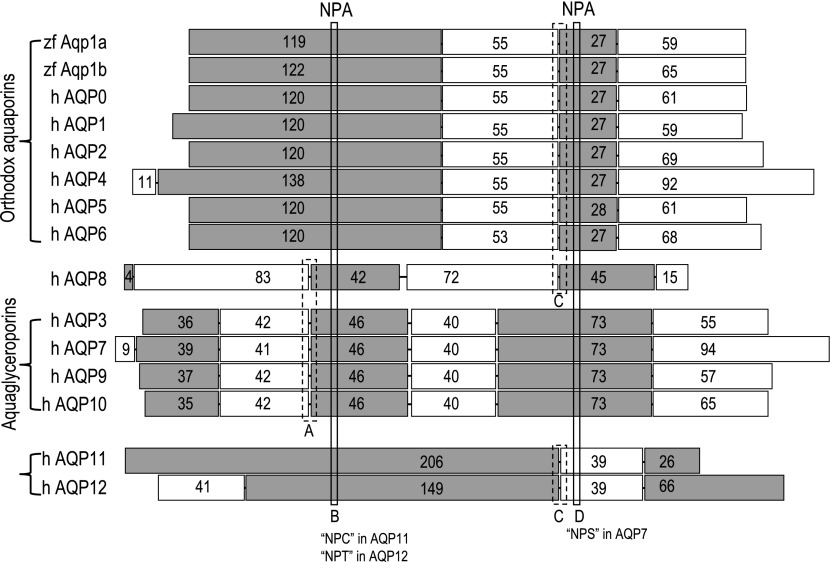
The aquaporin superfamily of integral membrane proteins includes at least 13 genes (AQP0–AQP12) in mammals. To understand the putative evolutionary relationships between aqp1a and aqp1b on the one hand and mammalian AQP genes on the other, we have analyzed the exon structures of the two zebrafish genes, as well as human AQP0–12 based upon the available genomic sequence (Fig. 9). The results indicate that the two zebrafish aqp1 genes and the AQP genes encoding the human orthodox aquaporins AQP0–AQP2 and AQP4–AQP6 group together. They and their murine (not shown) orthologs all have four exons (if we consider only the shorter M23 variant of AQP4) and nearly identical exon boundaries.
Fig. 9.
Comparison of the exon structures of genes encoding aqp1a, aqp1b, and human AQP0–12. All human aquaporin sequences are from GenBank (for accession numbers, see Supplemental Fig. 1 in the online version of this article). The white and gray rectangles indicate homologous exons. The sets of braces indicate the three major groups of AQPs: 1) orthodox AQPs, 2) the aquaglyceroporins, and 3) AQP11–12. Human AQP8 does not fit into any of these groups. Note that the M1 splice variant of human AQP4 has a fifth exon at the NH2 terminus resulting in extra 22 amino acids. The diagram was based on a sequence alignment performed using the online program ClustalW (EBI, http://www.ebi.ac.uk/clustalw/) with default gap parameters. The exon boundaries of human AQP genes were determined by comparing cDNA and the genomic sequences in GenBank (http://www.ncbi.nlm.nih.gov/mapview/map_search.cgi?taxid=9606). Although not shown here, similar results are obtained for mouse Aqp0-12. Two conserved NPA motifs were indicated (box B and D). Note that the first “NPA” motif (box B) is replaced by “NPC” in human AQP11 and “NPT” in human AQP12, whereas the second “NPA” motif (box D) is replaced by “NPS” in human AQP7. The boundary of exon 2 and 3 of human AQP8 is identical to those of the aquaglyceroporins group (indicated by box A), whereas the boundary of exon 4 and 5 of human AQP8 is identical to those of the orthodox aquaporins group (indicated by box C), suggesting that in evolution AQP8 lies between these two groups of aquaporins. The exon boundaries in human AQP11 and human AQP12 indicated by box C have one amino-acid shift compared with those of the orthodox aquaporins.
The aquaglyceroporins—AQP3, AQP7, AQP9, and AQP10—also clearly group together. Except for human AQP7 (in which a seventh exon encodes the NH2 terminus), the human and murine genes all have six homologous exons. Finally, the more recently cloned AQP11 (17) and AQP12 (23) in human and mouse have three homologous exons (except for human AQP12, in which a fourth exon encodes the NH2 terminus).
Thus, for all human AQP genes, except for AQP8, the three groups of homologous exons are consistent with the three major groups of homologous amino-acid sequences shown in Supplemental Fig. S1 (see online version of this article).
The human AQP8 (26) gene defies classification. In terms of amino-acid homology, AQP8 most closely groups with AQP11 and AQP12. In terms of the number of exons, AQP8 (with six exons), groups with the aquaglyceroporins. However, the exon structure of AQP8 is unique. Although the boundary between its exons 2 and 3 is homologous to that for the aquaglyceroporins, the boundary between exons 4 and 5 of AQP8 is homologous to the boundary between exons 2 and 3 for the orthodox aquaporins. Thus, AQP8 forms a group of its own. From this analysis, we conclude that the zebrafish aqp1a and aqp1b genes, as well as the genes encoding the mammalian orthodox aquaporins probably evolved from a common ancestor.
Distribution of aqp1a mRNA and Aqp1a Protein
By in situ hybridization, we found that aqp1a transcripts are expressed in the vasculature, peaking at ∼24 hpf and fading away by ∼72 hpf. On the other hand, aqp1a persists within the vessels, consistent with the hypothesis that, as in mammals, Aqp1a is present in blood cells. We also found strong expression of aqp1a transcripts in pneumatic duct, as well as the blood vessels surrounding the swim bladder in embryos at 72 hpf (Fig. 3). We discuss the possible role of Aqp1a in the swim bladder in Expression of Aqp1a in the Swim Bladder. In the vasculature, it is possible that Aqp1a, acting as a H2O channel, plays a role in angiogenesis, as has been proposed in mice (42). In the red blood cells (RBCs), Aqp1a may be playing a role as a gas channel, as is the case in mammals (11).
By performing Western blot analysis in adults, we found that Aqp1a protein is expressed at high levels in the brain, eye, gill, and swim bladder, but it is not detectable in intestine or muscle of adult zebrafish (Fig. 4). By RT-PCR, Tingaud-Sequeira et al. (49) found that aqp1b mRNA is expressed in the adult brain, ovary, and testis, but not in the adult gills. Taking our results together with those of Tingaud-Sequeira et al. (49), we conclude that aqp1a and aqp1b very likely have distinct tissue distributions. For example, although both aqp1a and aqp1b are expressed in brain, aqp1a but not aqp1b is expressed in gills.
Function of Aqp1a as a Dual-Water/Gas Channel
Water permeability.
We found that Aqp1a, compared to human AQP1 (30), engenders about the same Pf in Xenopus oocytes, but that pCMBS blocks a smaller fraction (∼30% vs. ∼50%). Treatment with β-mercaptoethanol fully reverses the inhibitory effect of pCMBS on Aqp1a. Tingaud-Sequeira et al. (49) found that HgCl2 reduces Aqp1b-dependent Pf by ∼36%, and that β-mercaptoethanol largely reverses this effect.
Gas permeability.
Gas transport through biological membrane is essential for virtually every form of life from prokaryotic archaea and bacteria to eukaryotic fungi, plants, and animals. It had been assumed that small neutral molecules such as CO2, O2, and NH3 permeate all membranes freely, by simply dissolving in and then diffusing through the lipid bilayer. The first evidence against the dogma was the observation of Waisbren et al. (56) that the apical membranes of gastric gland cells are impermeable to CO2 and NH3. Endeward and Gros (10) later found that the CO2 permeability of the apical membrane of guinea-pig colonic epithelial cells is only ∼0.3% as high as that of human red cell membrane. The first evidence for a gas channel was the observation by Nakhoul et al. (32) and then Cooper and Boron (6) that AQP1 expressed in Xenopus oocytes serves as a CO2 conduit. Prasad et al. (39) confirmed this observation in liposomes containing reconstituted AQP1. Later work suggested that NtAQP1 from the plant Nicotiana tabaccum facilitates CO2 transport for photosynthesis (52). Work on AQP1-deficient RBCs indicates that AQP1 is responsible for ∼60% of the CO2 permeability in human RBCs (11).
Structural studies reveal that the aquaporins are tetramers (13, 18, 20, 25, 44, 45, 47, 50) with a four-fold or pseudo-four-fold axis of symmetry. A molecular-dynamics simulation suggests that CO2 could permeate the four water pores of AQP1 (21). A second molecular-dynamics simulation suggests that CO2 could also pass through the central pore of AQP1 (57).
More recently, a second family of gas channels (30) has emerged: the Amt/MEP/Rh family (24, 31, 38, 59). The ammonium transporter (Amt) subfamily is present in archaea, bacteria, fungi (where the yeast members are referred to as methylamine permeases, or MEPs), plants, and invertebrate animals. The Rhesus (Rh) subfamily is present in bacteria, protists, and animals. The observation that DIDS blocks a large component of CO2 permeability in human erythrocytes (14) too large to be explained by the inhibitory effects of DIDS on the CO2 permeability of AQP1 (11), is consistent with the hypothesis that another protein(s) contributes to the CO2 permeability, perhaps the Rh complex (11) and/or AE1 (3). Indeed, experiments with Rh-deficient mouse RBCs suggests that the Rh complex mediates about half of the CO2 permeability (9). Thus, the combination of AQP1 and the Rh complex would account for nearly all CO2 permeability by RBCs.
Several groups have reported that, when expressed in Xenopus oocytes, several aquaporins are permeable to NH3-AQP1 (33), AQP3 (19), AQP8 (19), AQP9 (19), and the plant aquaporin TIP2 (19). The authors of a physiological study report that AQP8-knockout mice are phenotypically indistinguishable from wild-type mice and propose that although AQP8 may transport NH3, this action is not physiologically important (60).
The data in the present study indicate that Aqp1a is permeable not only to H2O (Fig. 5B and Fig. 8C), but also to CO2 (Fig. 6 and Fig. 8A) and NH3 (Fig. 6 and Fig. 8B). The transient rise in pHS induced by administering CO2/HCO3− in Fig. 6 indicates that the influx of CO2 has the dominant effect on pHS but does not rule out a contribution (having the opposite effect on pHS) from the influx of HCO3−. Conversely, the transient fall in pHS induced by administering NH3/NH4+ in Fig. 6 indicates that the influx of NH3 has the dominant effect on pHS but does not rule out a contribution (having the opposite effect on pHS) from the influx of NH4+.
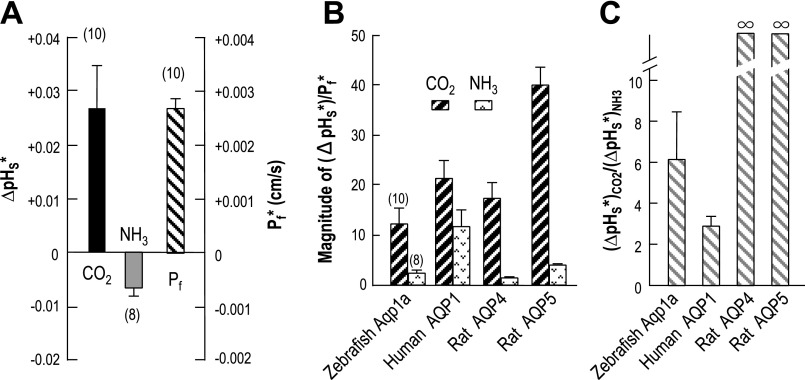
The data in the present paper provide insight into the relative CO2 and NH3 permeabilities of Aqp1a. The leftmost bar in Fig. 10A shows the Aqp1a-dependent component of the total ΔpHS due to the CO2 influx-computed by subtracting the ΔpHS value from day-matched control oocytes from the ΔpHS of individual Aqp1a oocytes. Similarly, the middle bar in Fig. 10A shows the Aqp1a-dependent component of the total ΔpHS due to the NH3 flux. The rightmost bar in Fig. 10A Aqp1a-dependent component of Pf.
Fig. 10.
Indices of the CO2 and NH3 permeability of Aqp1a. A: indices of the Aqp1a-dependent CO2 and NH3 permeability (ΔpHS*) and the Aqp1a-dependent (Pf*). The leftmost bar of CO2 is an index of the Aqp1a-dependent CO2 permeability. We subtracted the mean value of ΔpHS induced by CO2 in day-matched, H2O-injected control oocytes (data contributing to the H2O bar for Fig. 8A) from the ΔpHS induced by CO2 in individual oocytes expressing Aqp1a (data contributing to the Aqp1a bar for Fig. 8A). Similarly, the middle bar of NH3 is an index of the Aqp1a-dependent NH3 permeability, and the rightmost bar of Pf is the Aqp1a-dependent osmotic water permeability (Pf*). B: Aqp1a-dependent parameters normalized to Pf*. The leftmost hatched bar of Aqp1a is the result of dividing the Aqp1a-dependent index of CO2 permeability of individual oocytes (data contributing to the bar for CO2 in A) by the corresponding Aqp1a-dependent Pf values of the same oocytes (data contributing to the bar for Pf in A). The rightmost stippled bar is the result of a similar manipulation, but starting with the Aqp1a-dependent index of NH3 permeability of individual oocytes (data contributing to the bar for NH3 in A). The other three pairs of bars represent data reported in a previous study (30) on human AQP1, rat AQP4, and rat AQP5. C: ratio of Aqp1a-dependent indices of CO2 and NH3 permeability. The bar for Aqp1a is the result of dividing the Aqp1a-dependent index of CO2 permeability of individual oocytes (data contributing to the bar for CO2 in A) by the corresponding index of NH3 permeability of the same oocytes (data contributing to the bar for NH3 in A). The bars for human AQP1, rat AQP4, and rat AQP5 represent data reported in a previous study (30).
The leftmost pair bars in Fig. 10B are the results of normalizing Aqp1a-dependent ΔpHS* values for CO2 (or NH3) to Aqp1a-dependent Pf* values for individual oocytes. The value represented by the CO2 (or NH3) bar in Fig. 10B is an index of the absolute CO2 (or NH3) permeability of Aqp1a (normalized for Pf). For comparison, the other three pairs of bars in Fig. 10B represent normalized indices of CO2 and NH3 permeabilities from a previous study on human AQP1, rat AQP4, and rat AQP5 (30). Thus, normalized to Pf, the CO2 permeability of Aqp1a is somewhat less than that of human AQP1 or rat AQP4, and substantially less than that of rat AQP5. Similarly, normalized to Pf, the NH3 permeability of Aqp1a is similar to that of rat AQP4 and rat AQP5 but substantially less than that of human AQP1. Thus, compared with three mammalian orthodox AQPs, Aqp1a is on the low end of both CO2 and NH3 permeabilities when normalized for Pf.
The leftmost bar in Fig. 10C represents the mean value, computed for individual oocytes, of the Aqp1a-dependent ΔpHS* for CO2 (see left bar in Fig. 10A) divided by the Aqp1a-dependent -ΔpHS* for NH3 (see middle bar in Fig. 10A). Thus, the value represented by the leftmost bar in Fig. 10C is an index of the absolute CO2/NH3 permeability ratio but is not the actual permeability ratio. For comparison, the other three bars in Fig. 10C represent comparable ratios from the previous study on human AQP1, rat AQP4, and rat AQP5 (30). The CO2/NH3 permeability ratio of Aqp1a is about twice that of human AQP1, but considerably less than that of either rat AQP4 or human AQP5, neither of which has a significant NH3 permeability (i.e., the CO2/NH3 permeability ratio is infinity).
Expression of Aqp1a in the Swim Bladder
We were surprised to find Aqp1a expressed in the zebrafish swim bladder both of larvae (by in situ hybridization) and adults (by RT-PCR and Western blot analysis). Aoki et al. (2) also detected an AQP1 homologue in the swim bladder of the Japanese eel (Anguilla japonica) by Northern blot analysis. Most teleost fishes employ a gas-filled swim bladder as a buoyancy organ so that the fish can maintain a vertical position with minimal energy expenditure (for reviews, see Refs. 36 and 37). Like the lungs of higher vertebrates, the swim bladder develops as an outpocketing of the esophagus. Physostome bladders—like that of the zebrafish—maintain their connection (pneumatic duct) to the esophagus. Zebrafish larvae fill their swim bladders by gulping air. Aqp1a could contribute to the clearance of H2O from the swim bladder lumen. However, it is interesting to note that interposing a screen between zebrafish larvae and the air causes the larvae to become deformed and die (16). This result suggests to us that the larval zebrafish swim bladder could have a respiratory function. In this context, it is interesting to note that zebrafish Aqp1a—highly expressed in larval swim bladders—functions as a channel for CO2. If Aqp1a also serves as a channel for O2, it is possible that Aqp1a could contribute to the respiratory function of the larval swim bladder.
Perspectives and Significance
In the present paper, we report the cloning of the cDNA encoding zebrafish Aqp1a. The analysis of intron-exon boundaries provides insights into the evolution of the AQPs. The functional characterization of Aqp1a demonstrates that the protein can function not only as a water channel but a gas channel with a characteristic set of CO2/H2O, NH3/H2O, and CO2/NH3 permeability ratios. The strong expression of Aqp1a in gill and swim bladder—not easily reconciled with the water-channel function of Aqp1a—is consistent with the idea that the gas-channel function of Aqp1a is physiologically important. The development of an Aqp1a-null zebrafish would permit the testing of this hypothesis.
GRANTS
This work was supported by Grant 1N00014-05-0345 from the Office of Naval Research to W. F. Boron and National Institutes of Health Grant R01 DK52093 to I. A. Drummond. J. Zhao was supported by fellowship #38a2f from the Polycystic Kidney Disease Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Mark D. Parker for the help with DNA database search, and Mr. Duncan Wong for computer assistance. We thank Dr. Gordon Cramb at University of St. Andrews, Scotland, UK, and Dr. Toyoji Kaneko at University of Tokyo, Japan, for kindly providing us with anti-eel AQP1 antibodies.
REFERENCES
- 1. Agre P. The aquaporin water channels. Proc Am Thorac Soc 3: 5–13, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aoki M, Kaneko T, Katoh F, Hasegawa S, Tsutsui N, Aida K. Intestinal water absorption through aquaporin 1 expressed in the apical membrane of mucosal epithelial cells in seawater-adapted Japanese eel. J Exp Biol 206: 3495–3505, 2003. [DOI] [PubMed] [Google Scholar]
- 3. Blank ME, Ehmke H. Aquaporin-1 and HCO3−-Cl− transporter-mediated transport of CO2 across the human erythrocyte membrane. J Physiol London 550: 419–429, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boron WF, De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3 and metabolic inhibitors. J Gen Physiol 67: 91–112, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandy G, Zampighi GA, Kreman M, Hall JE. Comparison of the water transporting properties of MIP and AQP1. J Membrane Biol 159: 29–39, 1997. [DOI] [PubMed] [Google Scholar]
- 6. Cooper GJ, Boron WF. Effect of PCMBS on CO2 permeability of Xenopus oocytes expressing aquaporin 1 or its C189S mutant. Am J Physiol Cell Physiol 275: C1481–C1486, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Cutler CP, Cramb G. Branchial expression of an aquaporin 3 (AQP-3) homologue is downregulated in the European eel Anguilla anguilla following seawater acclimation. J Exp Biol 205: 2643–2651, 2002. [DOI] [PubMed] [Google Scholar]
- 8. Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23: 7233–7246, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J 22: 64–73, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Endeward V, Gros G. Low carbon dioxide permeability of the apical epithelial membrane of guinea-pig colon. J Physiol 567: 253–265, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Endeward V, Musa-Aziz R, Cooper GJ, Chen L, Pelletier MF, Virkki LV, Supuran CT, King LS, Boron WF, Gros G. Evidence that Aquaporin 1 is a major pathway for CO2 transport across the human erythrocyte membrane. FASEB J 20: 1974–1981, 2006. [DOI] [PubMed] [Google Scholar]
- 12. Fabra M, Raldua D, Power DM, Deen PM, Cerda J. Marine fish egg hydration is aquaporin-mediated. Science 307: 545, 2005. [DOI] [PubMed] [Google Scholar]
- 13. Fischer G, Kosinska-Eriksson U, Aponte-Santamaria C, Palmgren M, Geijer C, Kedfalk K, Hohmann S, de Groot BL, Neutze R, Lindkvist-Petersson K. Crystal structure of a yeast aquaporin at 115Å reveals a novel gating mechanism. PLoS Biol 7: e10000130, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forster RE, Gros G, Lin L, Ono Y, Wunder M. The effect of 4,4′-diisothiocyanato-stilbene-2,2′-disulfonate on CO2 permeability of the red blood cell membrane. Proc Natl Acad Sci USA 95: 15815–15820, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol 207: 266–279, 1992. [DOI] [PubMed] [Google Scholar]
- 16. Goolish EM, Okutake K. Lack of gas bladder inflation by the larvae of zebrafish in the absence of an air-water interface. J Fish Biol 55: 1054–1063, 1999. [Google Scholar]
- 17. Gorelick DA, Praetorius J, Tsunenari T, Nielsen S, Agre P. Aquaporin-11: a channel protein lacking apparent transport function expressed in brain. BMC Biochem 7: 14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harries WE, Akhavan D, Miercke LJ, Khademi S, Stroud RM. The channel architecture of aquaporin 0 at a 2.2-Å resolution. Proc Natl Acad Sci USA 101: 14045–14050, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holm LM, Jahn TP, Moller AL, Schjoerring JK, Ferri D, Klaerke DA, Zeuthen T. NH3 and NH4+ permeability in aquaporin-expressing Xenopus oocytes. Pflügers Arch 450: 415–428, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Horsefield R, Norden K, Fellert M, Backmark A, Tornroth-Horsefield S, Terwisscha van Scheltinga AC, Kvassman J, Kjellbom P, Johanson U, Neutze R. High-resolution X-ray structure of human aquaporin 5. Proc Natl Acad Sci USA 105: 13327–13332, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hub JS, de Groot BL. Does CO2 permeate through Aquaporin-1? Biophys J 91: 842–848, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hurley I, Hale ME, Prince VE. Duplication events and the evolution of segmental identity. Evol Dev 7: 556–567, 2005. [DOI] [PubMed] [Google Scholar]
- 23. Itoh T, Rai T, Kuwahara M, Ko SB, Uchida S, Sasaki S, Ishibashi K. Identification of a novel aquaporin, AQP12, expressed in pancreatic acinar cells. Biochem Biophys Res Commun 330: 832–838, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Khademi S, Stroud RM. The Amt/MEP/Rh family: Structure of AmtB and the mechanism of ammonia gas conduction. Physiology 21: 419–429, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Lee JK, Kozono D, Remis J, Kitagawa Y, Agre P, Stroud RM. Structural basis for conductance by the archaeal aquaporin AqpM at 1.68 Å. Proc Natl Acad Sci USA 102: 18932–18937, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu K, Nagase H, Huang CG, Calamita G, Agre P. Purification and functional characterization of aquaporin-8. Biol Cell 98: 153–161, 2006. [DOI] [PubMed] [Google Scholar]
- 27. Lu J, Daly CM, Parker MD, Gill HS, Piermarini PM, Pelletier MF, Boron WF. Effect of human carbonic anhydrase II on the activity of the human electrogenic Na/HCO3 cotransporter NBCe1-A in Xenopus oocytes. J Biol Chem 281: 19241–19250, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Martinez AS, Cutler CP, Wilson GD, Phillips C, Hazon N, Cramb G. Cloning and expression of three aquaporin homologues from the European eel (Anguilla anguilla): effects of seawater acclimation and cortisol treatment on renal expression. Biol Cell 97: 615–627, 2005. [DOI] [PubMed] [Google Scholar]
- 29. Morris AC, Fadool JM. Studying rod photoreceptor development in zebrafish. Physiol Behav 86: 306–313, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Musa-Aziz R, Chen L, Pelletier MF, Boron WF. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc Natl Acad Sci USA 106: 5406–5411, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Musa-Aziz R, Jiang L, Chen LM, Behar KL, Boron WF. Concentration-dependent effects on intracellular and surface pH of exposing Xenopus oocytes to solutions containing NH3/NH4+. J Membr Biol 228: 15–31, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol Cell Physiol 274: C543–C548, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Nakhoul NL, Hering-Smith KS, Abdulnour-Nakhoul SM, Hamm LL. Transport of NH3/NH4+ in oocytes expressing aquaporin-1. Am J Physiol Renal Physiol 281: F255–F263, 2001. [DOI] [PubMed] [Google Scholar]
- 34. Parker MD, Musa-Aziz R, Rojas JD, Choi I, Daly CM, Boron WF. Characterization of human SLC4A10 as an electroneutral Na/HCO3− cotransporter (NBCn2) with Cl- self-exchange activity. J Biol Chem 283: 12777–12788, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Parker MD, Young MT, Daly CM, Meech RW, Boron WF, Tanner MJ. A conductive pathway generated from fragments of the human red cell anion exchanger AE1. J Physiol 581: 33–50, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pelster B. The generation of hyperbaric oxygen tensions in fish. News Physiol Sci 16: 287–291, 2001. [DOI] [PubMed] [Google Scholar]
- 37. Pelster B. pH regulation and swimbladder function in fish. Respir Physiol Neurobiol 144: 179–190, 2004. [DOI] [PubMed] [Google Scholar]
- 38. Peng J, Huang CH. Rh proteins vs Amt proteins: an organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus Clin Biol 13: 85–94, 2006. [DOI] [PubMed] [Google Scholar]
- 39. Prasad GV, Coury LA, Fin F, Zeidel ML. Reconstituted aquaporin 1 water channels transport CO2 across membranes. J Biol Chem 273: 33123–33126, 1998. [DOI] [PubMed] [Google Scholar]
- 40. Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 256: 385–387, 1992. [DOI] [PubMed] [Google Scholar]
- 41. Preston GM, Jung JS, Guggino WB, Agre P. The mercury-sensitive residue at cysteine 189 in the CHIP28 water channel. J Biol Chem 268: 17–20, 1993. [PubMed] [Google Scholar]
- 42. Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature 434: 786–792, 2005. [DOI] [PubMed] [Google Scholar]
- 43. Santos CR, Estevao MD, Fuentes J, Cardoso JC, Fabra M, Passos AL, Detmers FJ, Deen PM, Cerda J, Power DM. Isolation of a novel aquaglyceroporin from a marine teleost (Sparus auratus): function and tissue distribution. J Exp Biol 207: 1217–1227, 2004. [DOI] [PubMed] [Google Scholar]
- 44. Savage DF, Egea PF, Robles-Colmenares Y, O'Connell JD, III, Stroud RM. Architecture and selectivity in aquaporins: 2.5 Å X-ray structure of aquaporin Z. PLoS Biol 1: E72, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sui H, Han BG, Lee JK, Walian P, Jap BK. Structural basis of water-specific transport through the AQP1 water channel. Nature 414: 872–878, 2001. [DOI] [PubMed] [Google Scholar]
- 46. Sumanas S, Jorniak T, Lin S. Identification of novel vascular endothelial-specific genes by the microarray analysis of the zebrafish cloche mutants. Blood 106: 534–541, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tajkhorshid E, Nollert P, Jensen MO, Miercke LJ, O'Connell J, Stroud RM, Schulten K. Control of the selectivity of the aquaporin water channel family by global orientational tuning. Science 296: 525–530, 2002. [DOI] [PubMed] [Google Scholar]
- 48. Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development 119: 1203–1215, 1993. [DOI] [PubMed] [Google Scholar]
- 49. Tingaud-Sequeira A, Chauvigne F, Fabra M, Lozano J, Raldua D, Cerda J. Structural and functional divergence of two fish aquaporin-1 water channels following teleost-specific gene duplication. BMC Evol Biol 8: 259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tornroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. Structural mechanism of plant aquaporin gating. Nature 439: 688–694, 2006. [DOI] [PubMed] [Google Scholar]
- 51. Toye AM, Parker MD, Daly CM, Lu J, Virkki LV, Pelletier MF, Boron WF. The human NBCe1-A mutant R881C, associated with proximal renal tubular acidosis, retains function but is mistargeted in polarized renal epithelia. Am J Physiol Cell Physiol 291: C788–C801, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Uehlein N, Lovisolo C, Siefritz F, Kaldenhoff R. The tobacco aquaporin NtAQP1 is a membrane CO2 pore with physiological functions. Nature 425: 734–737, 2003. [DOI] [PubMed] [Google Scholar]
- 53. Venkatesh B. Evolution and diversity of fish genomes. Curr Opin Genet Dev 13: 588–592, 2003. [DOI] [PubMed] [Google Scholar]
- 54. Virkki LV, Cooper GJ, Boron WF. Cloning and functional expression of an MIP (AQP0) homolog from killifish (Fundulus heteroclitus) lens. Am J Physiol Regul Integr Comp Physiol 281: R1994–R2003, 2001. [DOI] [PubMed] [Google Scholar]
- 55. Virkki LV, Franke C, Somieski P, Boron WF. Cloning and functional characterization of a novel aquaporin from Xenopus laevis oocytes. J Biol Chem 277: 40610–40616, 2002. [DOI] [PubMed] [Google Scholar]
- 56. Waisbren SJ, Geibel JP, Modlin IM, Boron WF. Unusual permeability properties of gastric gland cells. Nature 368: 332–335, 1994. [DOI] [PubMed] [Google Scholar]
- 57. Wang Y, Schulten K, Tajkhorshid E. Channel mediated gas transport across lipid membranes (Abstract). Biophys J 20: 1375, 2006. [Google Scholar]
- 58. Watanabe S, Kaneko T, Aida K. Aquaporin-3 expressed in the basolateral membrane of gill chloride cells in Mozambique tilapia Oreochromis mossambicus adapted to freshwater and seawater. J Exp Biol 208: 2673–2682, 2005. [DOI] [PubMed] [Google Scholar]
- 59. Winkler FK. Amt/MEP/Rh proteins conduct ammonia. Pflügers Arch 451: 701–707, 2006. [DOI] [PubMed] [Google Scholar]
- 60. Yang B, Zhao D, Solenov E, Verkman AS. Evidence from knockout mice against physiologically significant aquaporin 8-facilitated ammonia transport. Am J Physiol Cell Physiol 291: C417–C423, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.