Abstract
Fluorescent intercalator displacement (FID) is a convenient and practical tool for identifying new nucleic-acid-binding ligands. The success of FID is based on the fact that it can be fashioned into a versatile screening assay for assessing the relative binding affinities of compounds to nucleic acids. FID is a tagless approach; the target RNAs and the ligands or small molecules under investigation do not have to be modified in order to be examined. In this study, a modified FID assay for screening RNA-binding ligands was established using 3-methyl-2-((1-(3-(trimethylammonio)propyl)-4-quinolinylidene)methyl)benzothiazolium (TO-PRO) as the fluorescent indicator. Electrospray ionization mass spectrometry (ESI-MS) results provide direct evidence that correlates the reduction in fluorescence intensity observed in the FID assay with displacement of the dye molecule from RNA. The assay was successfully applied to screen a variety of RNA-binding ligands with a set of small hairpin RNAs. Ligands that bind with moderate affinity to the chosen RNA constructs (A-site, TAR, h31, and H69) were identified.
Keywords: FID, ESI-MS, RNA, ribosome, drug discovery
Subject category: nucleic acids, biophysical techniques, mass spectrometry
Introduction
The increasing level of bacterial resistance to current antibiotics poses a big challenge for the healthcare industry. Recently, there have been increased efforts toward finding new and effective antibiotics that will circumvent the resistance problem [1–2]. Being an established drug target and having numerous unexploited functional sites, the ribosome serves as a promising target for new antibiotics [3]. Developing new antibiotics requires a detailed understanding and characterization of drug-RNA interactions. A significant challenge, however, is the development of systems for efficient screening and discovery of ligands of interest for new RNA targets.
Some current methods used for studying drug-RNA interactions include mass spectrometry [4–5], surface plasmon resonance (SPR) [6], and equilibrium microdialysis [7]. These techniques provide valuable information about RNA-small-molecule interactions; however, they are often time consuming or require a high level of technical expertise. Thus, a relatively simple and high-throughput method such as fluorescent intercalator displacement (FID)1 [8–9] for RNA applications would be very useful in the drug-discovery process.
FID has become an increasingly important tool for identifying new nucleic-acid-binding ligands. Its success is based on the fact that it can be fashioned into a versatile high-throughput assay that can be used to assess the relative binding affinities of compounds to nucleic acids in a relatively simple manner, requiring only a moderate level of specialized expertise. FID has been used successfully to identify and establish relative binding selectivities and affinities, as well as distinguish binding modes for a variety of DNA-binding ligands [8–12]. More recently, work in the laboratories of Beal, Hergenrother, and Nakatani has shown that FID is useful for ranking and determining selectivity of RNA-binding ligands [13–15]. FID is becoming an increasingly attractive method because it is a tagless approach; neither the RNA nor the small molecule under investigation are modified. A dye molecule is employed, which has a greater fluorescence intensity when intercalated (or bound) to the nucleic acid than when free in solution [8–9; 12–13]. The FID assay is based on the model in which a fluorescent probe is bound to RNA and displaced by a ligand, leading to a reduction in fluorescence intensity. Although the traditional assay involved intercalation of the dye molecule into DNA, the mode of binding to RNA may actually differ. Nonetheless, the extent of decrease in fluorescence is still proportional to the affinity of the ligand for the RNA.
In this study, a modified FID method for screening RNA-binding ligands was established, and electrospray ionization mass spectrometry (ESI-MS) was used to investigate its mechanism. ESI-MS is a soft ionization process that was first used to detect non-covalent interactions in 1991 [16]. Since then, ESI-MS has been extended to the study of non-covalent interactions between nucleic acids and ligands as a screening tool for drug discovery [5; 17]. ESI-MS has been successful due to the fact that it is a powerful and reliable method that can be used to determine stoichiometry, relative binding affinities with multiple ligands or targets, and equilibrium binding constants in one set of experiments [4–5; 18–19]. In traditional spectroscopic methods, the bound concentration of ligand has to be relatively high in order for its contribution to be detected, and multiple stoichiometries complicate the analysis. ESI-MS on the other hand allows for examination of multiple stoichiometry complexes even at very low abundance [4]. Unfortunately, ESI-MS is limited to non-physiological buffers due to cationic complexation with nucleic acids [20–21]. ESI-MS is nevertheless a powerful tool that can be used to elucidate the mechanism of FID and verify relative binding affinities obtained from this method.
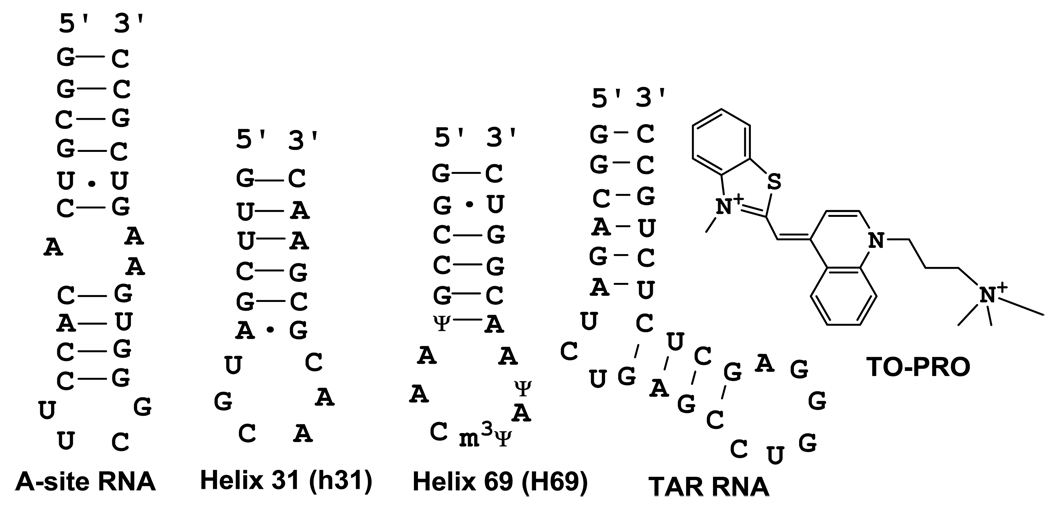
The traditional FID assays employed ethidium bromide or thiazole orange as dyes [8–10; 13]; however, these dyes were either not suitable for RNA FID and/or ESI-MS assays, or not compatible with certain types of ligands. In this study, the binding of a range of ligands from aminoglycosides to peptides was examined by FID using an alternative dye, namely 3-methyl-2-((1-(3-(trimethylammonio)propyl)-4-quinolinylidene)methyl)benzothiazolium (TO-PRO), and a variety of bacterial and viral RNA targets (A-site RNA and helix 31 (h31) of 16S rRNA, helix 69 (H69) of 23S rRNA, and TAR of HIV-1 RNA).
Materials and Methods
RNA preparation
The RNA hairpins with the following sequences were purchased from Dharmacon Research Inc. (Lafayette, CO): 5′-GGCGUCACACCUUCGGGUGAAGUCGCC-3′ (A-site RNA), 5′-GGCAGAUCUGAGCCUGGGAGCUCUCUGCC-3′ (TAR RNA), 5′-GUUCGAUGCAACGCGAAC-3′ (h31), and 5′- GGCCGΨAACm3ΨAΨAACGGUC-3′ (H69), in which Ψ is pseudouridine and m3Ψ is 3-methylpseudouridine.
The 2′-O-ACE-protected RNAs were deprotected by incubating in tetramethylethylenediamine acetate buffer (TEMED-acetate) (pH 3.8) for 30 min at 60 °C [22] The m3Ψ phosphoramidite was provided to Dharmacon for the synthesis of H69 [23]. The RNAs were purified by electrophoresis on 20% denaturing polyacrylamide gels, followed by electroelution with 1/2× TBE (45 mM Tris-HCl, 45 mM boric acid, 1.25 mM Na2EDTA, pH 8.3) in an Amicon centrilutor. The RNAs were desalted by ethanol precipitation using 2.5 M ammonium acetate. The concentration of RNAs were determined using Beer’s law with the following single-stranded extinction coefficients: ε260 nm of 253,390 M−1cm−1 for A-site RNA, 268,900 M−1cm−1 for TAR RNA, 176,900 M−1cm−1 for h31, and 189,400 M−1cm−1 for H69 [24].
Ligands
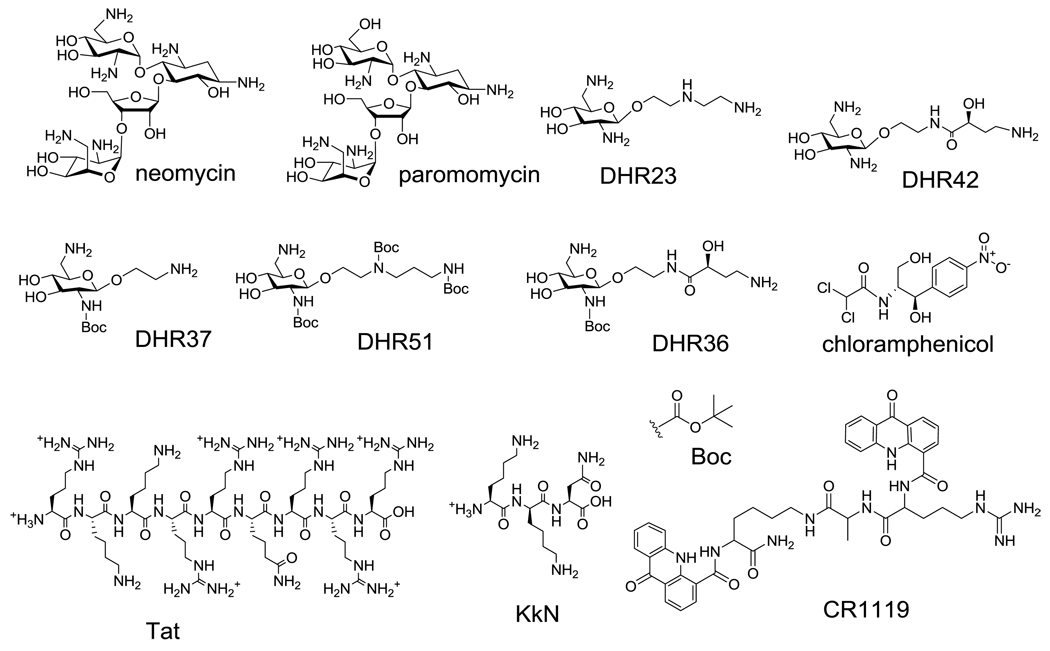
TO-PRO was purchased from Invitrogen (Carlsbad, CA). Paromomycin, neomycin and chloramphenicol were purchased from Sigma Aldrich (St. Louis MO). Tat peptide and KkN were purchased from Bachem (Torrance CA). CR1119 was obtained from the lab of Dr. Mark Spaller (Dartmouth Medical School). Single-ring aminoglycoside analogues were obtained from Shahriar Mobashery (University of Notre Dame).
FID assay
All fluorescence readings were taken on a Cary Eclipse Spectrophotometer (Varian Inc., Walnut Creek, CA). Typically, 2 µM of RNA was incubated with 2 µM of TO-PRO in 150 mM ammonium acetate buffer, pH 7, or 100 mM KCl and 20 mM Tris-Cl, pH 7, for 5 minutes. The initial fluorescence reading was taken and the appropriate concentration of drug was then added and mixed thoroughly and allowed to equilibrate for another 5 minutes. Five scans per sample were taken with an excitation wavelength of 512 nm and emission wavelength of 533 nm. The level of fluorescence change (either enhancement or quenching) was determined and converted to a percent change in fluorescence using the formula (F1/F0)*100, where F0 is the initial fluorescence of dye bound to RNA before the addition of drug and F1 is the fluorescence after addition of drug.
Electrospray ionization mass spectrometry (ESI-MS)
Electrospray ionization mass spectrometry (ESI-MS) experiments were performed on a Quattro LC tandem quadrupole mass spectrometer equipped with electrospray ionization in the negative ion mode (Micromass, Manchester, UK). The samples were injected via a Harvard 11 syringe pump at a flow rate of 6 µL/min. The following tuning parameters were used: capillary voltage 2.5 kV, cone voltage 50 V, extractor voltage 2 V, RF lens voltage 0.6 V, source block temperature 100 °C, and disolvation temperature 120 °C. The RNA-ligand samples were prepared in 150 mM ammonium acetate, pH 7.0. To aid sample disolvation, isopropyl alcohol was added to the samples to achieve a 1:1 (isopropyl alcohol: sample) solution prior to injection.
For competition experiments, the RNA and dye solution were incubated for 5 minutes after which the appropriate drug was added and incubated for another 5 minutes before injection. The final concentrations of RNA and dye were 1 µM. Sixty scans per sample were taken and averaged. The spectra obtained were smoothed and the area under each peak was calculated using Masslynx (Micromass Ltd. Manchester, UK). The faction bound was calculated as follows: the fraction bound of TO-PRO is equal to the peak area corresponding to RNA/TO-PRO complex divided by the peak area corresponding to total RNA [25]. The peak area of total RNA is equal to the summation of the peak area for free RNA, RNA/TO-PRO complex and RNA/paromomycin complex.
Results and Discussion
TO-PRO association with the A-site RNA
A series of commercial dye molecules was screened for one that had ideal fluorescent changes in the presence of RNA and no detectable fluorescence in the absence of RNA. A common staining dye referred to as TO-PRO (3-methyl-2-((1-(3-(trimethylammonio)propyl)-4quinolinylidene)methyl)benzothiazolium) (Figure 1) has essentially no fluorescence in the unbound form in buffer; however, it has fluorescence enhancement of approximately 500-fold when bound to A-site RNA. The A-site RNA is the aminoacyl-tRNA site of the decoding region of bacterial 16S rRNA, and a known antibiotic target site for aminoglycosides [26–28]. A series of titrations were performed with TO-PRO and the A-site RNA construct (Figure 1). The intersection of the pre- and post-saturation data points for fluorescence titrations indicate that TO-PRO associates with the A-site RNA with a stoichiometry of one (Supplementary material: Figure S1). Similarly, by measuring the fluorescence as a function of the molar fraction of TO-PRO, a Job plot of TO-PRO binding to the A-site RNA was generated [29–30]. The two straight lines intersected at a mole fraction of 0.52, which indicates the presence of a 1:1 complex (Supplementary material: Figure S2).
Figure 1.
The RNA constructs and dye indicator (TO-PRO) used in this study are shown.
FID experiments with paromomycin and A-site RNA
Paromomycin, an aminoglycoside that is known to bind to the A-site RNA [27; 31–33], was titrated against 2 µM of the A-site construct bound to 2 µM TO-PRO. The level of fluorescence change (either enhancement or quenching) was determined and converted to a percent change in fluorescence (see Materials and Methods). The results are shown in Table 1. Paromomycin was able to displace pre-bound TO-PRO from the A-site RNA. Upon addition of 31 µM of paromomycin, the fluorescence intensity of TO-PRO was reduced to less than 30%. The buffer conditions used were 150 mM ammonium acetate, pH 7. These conditions were chosen to match the ESI-MS conditions for data comparison purposes. Binding experiments using A-site RNA and paromomycin were performed in 20 mM Tris, 100 mM KCl, pH 7, and similar results were obtained. All results shown are the average of three experiments.
Table 1.
FID Assay Results with Paromomycin
| dye (µM) |
RNA (µM) |
paromomycin (µM)a |
relative fluorescence (%)b |
|---|---|---|---|
| 1 | 0 | 0 | N.D.c |
| 1 | 1 | 0 | 100 |
| 1 | 1 | 2 | 87 |
| 1 | 1 | 10 | 46 |
| 1 | 1 | 19 | 34 |
| 1 | 1 | 31 | 28 |
Equimolar concentrations of A-site RNA and TO-PRO (1 µM) were pre-bound in buffer (150 mM ammonium acetate, pH 7) prior to paromomycin addition.
Average of three separate experiments.
N.D.: not detectable.
FID mechanism examined by ESI-MS
To determine if the change in fluorescence observed was due to displacement of TO-PRO by paromomycin, ESI-MS was utilized. There could be several outcomes when dye (TO-PRO) bound to RNA is treated with various RNA-binding ligands (summarized in Figure 2). One possibility is that the ligand may compete for the same binding site as the dye, thereby displacing the dye from the RNA, leading to a decrease in fluorescence intensity (1). Alternatively, the ligand may bind to a different part of the RNA, in which case fluorescence intensity is unchanged (2), or the ligand may interact with the dye leading to quenching of fluorescence (3). Some ligands may not have affinity for the RNA; in this case, the fluorescence value will be unchanged (4).
Figure 2.
Four possible outcomes of the FID process are depicted. Initially, TO-PRO has negligible fluorescence in the absence of RNA. The dye displays a 500-fold increase in fluorescence intensity in the presence of structured RNA. In the first scenario, the dye is displaced by a ligand, leading to a loss in dye fluorescence. In the second scenario, the ligand and dye have different binding sites, such that no change in fluorescence is observed. In the third case, the ligand and dye have overlapping binding sites, such that fluorescence is quenched. In case 4, the ligand has no affinity for the RNA target, such that TO-PRO remains bound to the RNA and the fluorescence intensity remains the same.
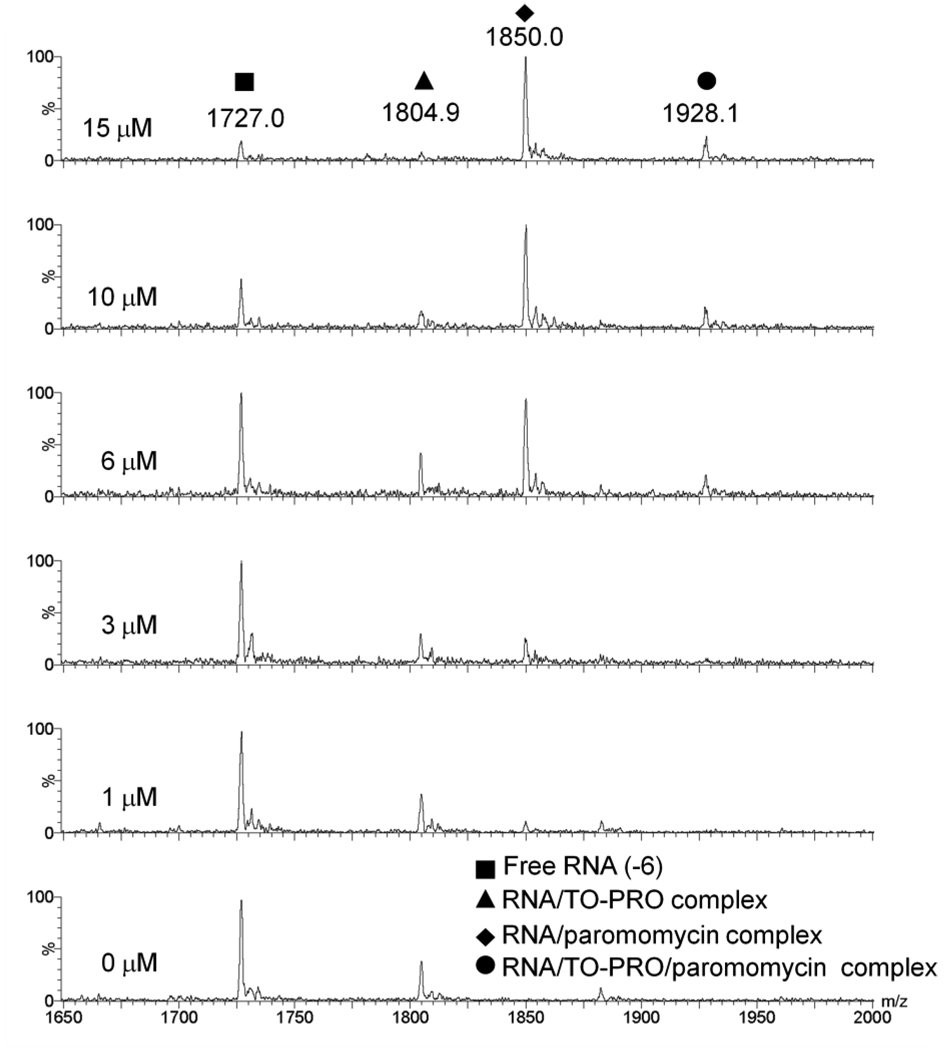
FID is based on a model in which displacement of a fluorescent dye molecule from the host or target results in a decrease of fluorescence intensity [8–11]. In the ESI-MS experiment, we sought evidence to support this model. Competition assays with ESI-MS allows for analysis of the FID mechanism. ESI-MS is useful in this case because the stoichiometry of ligands to RNA can be determined. By monitoring a titration of paromomycin into 1 µM of 1:1 A-site hairpin:TO-PRO complex with ESI-MS, it can be observed that with increasing concentrations of paromomycin, the amount of RNA/TO-PRO complex diminishes and the RNA/paromomycin complex increases (Figure 3). This result indicates competition between the TO-PRO dye and paromomycin for binding to the RNA hairpin. The appearance of a peak representing a complex of RNA/paromomycin/TO-PRO indicates that paromomycin and TO-PRO have additional non-competing binding sites on the RNA hairpin. This result is not surprising since paromomycin is known to be a promiscuous RNA binder [34–35].
Figure 3.
ESI-MS spectra showing a titration of paromomycin (0–15 µM) into equimolar concentrations of A-site RNA and TO-PRO complex (1 µM). Buffer conditions are 150 mM ammonium acetate, pH 7.
The extent of TO-PRO displacement by binding of paromomycin in ESI-MS was quantified and the results are shown in the Supplementary material (Table S1). At 15 µM paromomycin, only a negligible amount of TO-PRO remains bound to the RNA. These data are consistent with a displacement model and corresponding decrease in fluorescence intensity of TO-PRO as it is dissociated from the RNA target (as shown in Figure 2, example 1).
A-site RNA and chloramphenicol
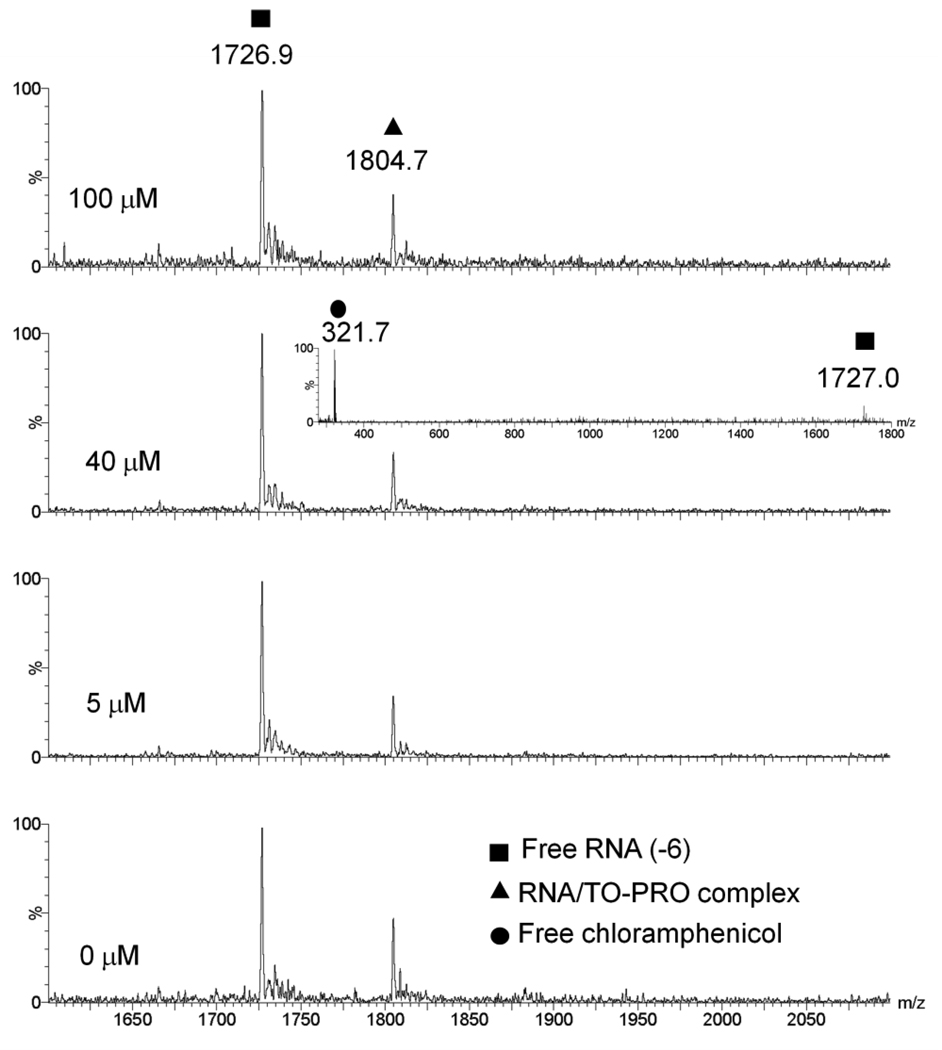
Chloramphenicol is a potent antibiotic that binds to 23S rRNA in the petidyl transferase center of the ribosome and inhibits protein synthesis [36–38]. Chloramphenicol was chosen to serve as negative control because it is a strong RNA binder, but is not known to bind to the A-site RNA. A titration of chloramphenicol into the A-site/TO-PRO complex showed no significant change in fluorescence, even after addition of 31 µM of chloramphenicol (Table 2). In contrast, more than 70% reduction in fluorescence was observed in the case of paromomycin (Table 1). The ESI-MS data also confirms this result. There was no significant change in peak areas of the RNA:TO-PRO complex upon addition of 100 µM of chloramphenicol (Figure 4 and Supplementary material, Table S2). There was no observable peak representing the binding of chloramphenicol to the A-site RNA, which was expected at an m/z value of 1790. A control scan confirmed that at 40 µM of chloramphenicol, there is a high concentration of chloramphenicol in the injected solution as compared to RNA (inset of Figure 4). This result would be consistent with example 4 in Figure 2.
Table 2.
FID Assay Results with Chloramphenicol
| dye (µM) |
RNA (µM) |
Chloramphenicol (µM)a |
relative fluorescence (%)b |
|---|---|---|---|
| 1 | 0 | 0 | N.D.c |
| 1 | 1 | 0 | 100 |
| 1 | 1 | 2 | 100 |
| 1 | 1 | 10 | 100 |
| 1 | 1 | 19 | 99 |
| 1 | 1 | 31 | 99 |
Equimolar concentrations of A-site RNA and TO-PRO (1 µM) were pre-bound in buffer (150 mM ammonium acetate, pH 7) prior to Chloramphenicol addition.
Average of three separate experiments.
N.D.: not detectable.
Figure 4.
ESI-MS spectra showing a titration of Chloramphenicol (1–100 µM) into equimolar concentrations of A-site RNA and TO-PRO complex (1 µM). Buffer conditions are 150 mM ammonium acetate, pH 7. Insert shows the relative concentrations free RNA and free chloramphenicol after 40 µM of chloramphenicol was titrated into the pre-bound A-site RNA and TO-PRO complex.
TAR RNA and Tat
Human immunodeficiency virus type 1 (HIV-1) gene expression is controlled by binding of a viral regulatory protein, known as the trans-activator of transcription (Tat), to its RNA target, the trans-activation responsive region (TAR) [39–41]. This Tat-TAR interaction is crucial for the successful replication of HIV-1 [39–41]. Consequently, disruption of this Tat-TAR interaction has been a good prospect for developing new HIV therapies [42–44]. Studies have identified the minimal motifs for the specific interaction of Tat-TAR to be the 9mer basic amino-acid region of Tat (residues 49–57) and the UCU bulge of the TAR RNA (Figures 1 and 5) [42–44]. This well-characterized Tat-TAR system was used in the modified FID assay.
Figure 5.
The RNA binding ligands used in this study are shown.
FID and ESI-MS experiments were both carried out using the Tat-TAR system. The FID results from the Tat-TAR system show a similar trend as observed with the A-site/aminoglycoside system. Upon addition of 31 µM Tat peptide, a 70% decrease in fluorescence is observed (Supplementary material, Table S3). The reduction in fluorescence was confirmed by using ESI-MS to be due to the displacement of pre-bound TO-PRO from the TAR RNA (Supplementary material: Figure S3 and Table S4).
FID screening results with RNA constructs and ligands
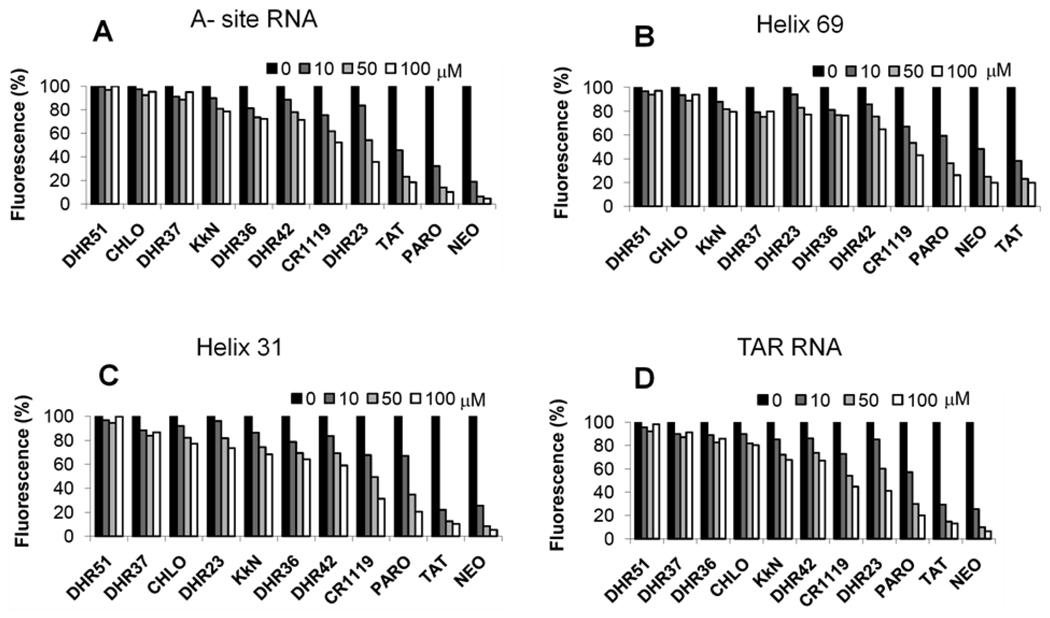
As shown in the previous sections, two well-studied RNAs and known ligands were used to validate the modified FID assay with TO-PRO, namely the Tat-TAR system [39; 45–46] and the A-site RNA-aminoglycoside (paromomycin) system [47–50]. Since the binding sites of paromomycin and Tat are well characterized, these results suggest that the TO-PRO dye molecule associates with the RNAs in the secondary structure elements, namely the nucleotide bulge regions. Our next goal was to test new ligands against the A-site RNA and TAR RNA, and carry out FID with previously untested RNA model systems, namely helix 69 (H69) and helix 31 (h31). These RNAs represent functionally important sites of the bacterial ribosome. H69, from the large subunit of the ribosome, is located in the intersubunit bridge B2a region and makes important contacts with the small ribosomal subunit, A- and P-site bound tRNAs, as well as translation factors [51–52]. This RNA contains two modified nucleotides, pseuduridine (Ψ) and 3-methylpseudouridine (m3Ψ), which can be inserted into the RNA model systems by using synthetic approaches [23]. The small subunit hairpin h31 is located in the 970-loop region of 16S rRNA. Helix 31 serves as a promising drug target because it is located near the P-site (peptidyl-tRNA site) and is proposed to be involved in the decoding process [53–55]. This region is also modified, but we chose the unmodified variant for this study. The h31 and H69 RNAs contain secondary structure elements that differ from the A-site RNA and TAR RNA, such as larger loop regions and nucleotide mismatches.
The FID assay was used to identify ligands for the four RNA constructs shown in Figure 1. The structures of the ligands employed are shown in Figure 5. Among the compounds screened, the cationic aminoglycosides, paromomycin and neomycin, are known to target the A-site RNA [50]. Simplified aminoglycoside, or single-ring derivatives of paromomycin, referred to as DHR23, DHR36, DHR37, DHR42, and DHR51, were also screened. These compounds were designed with the goal of retaining their RNA-binding ability, but reducing their affinity for resistance enzymes, which are known to modify aminoglycosides and reduce their association with RNA [56–57]. KkN is a tri-peptide containing D-lysine (k) that was selected by Hwang and coworkers to target TAR RNA [58]. CR1119 is a peptide-based compound that contains potential intercalating groups for nucleic-acid binding.
The results of the screen reveal that FID is a promising method for rRNA or TAR RNA ligand discovery. The assay was suitable for RNAs with different sequences and secondary structures, as well as modified nucleotides. Perhaps not surprising, the cationic RNA ligands, paromomycin, neomycin, and Tat, show binding to all four constructs, the A-site, h31, H69, and TAR RNAs (Figure 6). As expected chloramphenicol, which is known to be specific for the peptide exit tunnel in the 50S rRNA [37–38], had only slight affinity for the RNA constructs. DHR23, a single-ring analogue of paromomycin, had a slight preference for the A-site RNA and TAR RNA, and CR1119 had a preference for h31 (Figure 6). In contrast, DHR51, which is similar in structure to DHR23 but contains tert-butyloxycarbonyl (BOC) groups at the amine positions (Figure 5), had reduced affinity to all the RNA constructs.
Figure 6.
FID screening results obtained for the different RNA constructs upon addition of ligand concentrations ranging from 0–100 µM: A) A-site RNA, B) H69 RNA, C) h31 RNA, and D) TAR RNA (buffer conditions are 20 mM Tris, 100 mM KCl, pH 7).
The FID results for paromomycin and DHR23 with H69 and A-site RNA were further evaluated by ESI-MS. Dissociation constants obtained from ESI-MS experiments show that DHR23 has a higher affinity for A-site RNA as compared to H69 (Kd = 19 and 43 µM, respectively). Paromomycin also demonstrated a higher affinity for the A-site RNA as compared to H69 (Kd = 1.3 and 21 µM, respectively). These dissociation constants are therefore consistent with the FID data (Table S5).
In summary, a modified FID assay for RNA applications has been developed and the ESI-MS data provide molecular evidence that correlates the reduction in fluorescence observed in the FID assay with the displacement of the TO-PRO dye molecule from RNA. FID with TO-PRO is an appropriate method for obtaining relative binding affinities of a variety of ligands to RNA, including amino sugars, peptides, and planar aromatic compounds. The FID assay will be amenable to high-throughput screening because it is a sensitive, fluorescence-based method that can be done on a 96- or 384-well plate format. The system is compatible with cationic buffer components, including Mg2+, Na+, and K+, which are not suitable for ESI-MS screening. The results with DHR23 show that RNA binding with simplified amino sugars is possible, and with further modifications, more selective reagents may be developed. The moderate selectivity of DHR23 for the A-site and TAR RNAs relative to the ribosomal targets H69 and h31 is encouraging. In contrast, the aromatic compound CR1119 has a slight preference for h31, which contains a six-nucleotide loop and mismatch at the loop-closing base pair. These results suggest that generation of compounds based on these simplified structures in combination with FID screening may lead to selective reagents for RNA internal bulges, loops, mismatches, or other unique secondary structure elements.
Supplementary Material
Aknowledgements
We thank Shahriar Mobashery, Dusan Hesek, and Mijoon Lee for providing the DHR compounds, Chamila Rupasinghe and Mark Spaller for providing CR1119, and Jay Herath for synthesis of H69. This work was supported by NIH GM087596 and AI055496.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: FID, fluorescent intercalator displacement; ESI-MS, electrospray ionization mass spectrometry; TAR, trans- activation responsive region; h31, helix 31; H69, helix 69; TO-PRO, 3-methyl-2-((1-(3-(trimethylammonio)propyl)-4-quinolinylidene)methyl)benzothiazolium; Tat, trans-activator of transcription.
References
- 1.Bonomo RA, Rossolini GM. Importance of antibiotic resistance and resistance mechanisms. Expert. Rev. Anti. Infect. Ther. 2008;6:549–550. doi: 10.1586/14787210.6.5.549. [DOI] [PubMed] [Google Scholar]
- 2.Maragakis LL, Perencevich EN, Cosgrove SE. Clinical and economic burden of antimicrobial resistance. Expert. Rev. Anti. Infect. Ther. 2008;6:751–763. doi: 10.1586/14787210.6.5.751. [DOI] [PubMed] [Google Scholar]
- 3.Bottger EC. The ribosome as a drug target. Trends Biotechnol. 2006;24:145–147. doi: 10.1016/j.tibtech.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Rosu F, Gabelica V, Houssier C, De Pauw E. Determination of affinity, stoichiometry and sequence selectivity of minor groove binder complexes with double-stranded oligodeoxynucleotides by electrospray ionization mass spectrometry. Nucleic Acids Res. 2002;30:e82. doi: 10.1093/nar/gnf081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosu F, De Pauw E, Gabelica V. Electrospray mass spectrometry to study drug-nucleic acids interactions. Biochimie. 2008;90:1074–1087. doi: 10.1016/j.biochi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen B, Tanious FA, Wilson WD. Biosensor-surface plasmon resonance: Quantitative analysis of small molecule-nucleic acid interactions. Methods. 2007;42:150–161. doi: 10.1016/j.ymeth.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Ragazzon PA, Garbett NC, Chaires JB. Competition dialysis: A method for the study of structural selective nucleic acid binding. Methods. 2007;42:173–182. doi: 10.1016/j.ymeth.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Boger DL, Fink BE, Brunette SR, Tse WC, Hedrick MP. A simple, high-resolution method for establishing DNA binding affinity and sequence selectivity. J. Am. Chem. Soc. 2001;123:5878–5891. doi: 10.1021/ja010041a. [DOI] [PubMed] [Google Scholar]
- 9.Boger DL, Tse WC. Thiazole orange as the fluorescent intercalator in a high resolution FID assay for determining DNA binding affinity and sequence selectivity of small molecules. Bioorg. Med. Chem. 2001;9:2511–2518. doi: 10.1016/s0968-0896(01)00243-7. [DOI] [PubMed] [Google Scholar]
- 10.Monchaud D, Allain C, Bertrand H, Smargiasso N, Rosu F, Gabelica V, De Cian A, Mergny JL, Teulade-Fichou MR. Ligands playing musical chairs with G-quadruplex DNA: A rapid and simple displacement assay for identifying selective G-quadruplex binders. Biochimie. 2008;90:1207–1223. doi: 10.1016/j.biochi.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 11.Tse WC, Boger DL. A fluorescent intercalator displacement assay for establishing DNA binding selectivity and affinity. Acc. Chem. Res. 2004;37:61–69. doi: 10.1021/ar030113y. [DOI] [PubMed] [Google Scholar]
- 12.Lewis MA, Long EC. Fluorescent intercalator displacement analyses of DNA binding by the peptide-derived natural products netropsin, actinomycin, and bleomycin. Bioorg. Med. Chem. 2006;14:3481–3490. doi: 10.1016/j.bmc.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Krishnamurthy M, Schirle NT, Beal PA. Screening helix-threading peptides for RNA binding using a thiazole orange displacement assay. Bioorg. Med. Chem. 2008;16:8914–8921. doi: 10.1016/j.bmc.2008.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyer ST, Hergenrother PJ. Small molecule ligands for bulged RNA secondary structures. Org. Lett. 2009;11:4052–4055. doi: 10.1021/ol901478x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Umemoto S, Nakatani K. Fluorescent indicator displacement assay for ligand-RNA interactions. J. Am. Chem. Soc. 2010;132:3660–3661. doi: 10.1021/ja100089u. [DOI] [PubMed] [Google Scholar]
- 16.Ganem B, Li YT, Henion JD. Detection of noncovalent receptor ligand complexes by mass-spectrometry. J. Am. Chem. Soc. 1991;113:6294–6296. [Google Scholar]
- 17.Hofstadler SA, Sannes-Lowery KA. Applications of ESI-MS in drug discovery: interrogation of noncovalent complexes. Nat. Rev. Drug Discovery. 2006;5:585–595. doi: 10.1038/nrd2083. [DOI] [PubMed] [Google Scholar]
- 18.Kempen EC, Brodbelt JS. A method for the determination of binding constants by electrospray ionization mass spectrometry. Anal. Chem. 2000;72:5411–5416. doi: 10.1021/ac000540e. [DOI] [PubMed] [Google Scholar]
- 19.Daniel JM, McCombie G, Wendt S, Zenobi R. Mass spectrometric determination of association constants of adenylate kinase with two noncovalent inhibitors. J. Am. Soc. Mass Spectrom. 2003;14:442–448. doi: 10.1016/S1044-0305(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 20.Stults JT, Marsters JC. Improved electrospray ionization of synthetic oligodeoxynucleotides. Rapid Commun. Mass Spectrom. 1991;5:359–363. [Google Scholar]
- 21.Limbach PA, Crain PF, McCloskey JA. Molecular mass measurments of intact ribonucleic acids via electrospray ionization quadrupole mass spectrometry. J. Am. Soc. Mass Spectrom. 1995;6:27–39. doi: 10.1016/1044-0305(94)00086-F. [DOI] [PubMed] [Google Scholar]
- 22.Scaringe SA, Wincott FE, Caruthers MH. Novel RNA synthesis method using 5'-O-silyl-2'-O-orthoester protecting groups. J. Am. Chem. Soc. 1998;120:1820–1821. [Google Scholar]
- 23.Chui HM, Desaulniers JP, Scaringe SA, Chow CS. Synthesis of helix 69 of Escherichia coli 23S rRNA containing its natural modified nucleosides, m3Ψ and Ψ. J. Org. Chem. 2002;67:8847–8854. doi: 10.1021/jo026364m. [DOI] [PubMed] [Google Scholar]
- 24.Fasman GD. Handbook of biochemistry and molecular biology. Cleveland: CRC Press; 1975. [Google Scholar]
- 25.Sannes-Lowery KA, Griffey RH, Hofstadler SA. Measuring dissociation constants of RNA and aminoglycoside antibiotics by electrospray ionization mass spectrometry. Anal. Biochem. 2000;280:264–271. doi: 10.1006/abio.2000.4550. [DOI] [PubMed] [Google Scholar]
- 26.Recht MI, Fourmy D, Blanchard SC, Dahlquist KD, Puglisi JD. RNA sequence determinants for aminoglycoside binding to an A-site rRNA model oligonucleotide. J. Mol. Biol. 1996;262:421–436. doi: 10.1006/jmbi.1996.0526. [DOI] [PubMed] [Google Scholar]
- 27.Fourmy D, Recht MI, Puglisi JD. Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J. Mol. Biol. 1998;277:347–362. doi: 10.1006/jmbi.1997.1552. [DOI] [PubMed] [Google Scholar]
- 28.Fourmy D, Recht MI, Blanchard SC, Puglisi JD. Structure of the A site of Escherichia coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science. 1996;274:1367–1371. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- 29.Huang CY. Determination of binding stoichiometry by the continuous variation method: the Job plot. Methods Enzymol. 1982;87:509–525. doi: 10.1016/s0076-6879(82)87029-8. [DOI] [PubMed] [Google Scholar]
- 30.Satz AL, Bruice TC. Synthesis of fluorescent microgonotropens (FMGTs) and their interactions with dsDNA. Bioorg. Med. Chem. 2000;8:1871–1880. doi: 10.1016/s0968-0896(00)00116-4. [DOI] [PubMed] [Google Scholar]
- 31.Moazed D, Noller HF. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 32.Purohit P, Stern S. Interactions of a small RNA with antibiotic and RNA ligands of the 30S subunit. Nature. 1994;370:659–662. doi: 10.1038/370659a0. [DOI] [PubMed] [Google Scholar]
- 33.Fourmy D, Yoshizawa S, Puglisi JD. Paromomycin binding induces a local conformational change in the A-site of 16 S rRNA. J. Mol. Biol. 1998;277:333–345. doi: 10.1006/jmbi.1997.1551. [DOI] [PubMed] [Google Scholar]
- 34.Hermann T, Westhof E. Aminoglycoside binding to the hammerhead ribozyme: a general model for the interaction of cationic antibiotics with RNA. J Mol Biol. 1998;276:903–912. doi: 10.1006/jmbi.1997.1590. [DOI] [PubMed] [Google Scholar]
- 35.Hermann T, Westhof E. Saccharide-RNA recognition. Biopolymers. 1998;48:155–165. doi: 10.1002/(SICI)1097-0282(1998)48:2<155::AID-BIP5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Gottlieb D, Bhattacharyya PK. Some properties of an antibiotic obtained from a species of streptomyces. J.Bacteriol. 1948;55:409–417. doi: 10.1128/JB.55.3.409-417.1948. [DOI] [PubMed] [Google Scholar]
- 37.Long KS, Porse BT. A conserved chloramphenicol binding site at the entrance to the ribosomal peptide exit tunnel. Nucleic Acids Res. 2003;31:7208–7215. doi: 10.1093/nar/gkg945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moazed D, Noller HF. Chloramphenicol, erythromycin, carbomycin and vernamycin-B protect overlapping sites in the peptidyl transferase region of 23S-ribosomal RNA. Biochimie. 1987;69:879–884. doi: 10.1016/0300-9084(87)90215-x. [DOI] [PubMed] [Google Scholar]
- 39.Dingwall C, Ernberg I, Gait MJ, Green SM, Heaphy S, Karn J, Lowe AD, Singh M, Skinner MA. Hiv-1 Tat protein stimulates transcription by binding to A U-rich bulge in the stem of the TAR RNA structure. EMBO J. 1990;9:4145–4153. doi: 10.1002/j.1460-2075.1990.tb07637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frankel AD. Activation of HIV transcription by Tat. Curr. Opin. Genet. Dev. 1992;2:293–298. doi: 10.1016/s0959-437x(05)80287-4. [DOI] [PubMed] [Google Scholar]
- 41.Berkhout B, Silverman RH, Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 42.Long KS, Crothers DM. Interaction of human-immunodeficiency-virus type-1 Tat-derived peptides with TAR RNA. Biochemistry. 1995;34:8885–8895. doi: 10.1021/bi00027a041. [DOI] [PubMed] [Google Scholar]
- 43.Mei HY, Galan AA, Halim NS, Mack DP, Moreland DW, Sanders KB, Truong HN, Czarnik AW. Inhibition of an HIV-1 Tat-derived peptide binding to TAR RNA by aminoglycoside antibiotics. Bioorg. Med. Chem. Lett. 1995;5:2755–2760. [Google Scholar]
- 44.Weeks KM, Ampe C, Schultz SC, Steitz TA, Crothers DM. Fragments of the HIV-1 Tat protein specifically bind TAR RNA. Science. 1990;249:1281–1285. doi: 10.1126/science.2205002. [DOI] [PubMed] [Google Scholar]
- 45.Chaloin O, Peter JC, Briand JP, Masquida B, Desgranges C, Muller S, Hoebeke J. The N-terminus of HIV-1 Tat protein is essential for Tat-TAR RNA interaction. Cell. Mol. Life Sci. 2005;62:355–361. doi: 10.1007/s00018-004-4477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sannes-Lowery KA, Hu PF, Mack DP, Mei HY, Loo JA. HIV 1 Tat peptide binding do to TAR RNA by electrospray ionization mass spectrometry. Anal. Chem. 1997;69:5130–5135. doi: 10.1021/ac970745w. [DOI] [PubMed] [Google Scholar]
- 47.Hendrix M, Priestley ES, Joyce GF, Wong CH. Direct observation of aminoglycoside-RNA interactions by surface plasmon resonance. J. Am. Chem. Soc. 1997;119:3641–3648. doi: 10.1021/ja964290o. [DOI] [PubMed] [Google Scholar]
- 48.Ryu DH, Rando RR. Aminoglycoside binding to human and bacterial A-site rRNA decoding region constructs. Bioorg. Med. Chem. 2001;9:2601–2608. doi: 10.1016/s0968-0896(01)00034-7. [DOI] [PubMed] [Google Scholar]
- 49.Ryu DH, Rando RR. Decoding region bubble size and aminoglycoside antibiotic binding. Bioorg. Med. Chem. Lett. 2002;12:2241–2244. doi: 10.1016/s0960-894x(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 50.Wong CH, Hendrix M, Priestley ES, Greenberg WA. Specificity of aminoglycoside antibiotics for the A-site of the decoding region of ribosomal RNA. Chem. Biol. 1998;5:397–406. doi: 10.1016/s1074-5521(98)90073-4. [DOI] [PubMed] [Google Scholar]
- 51.Gabashvili IS, Agrawal RK, Spahn CMT, Grassucci RA, Svergun DI, Frank J, Penczek P. Solution structure of the E. coli 70S ribosome at 11.5 Å resolution. Cell. 2000;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 52.Yusupov MM, Yusupova GZ, Baucom A, Lieberman K, Earnest TN, Cate JHD, Noller HF. Crystal structure of the ribosome at 5.5 Å resolution. Science. 2001;292:883–896. doi: 10.1126/science.1060089. [DOI] [PubMed] [Google Scholar]
- 53.Doring T, Mitchell P, Osswald M, Bochkariov D, Brimacombe R. The decoding region of 16S RNA - A cross-linking study of the ribosomal A-site, P-site and E-site using transfer-RNA derivatized at position-32 in the anticodon loop. EMBO J. 1994;13:2677–2685. doi: 10.1002/j.1460-2075.1994.tb06558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Korostelev A, Trakhanov S, Laurberg M, Noller HF. Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements. Cell. 2006;126:1065–1077. doi: 10.1016/j.cell.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 55.Selmer M, Dunham CM, Murphy FV, Weixlbaumer A, Petry S, Kelley AC, Weir JR, Ramakrishnan V. Structure of the 70S ribosome complexed with mRNA and tRNA. Science. 2006;313:1935–1942. doi: 10.1126/science.1131127. [DOI] [PubMed] [Google Scholar]
- 56.Haddad J, Kotra LP, Llano-Sotelo B, Kim C, Azucena EF, Jr, Liu M, Vakulenko SB, Chow CS, Mobashery S. Design of novel antibiotics that bind to the ribosomal acyltransfer site. J. Am. Chem. Soc. 2002;124:3229–3237. doi: 10.1021/ja011695m. [DOI] [PubMed] [Google Scholar]
- 57.Llano-Sotelo B, Azucena EF, Jr, Kotra LP, Mobashery S, Chow CS. Aminoglycosides modified by resistance enzymes display diminished binding to the bacterial ribosomal aminoacyl-tRNA site. Chem. Biol. 2002;9:455–463. doi: 10.1016/s1074-5521(02)00125-4. [DOI] [PubMed] [Google Scholar]
- 58.Hwang S, Tamilarasu N, Ryan K, Huq I, Richter S, Still WC, Rana TM. Inhibition of gene expression in human cells through small molecule-RNA interactions. Proc. Natl. Acad. Sci. U. S. A. 1999;96:12997–13002. doi: 10.1073/pnas.96.23.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.