Abstract
A divergent and highly stereoselective route to eleven glycosylated methymycin analogues has been developed. The key to the success of this method was the iterative use of the Pd-catalyzed glycosylation reaction and post-glycosylation transformation. This unique application of Pd-catalyzed glycosylation demonstrates the breath of α/β-& D/L-glycosylation of macrolides that can be efficiently prepared using a de novo asymmetric approach to the carbohydrate portion.
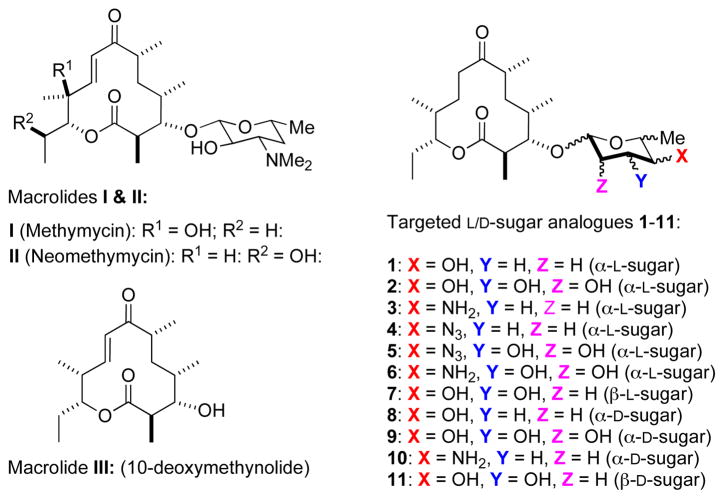
Glycosylated macrolactones, known as macrolides, are an important class of polyketide antibiotics used to treat infections caused by Gram-positive bacteria.2 Streptomyces venezuelae ATCC 15439 produces several 12-membered ring macrolides, including methymycin I and neomethymycin II derived from 10-deoxymethynolide III (Figure 1).3 These macrolides consist of a lactone aglycon carrying a rare deoxyamino sugar, desosamine, which is important for their bioactivity. In fact, the biological activities of I and II are dramatically decreased when the sugar appendage is removed.
Figure 1.
Macrolides (I–III) and targeted methymycin analogues 1–11
Since the deoxyaminosugar portion of macrolides is in general essential for their antimicrobial activities, 4 its modifications hold promise as a valuable approach towards preparing new macrolide antibiotics with improved and/or altered biological properties. 5 In this regard, several synthetic and biosynthetic approaches to novel glycosylated macrolides have been reported. 6 These approaches are limited in terms of the stereochemical diversity of structures that can be generated. In addition, the methods that use in vivo modified biosynthetic pathways and/or in vitro enzymatic reactions for the production of new glycosylated antibiotics7 are limited by the availability and the lability of the sugar nucleotide glycosyl donors. Similarly, these routes often suffer from the reduced catalytic efficiency of the glycosyltransferases involved when dealing with unnatural substrates.
To address these concerns we envisioned the development of a highly diastereoselective, yet stereo-divergent route that would allow for the mild installation of the sugar moieties onto complex antibiotic aglycons using simple achiral starting material. It is in this, as well as other, contexts that we developed a de novo asymmetric approach to carbohydrates, which we hoped would allow for the facile synthesis of various methymycin analogues for carbohydrate SAR-type studies (Figure 1). Herein, we report our successful efforts at the use of this approach for the synthesis of a stereochemically (L/D and α/β-) diverse glycosylated 8,9-dihydro-10-deoxymethymycin analogues.
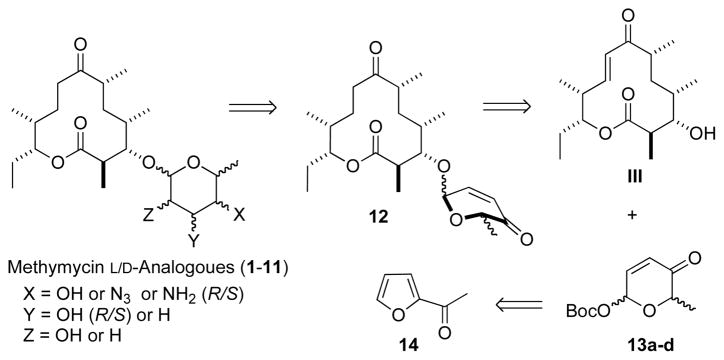
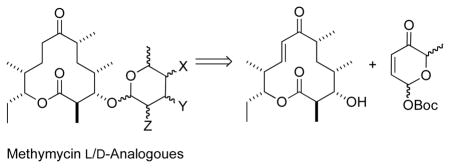
Our basic retrosynthetic analysis for the preparation of the stereochemically diverse methymycin analogues is shown in Scheme 1. The plan was that various amino- and/or deoxy-sugar glycosylated macrolide analogues could be prepared from methynolides like 12 with the desired pyranone stereochemistry. The macrolides 12, in turn, could be prepared by a stereospecific Pd-catalyzed coupling of macrolide III and 13a–d.8,9 The required pyranone stereoisomers 13a–d can be stereoselectively prepared from the achiral acetyl furan 14 via our de novo asymmetric synthesis approach.10
Scheme 1.
Retrosynthetic analysis of methymycin analogues
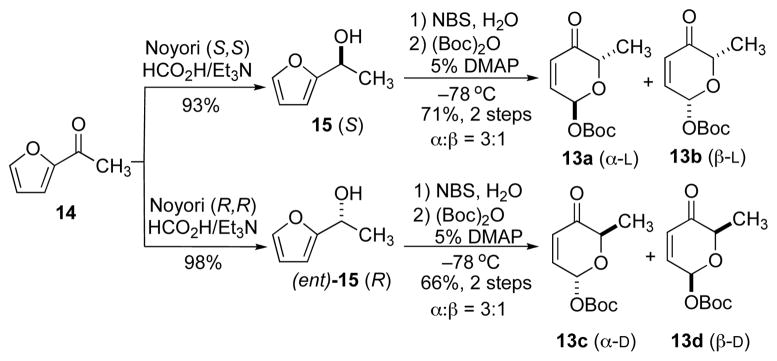
Accordingly, our synthetic effort began with the isolation of 10-deoxymethynolide III5 and preparation of the required D/L-, α/β-Boc-pyranones 13a–d for coupling.9 As previously described, the Pd-glycosyl donors 13a–d were synthesized from the achiral acetyl furan 14 by a very practical three-step sequence, employing an enantioselective Noyori reduction (14 to 15/ent-15), an Achmatowicz oxidation, and a stereodivergent tert-butyl carbonate formation (See Scheme 2).11
Scheme 2.
Synthesis of D/L-, α/β-Boc-pyranones 13a–d
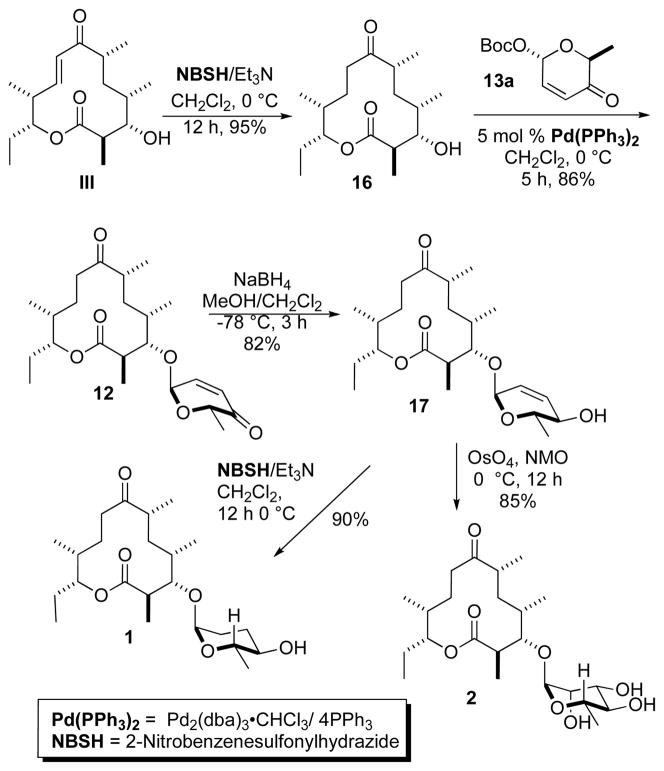
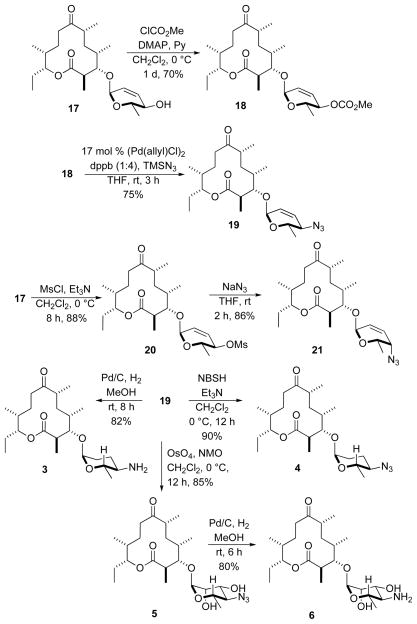
The double bond of 10-deoxymethynolide III5 was then selectively reduced by treatment with excess diimide (NBSH, Et3N) 12 to give the desired 8,9-dihydro-10-deoxymethynolide (16) in 95% yield (Scheme 3). The resulting macrolactone 16 was subjected to the diastereoselective Pd-catalyzed glycosylation with α-L-Boc-pyranone 13a, producing α-L-glycoside 12 as a single diastereomer in good yield (86%). A NaBH4 reduction13 of enone 12 gave the equatorial allylic alcohol 17 in 82% yield. Diimide reduction of 17 with an excess triethylamine and O-nitrophenylsulfonyl hydrazide led to the dideoxy analogue 1 in excellent yield (90%). Preparation of the rhamno-sugar analogue 2 was accomplished by diastereoselective dihydroxylation of 17 under Upjohn conditions14 (OsO4/NMO) in 85% yield.
Scheme 3.
Synthesis of α-L-sugar analogues 1 and 2
We next investigated methods for the construction of various C-4-amino/azido sugar analogues (Scheme 4). Towards this goal, a methyl carbonate leaving group was installed on the allylic alcohol by reaction of 17 with methyl chloroformate to form the C-4-carbonate 18 in 70% yield. Exposing carbonate 18 to the Sinou conditions15 (TMSN3, (Pd(allyl)Cl)2/1,4-bis(diphenylphosphino)-butane) afforded a single regio- and stereoisomeric allylic azide 19 in 75% yield. However, when alcohol 17 was subjected to Mitsunobu conditions using TMS azide as the nucleophile, no desired C-4-azido compound 21 was formed. Therefore a two-step SN2 reaction route was employed, in which the allylic alcohol 17 was converted into mesylate 20 (MsCl/Et3N) in an excellent yield (88%), followed by treatment of 20 with NaN3/THF to afford the inverted C-4-azido isomer 21 in 86% yield.
Scheme 4.
Syntheses of α-L-amino sugar analogues 3–6
These azide intermediates can now be converted to different amino sugar analogues. As depicted in Scheme 4, hydrogenolysis (Pd/C, H2, MeOH) of allylic azide 19 via a one-pot reduction of both azide and allylic double bond gave the dideoxy amino sugar analogue 3 in 82% yield. As before, diimide reduction of the allylic azide 19 gave 2,3-dideoxy analogue 4 in 90% yield. Alternatively, diastereoselective dihydroxylation (OsO4/NMO) of 19, followed by reduction of azide (Pd/C, H2, MeOH) produced the aminomannose analogue 6 via a rhamno-azidosugar 5 intermediate.
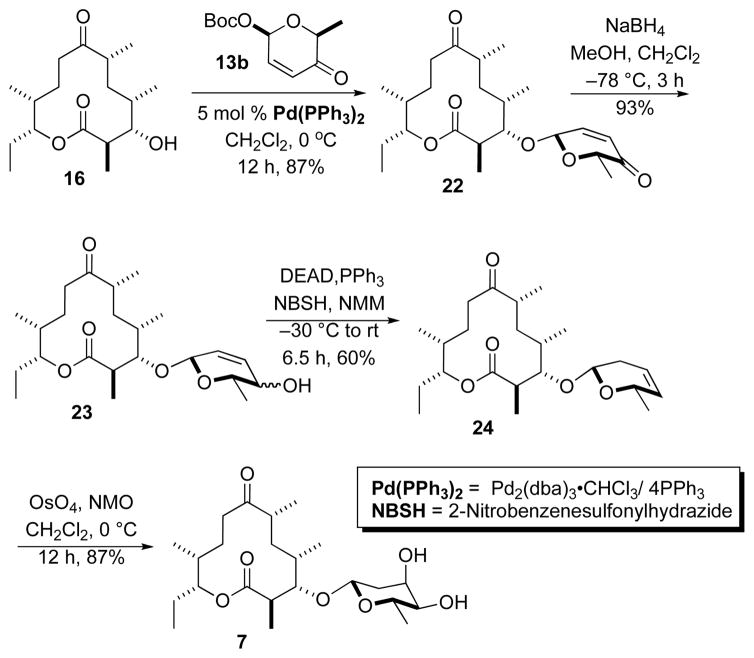
We next investigated the synthesis of the 2,6-dideoxy β-L-allo-sugar analogue 7 of methymycin (Scheme 5), which builds on our digitoxin work.16 Thus, macrolide 16 and β-L-pyranone 13b were subjected to Pd-catalyzed glycosylation to give β-L-glycoside 22 as a single diastereomer in good yield (87%). A NaBH4 reduction of ketone 22 provided a mixture of diastereomeric allylic alcohols 23 in 93% yield. Exposing the mixture of allylic alcohols 23 to the Myers’ reductive rearrangement conditions17 (NBSH/PPh3/DEAD, NMM, −30 °C to rt) provided olefin 24 in a moderate yield (60%). Finally, Upjohn dihydroxylation of olefin 24 (OsO4/NMO) gave exclusively the 2,6-dideoxy allo-sugar analogue 7 in 87% yield.
Scheme 5.
Synthesis of β-L-sugar analogue 7
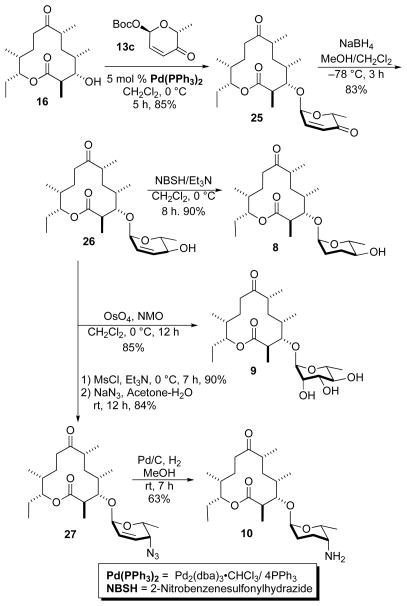
In a similar fashion, aglycon 16 was subjected to a diastereoselective Pd-catalyzed glycosylation with α-D-Boc-pyranone 13c producing α-D-glycoside 25 as a single diastereomer in good yield (85%) (Scheme 6). A NaBH4 reduction of enone 25 afforded the equatorial allylic alcohol 26 in 83% yield. Diimide reduction of the allylic alcohol 26 (NBSH/Et3N) as described above led to the dideoxy analogue 8 in an excellent yield (90%). The final conversion of 26 to the rhamno-sugar analogue 9 was achieved by diastereoselective dihydroxylation using the Upjohn conditions (OsO4/NMO). The desired product was obtained in 85% yield.
Scheme 6.
Syntheses of α-D-sugar analogues 8–10
Via a similar two-step SN2 reaction route, allylic alcohol 26 was converted into the inverted C-4-azido compound 27 in 86% yield by mesylation (MsCl/Et3N; 88%) followed by treatment with NaN3/Acetone(aq). Reduction of the C-4-azido group and allylic double bond in compound 27 under hydrogenolysis conditions (Pd/C, H2, MeOH) gave the C-4-deoxy-amino analogue of methymycin 10. The desired compound was isolated in a modest yield (63%) through reverse phase chromatography.
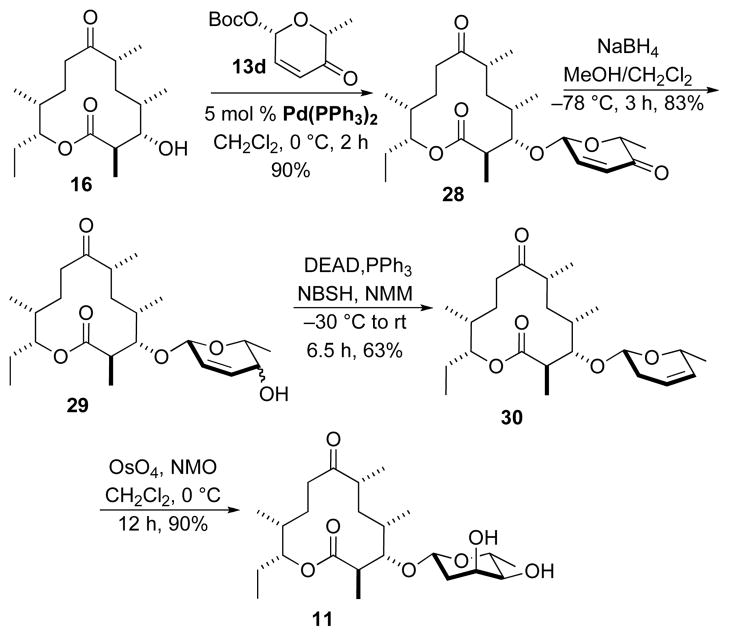
The synthesis of the 2,6-dideoxy β-D-allo-sugar analogue 11 of methymycin was performed according to an analogous sequence as described above for the synthesis of 7 (Scheme 7). Namely, Pd-catalyzed glycosylation of macrolactone 16 using β-D-pyranone 13d gave β-D-glycoside 28 as a single diastereomer in good yield (90%). A NaBH4 reduction of ketone 28 afforded a mixture of diastereomeric allylic alcohols 29 that was converted to olefin 30 under the Myers’ reductive rearrangement conditions (NBSH/PPh3/DEAD, NMM, −30 °C to rt) in moderate yield (63%). Finally, Upjohn dihydroxylation of olefin 30 gave exclusively the 2,6-dideoxy allose-sugar analogue 11 in 90% yield.
Scheme 7.
Synthesis of β-D-sugar analogue 11
In conclusion, a divergent yet highly stereoselective route to eleven variously substituted (amino/azido/dideoxy) methymycin analogues has been developed. The key to the success of this method is the iterative use of the Pd-catalyzed glycosylation reaction, ketone reduction/Myers’ reductive rearrangement, diastereoselective dihydroxylation, and regioselective reductions. This unique application of the established Pd-catalyzed glycosylation strategy allows for efficient preparation of challenging and stereochemically diverse glycoside macrolide targets. While some post-glycosylation transformations were not compatible with the macrolide double bond, there was no need for ketone protection.18 The testing of these macrolide analogs will be reported in due course as well as the use of this methodology towards other biologically important aglycons.
Supplementary Material
Acknowledgments
We are grateful to NIH (GM054346 and GM035906 to H.-w.L., and GM088839 to G.A.O.) and NSF (CHE-0749451 to G.A.O.) for the support of our research.
Footnotes
Supporting Information Available: Complete experimental procedures and spectral data for all new compounds can be found in the Supporting Information. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
John H. Penn, Email: john.penn@mail.wvu.edu.
Hung-wen Liu, Email: h.w.liu@mail.utexas.edu.
George A. O’Doherty, Email: g.odoherty@neu.edu.
References
- 2.(a) Rawlings BJ. Nat Prod Rep. 2001;18:190–227. doi: 10.1039/b009329g. [DOI] [PubMed] [Google Scholar]; (b) Rawlings BJ. Nat Prod Rep. 2001;18:231–281. doi: 10.1039/b100191o. [DOI] [PubMed] [Google Scholar]; (c) Staunton J, Weissman KJ. Nat Prod Rep. 2001;18:380–416. doi: 10.1039/a909079g. [DOI] [PubMed] [Google Scholar]; (d) Walsh C. Antibiotics: Actions, Origins, Resistance. ASM Press; Washington, D.C: 2003. [Google Scholar]; (e) Weissman KJ. Philos Trans R Soc London, Ser A. 2004;362:2671–2690. doi: 10.1098/rsta.2004.1470. [DOI] [PubMed] [Google Scholar]
- 3.(a) Donin MN, Pagano J, Dutcher JD, McKee CM. Antibiotics Annu. 1953–1954;1:179–185. [Google Scholar]; (b) Djerassi C, Zderic JA. J Am Chem Soc. 1956;78:6390–6395. [Google Scholar]; (c) Xue Y, Zhao L, Liu H-w, Sherman DH. Proc Natl Acad Sci USA. 1998;95:12111–12116. doi: 10.1073/pnas.95.21.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Weymouth-Wilson AC. Nat Prod Rep. 1997;14:99–110. doi: 10.1039/np9971400099. [DOI] [PubMed] [Google Scholar]; (b) Thorson JS, Hosted J, Jiang J, Biggins JB, Ahlert J. Curr Org Chem. 2001;5:139–167. [Google Scholar]; (c) Kren V, Martinkova L. Curr Med Chem. 2001;8:1303–1328. doi: 10.2174/0929867013372193. [DOI] [PubMed] [Google Scholar]
- 5.(a) He XM, Liu H-w. Annu ReV Biochem. 2002;71:701–754. doi: 10.1146/annurev.biochem.71.110601.135339. [DOI] [PubMed] [Google Scholar]; (b) Thibodeaux CJ, Melancon CE, III, Liu H-w. Nature. 2007;446:1008–1016. doi: 10.1038/nature05814. [DOI] [PubMed] [Google Scholar]; (c) Thibodeaux CJ, Melancon CE, III, Liu H-w. Angew Chem Int Ed. 2008;47:9814–9859. doi: 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Borisova SA, Zhao L, Sherman DH, Liu H-w. Org Lett. 1999;1:133–136. doi: 10.1021/ol9906007. [DOI] [PubMed] [Google Scholar]
- 6.(a) Velvadapu V, Andrade RB. Carb Res. 2008;343:145–150. doi: 10.1016/j.carres.2007.10.004. [DOI] [PubMed] [Google Scholar]; (b) Packard GK, Scott D, Rychnovsky SD. Org Lett. 2001;3:3393–3396. doi: 10.1021/ol016617i. [DOI] [PubMed] [Google Scholar]
- 7.(a) Mendez C, Salas JA. Trends Biotechnol. 2001;19:449–456. doi: 10.1016/s0167-7799(01)01765-6. [DOI] [PubMed] [Google Scholar]; (b) Griffith BR, Langenhan JM, Thorson JS. Curr Opin Biotechnol. 2005;16:622–630. doi: 10.1016/j.copbio.2005.10.002. [DOI] [PubMed] [Google Scholar]; (c) Melançon CE, Thibodeaux C, Liu H-w. ACS Chem Biol. 2006;1:499–504. doi: 10.1021/cb600365q. [DOI] [PubMed] [Google Scholar]; (d) Mendez C, Luzhetskyy A, Bechthold A, Salas JA. Curr Top Med Chem. 2008;8:710–724. doi: 10.2174/156802608784221532. [DOI] [PubMed] [Google Scholar]; (e) Williams GJ, Gantt RW, Thorson JS. Curr Opin Chem Biol. 2008;12:556–564. doi: 10.1016/j.cbpa.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Babu RS, O’Doherty GA. J Am Chem Soc. 2003;125:12406–12407. doi: 10.1021/ja037097k. [DOI] [PubMed] [Google Scholar]; (b) Comely AC, Eelkema R, Minnaard AJ, Feringa BL. J Am Chem Soc. 2003;125:8714–8715. doi: 10.1021/ja0347538. [DOI] [PubMed] [Google Scholar]; (c) Kim H, Men H, Lee C. J Am Chem Soc. 2004;126:1336–1337. doi: 10.1021/ja039746y. [DOI] [PubMed] [Google Scholar]; (d) Babu RS, Zhou M, O’Doherty GA. J Am Chem Soc. 2004;126:3428–3429. doi: 10.1021/ja039400n. [DOI] [PubMed] [Google Scholar]
- 9.Harris JM, Keranen MD, O’Doherty GA. J Org Chem. 1999;64:2982–2983. doi: 10.1021/jo990410+.Harris JM, Keranen MD, Nguyen H, Young VG, O’Doherty GA. Carbohydr Res. 2000;328:17–36. doi: 10.1016/s0008-6215(00)00031-8.For its use in oligosaccharide synthesis see: Guo H, O’Doherty GA. Angew Chem Int Ed. 2007;46:5206–5208. doi: 10.1002/anie.200701354.Zhou M, O’Doherty GA. Org Lett. 2008;10:2283–2286. doi: 10.1021/ol800697k.
- 10.(a) Fujii A, Hashiguchi S, Uematsu N, Ikariya T, Noyori R. J Am Chem Soc. 1996;118:2521–2522. [Google Scholar]; (b) Li M, Scott JG, O’Doherty GA. Tetrahedron Lett. 2004;45:1005–1009. [Google Scholar]; (c) Li M, O’Doherty GA. Tetrahedron Lett. 2004;45:6407–6411. [Google Scholar]
- 11.(a) Babu RS, O’Doherty GA. J Carbohydr Chem. 2005;24:169–177. [Google Scholar]; (b) Li M, Scott JG, O’Doherty GA. Tetrahedron Lett. 2004;45:1005–1009. [Google Scholar]
- 12.Haukaas MH, O’Doherty GA. Org Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x. [DOI] [PubMed] [Google Scholar]
- 13.(a) Luche JL. J Am Chem Soc. 1978;100:2226–2227. [Google Scholar]; (b) Haukaas MH, O’Doherty GA. Org Lett. 2001;3:401–404. doi: 10.1021/ol006907j. [DOI] [PubMed] [Google Scholar]
- 14.VanRheenen V, Kelly RC, Cha DY. Tetrahedron Lett. 1976;17:1973–1976. [Google Scholar]
- 15.de Oliveira RN, Cottier L, Sinou D, Srivastava RM. Tetrahedron. 2005;61:8271–8281. [Google Scholar]
- 16.Zhou M, O’Doherty GA. Org Lett. 2006;8:4339–4342. doi: 10.1021/ol061683b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Myers AG, Zheng B. J Am Chem Soc. 1996;118:4492–4493. [Google Scholar]; (b) Myers AG, Zheng B. Tetrahedron Lett. 1996;37:4841–4844. [Google Scholar]; (c) Myers AG, Zheng B, Movassaghi M. J Org Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]; (d) Haukaas MH, O’Doherty GA. Org Lett. 2002;4:1771–1774. doi: 10.1021/ol025844x. [DOI] [PubMed] [Google Scholar]
- 18.This compatibility with the macrocyclic ketone functionality is important as there are many ketone conatining macrolide aglycons which can be used with this methodology, see: Ma Z, Nemoto PA. Curr Med Chem Anti-Infective Agents. 2002;1:15–34.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.