Abstract
The asymmetric total synthesis of the α-glucosidase inhibitor (+)-castanospermine is reported. The central theme in our approach to this polyhydroxylated alkaloid is the simultaneous generation of the piperidine ring and the C-1/8a erythro stereodiad through the diastereoselective, oxamidation of an unsaturated O-alkyl hydroxamate. This process is believed to proceed sequentially via singlet acylnitrenium and aziridinium ion intermediates.
(+)-Castanospermine (1), a polyhydroxylated indolizidine alkaloid originally isolated from the seeds of the Moreton Bay chestnut tree (Castanospermum australe),1 displays a prestigious range of biological activities which stem from its role as a glycosidase inhibitor (Scheme 1).2 Compound 1’s ability to inhibit endoplasmic reticulum (ER) glucosidase I is of particular significance since this leads to the abrogation of normal glycoprotein trafficking,3 a process critical to a host of cellular functions as well as the coat protein biogenesis of enveloped viruses.4 From a drug-development standpoint, compound 1 has elicited considerable interest since it displays activity against several human viral pathogens, including HCV, parainfluenza, dengue virus, HSV-2, and HIV-1.5 Most recently, castanospermine has also been found to inhibit the Rho/Ras-glycosylating action of Clostridium difficile toxin B,6 which is the major virulence factor of this Gram-positive bacteria and the causative agent of antibiotic-associated pseudomembranous colitis, a leading cause of infectious diarrhoea in hospitals worldwide.7
Scheme 1.
Retrosynthetic Analysis of Castanospermine (1).
Given the biological activity of castanospermine, it is understandable that almost 30 years after its initial isolation, this alkaloid remains a relevant and popular synthetic target.8,9 That minor structural/stereochemical alterations to 1 lead to dramatic alterations in glycosidase selectivity, only adds further impetus to the development of new synthetic routes to this natural product.10 In light our of our ongoing interest in the synthesis of α-glucosidase inhibitors11,12 and having recently reported a versatile oxamidation method for the preparation of α-hydroxyalkyl lactams involving the intramolecular addition of acylnitrenium ions to alkenes,13 we were prompted to consider whether this methodology might be gainfully employed in the enantioselective preparation of (+)-castanospermine. Herein, we report the successful implementation of this idea through the use of a substrate-controlled nitrenium ion oxamidation reaction.
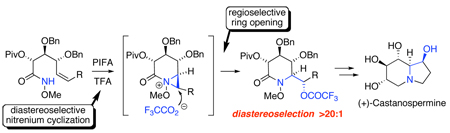
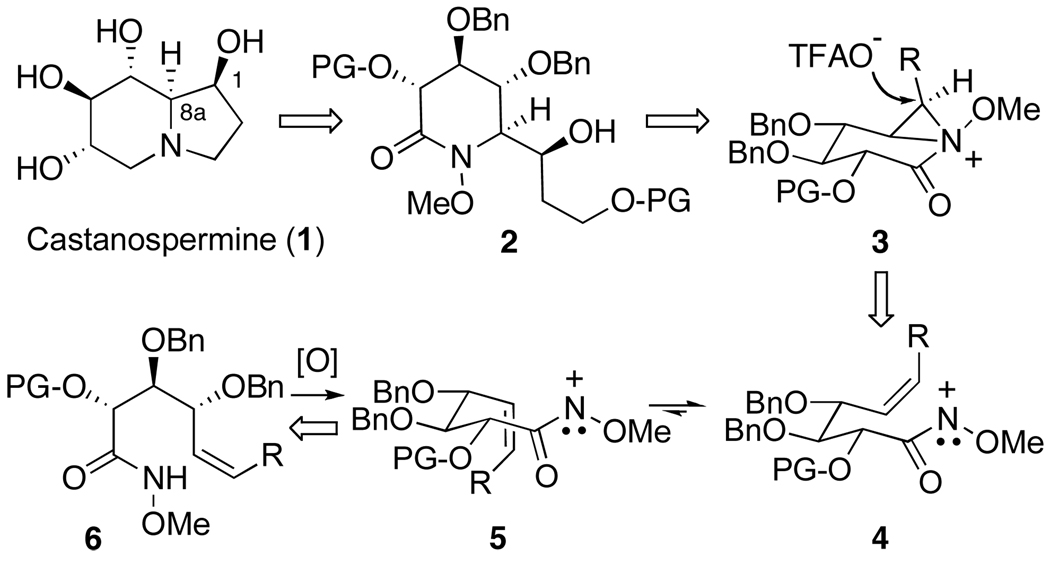
From a reterosynthetic perspective, we envisioned that the indolizidine ring of 1 could be generated from α-hydroxyalkyl lactam 2 through a sequence of reduction and ring closure (Scheme 1). In turn, this compound would be accessed through the cyclization of the nitrenium ion generated upon the oxidation of methyl d-gluco-hydroxamate 6. Since singlet nitrenium ions are known to undergo concerted addition to alkenes,14 this reaction would generate bicyclic aziridinium ion 3, which upon concerted, regioselective ion-pair collapse at the external (α) position15 and hydrolysis of the resulting triflouroacetate ester adduct would provide δ-lactam 2 and thereby establish the C-1/8a erythro stereodiad of the natural product. Regarding the diastereoselectivity of the addition process, we anticipated that cyclization of the nitrenium ion generated from 6 would preferentially proceed via a transition state resembling pseudo-chair 4, thereby avoiding the 1,3-allylic strain16 present in boat-like conformer 5.17
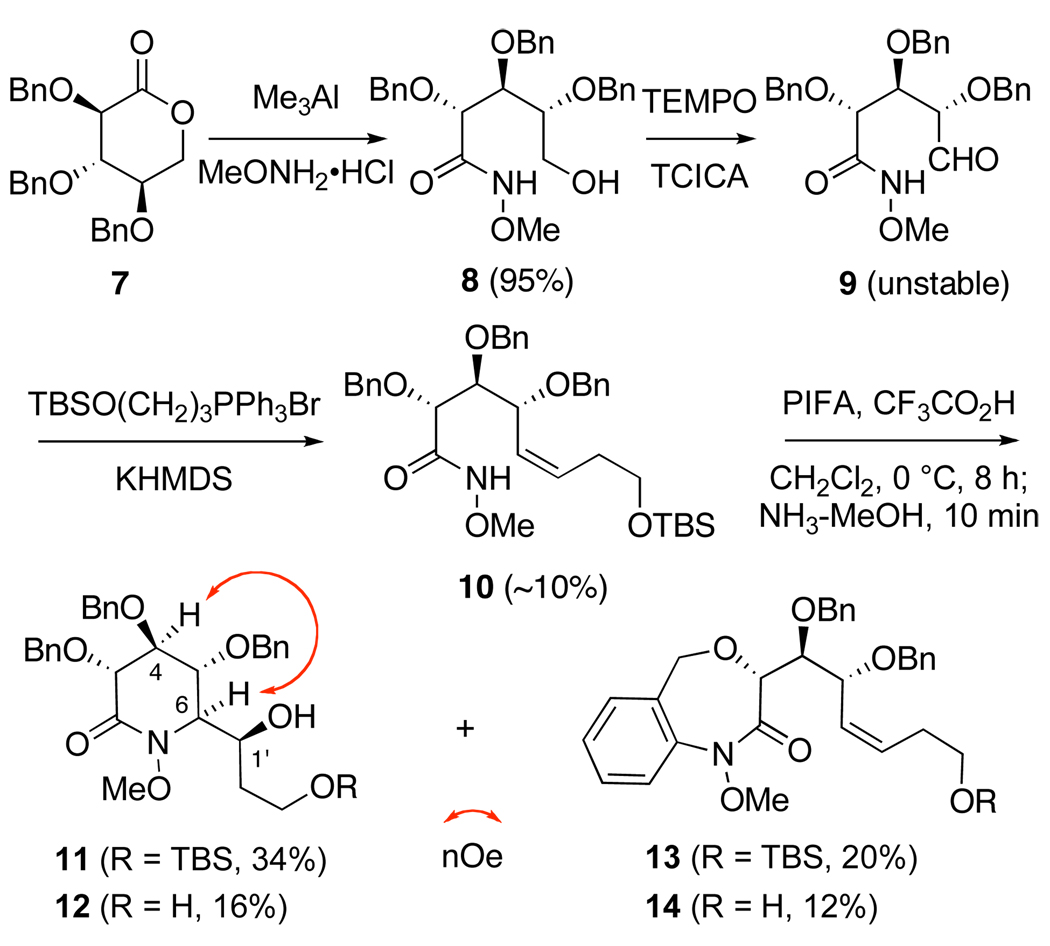
Our initial route towards (+)-castanospermine (1) commenced from tribenzyl d-glucono-δ-lactone (7),18 which underwent ring opening with the methoxylamine in the presence of Me3Al19 to provide O-methyl hydroxamate 8 in excellent yield (Scheme 2). Chemoselective oxidation of the primary alcohol, using TEMPO and trichloroisocyanuric acid (TCICA),20 now generated unstable aldehyde 9.21 Exposure of this substrate to the ylide generated from 3-(tert-butyldimethylsilyloxy)propyl phosphonium bromide and KHMDS22 provided 10 in poor yield. Inefficiencies of preparation notwithstanding, we proceeded to investigate the key cyclization step using this substrate.
Scheme 2.
Initial Route to (+)-Castanospermine (1).
Upon exposure to phenyliodine bis(triflouroacetate) (PIFA) and trifluoroacetic acid in CH2Cl2, hydroxamate 10 underwent slow cyclization to form a mixture of products which following in-situ ammonolysis of the trifluoroacetate adducts, were separated by flash chromatography to provide compounds 11 and 13 and their desilyated counterparts 12 and 14. The unanticipated formation of 1,4-oxazepan-3-ones 13 and 14 presumably arises from competitive interception of the nitrenium ion intermediate by the C-3 benzyl ether in 10.23 More encouragingly, oxamidation products 11 and 12 were isolated as single diastereomers, which NOSEY experiments indicated were of the desired stereochemistry at C-6.
In light of the involvement of the C-3 O-protecting group during cyclization and the inefficiency of the preceding Wittig reaction, a number of alterations to our synthetic plan were clearly mandated. Accordingly, we decided to evaluate alternative protecting groups that would not irreversibly trap the putative N-acylnitrenium ion. In order to impede loss of the TBS silyl ether under the acidic reaction conditions, a TIPS protecting group was chosen as a more robust surrogate.
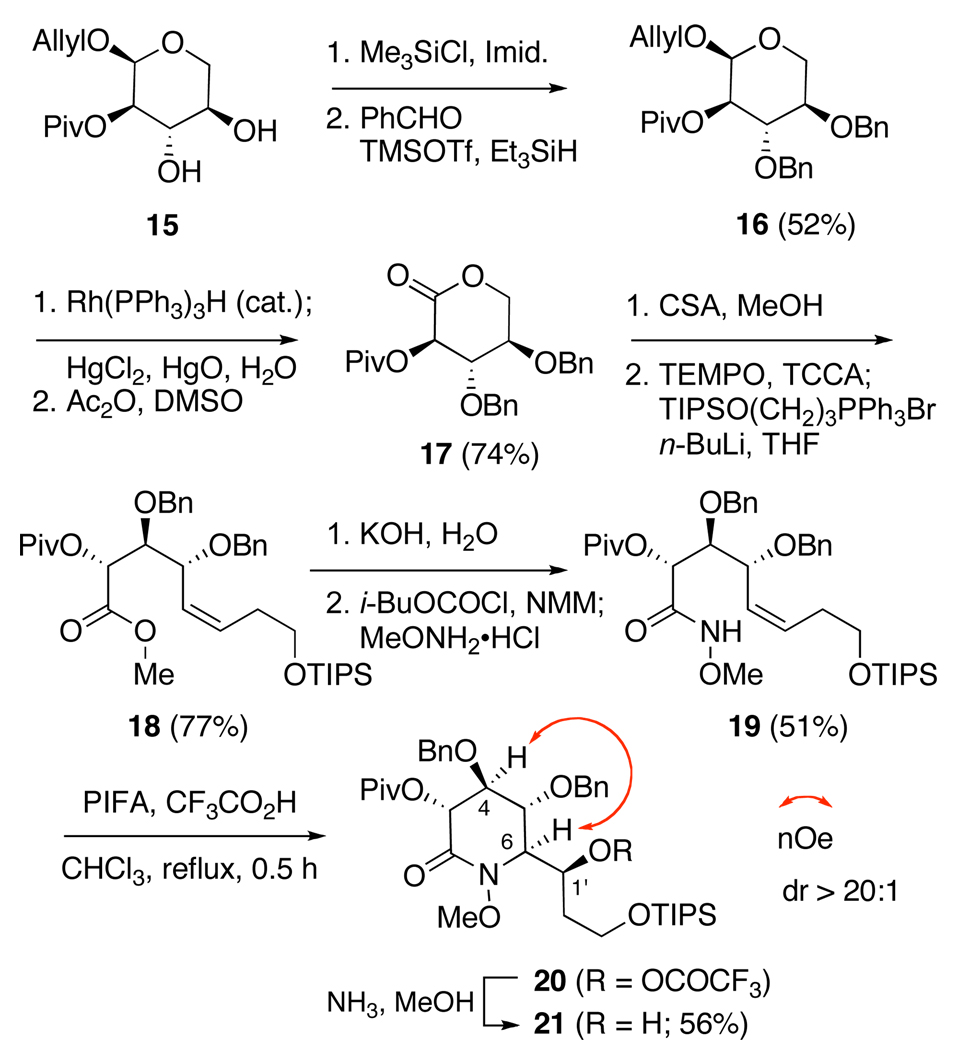
Our plan thus amended, the second-generation, and ultimately successful, route to (+)-castanospermine commenced from α-d-xylopyranoside 15,24 which through reductive etherification of the corresponding (bis)trimethylsilyl ether using benzaldehyde,25 was converted to dibenzyl ether 16 (Scheme 3). While direct deallylation of 16 proved to be unexpectedly challenging and failed with a number of reagents, a stepwise approach to this task ultimately proved successful. Thus, exposure of 16 to catalytic HRh(PPh3)3 in THF provided the corresponding enol ether which without isolation, was hydrolyzed with HgCl2-HgO to afford a mixture of lactol anomers in good overall yield.26 Conversion of these products to lactone 17 while sluggish with PDC or PCC, proceeded with high efficiency under the Albright-Goldman conditions (DMSO, Ac2O).27
Scheme 3.
2nd Generation Route to (+)-Castanospermine (1).
In preparation for installation of the pendant alkene, methanolysis of 17 in the presence of camphorsulfonic acid provided the corresponding δ-hydroxy ester in high yield. Addition of a phosphate buffer (pH 7) prior to workup, to prevent transesterification to 17, was found to be a prerequisite for the success of this reaction. Oxidation of the primary alcohol, again using TEMPO and TCICA, and treatment of the aldehyde with the ylide generated from (3-isopropylsiloxy)propyltriphenylphosphonium bromide28 then provided 18 in high overall yield. Chemoselective saponification of the methyl ester was now accomplished by the treatment of 18 with aqueous KOH (0.1 M, 3 equiv) in THF and MeOH. Formation of oxamidation substrate 19 was then accomplished by conversion of 18 to the corresponding mixed anhydride, which was treated with methoxylamine hydrochloride.
Disappointingly, exposure of compound 19 to PIFA in the presence of trifluoroacetic acid at room temperature failed to effect cyclization and instead generated an intractable mixture of products.29 Fortunately, performing this reaction at higher temperature, accomplished by adding 19 to a solution of PIFA and trifluoroacetic acid in CHCl3 at reflux, rapidly afforded 20, which was isolated as a single diasteroisomer after in-situ ammonolysis of the trifluoroacetate adduct. That 19 does not undergo cyclization at ambient temperature, in contrast to compound 10, may reflect a decrease in the stability of the intermediate nitrenium ion, which in this case, could be destabilized by the presence of a more electron withdrawing, α-acyloxy substituent. This interpretation is consistent with our current belief that formation of this N-electrophile is rate limiting and may occur from an N-methoxy-N-trifluoroacetoxy, or anomeric, amide.30
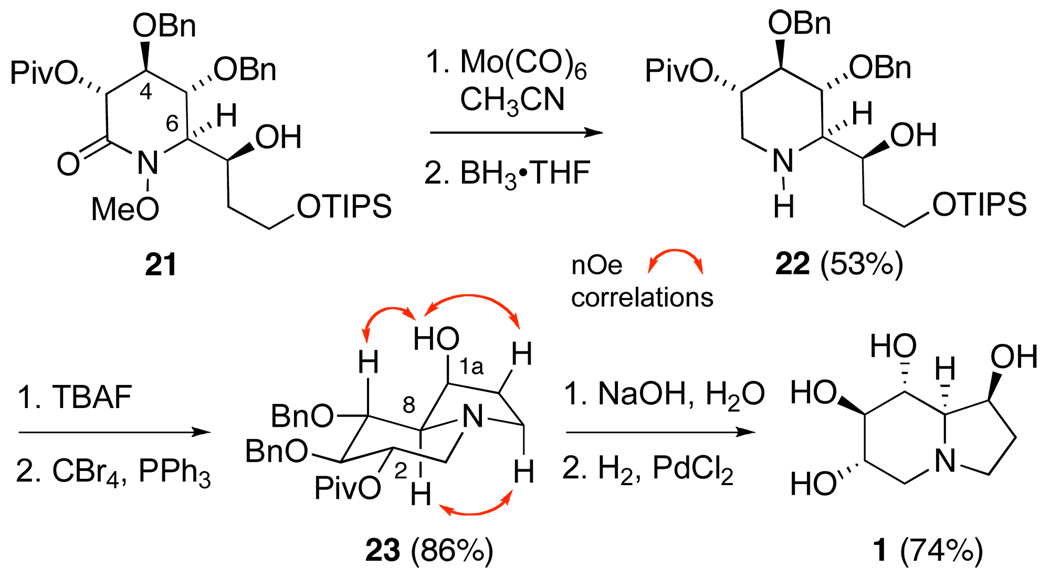
As with piperidinones 11 and 12, a 2D NOESY experiment conducted upon 21 revealed a correlation between H-4 and H-6, which together with the absence of interactions between H-3 and H-5, is diagnostic of a half-chair conformation31 and confirms the relative stereochemistry at C-6 (Scheme 3).
Treatment of compound 21 with Mo(CO)6 in aqueous acetonitrile now cleaved the N-O bond and provided the corresponding NH-lactam,32 which was reduced with borane to yield piperidine 22. Removal of the TIPS ether, using TBAF in THF, then provided the corresponding amino-2,4-diol in excellent yield. Selective bromination of this primary alcohol and in-situ cyclization to indolizidine 23 was now accomplished by recourse to Appel’s conditions.33 Reflecting the relatively hindered environment at the C-2 position, saponification of the pivalate ester now proceeded slowly at room temperature to provide the 6,7-di-O-benzyl ether of castanospermine, albeit in high yield. With the C-1a stereocenter now constrained within the indolizidine ring system, unambiguous assignment of stereochemistry was possible through a NOSEY experiment (Scheme 4). Finally, removal of the benzyl ethers, via hydrogenolysis in the presence of PdCl2, provided (+)-castanospermine (1). The 1H and 13C NMR spectral data obtained from this material were identical to those reported for the natural product. In addition, the optical rotation of synthetic 1 ([α]24D − 86.0; c 0.1, MeOH) closely matched that of the natural product ([α]25D −87.2; c 2.1, MeOH).34
Scheme 4.
Total Synthesis of (+)-Castanospermine (1).
In conclusion, we have developed a 15-step synthesis of (+)-castanospermine (1) in which the C-1/8a stereodiad is established through the diastereoselective oxamidation of an unsaturated O-alkyl hydroxamate. That this process was found to be stereospecific with respect to alkene geometry supports our current belief that the oxamidation proceeds via the concerted ring opening of a bicyclic aziridinium ion formed upon the addition of a singlet nitrenium ion to the pendant alkene. Extension of this valuable methodology to the preparation of other azasugars is now in progress.
Supplementary Material
Acknowledgment
We thank the National Institutes of Health (GM-67176) and the University of Illinois at Chicago for financial support of this work.
Footnotes
Supporting Information Available Experimental procedures and characterization data for all new compounds.
References
- 1.(a) Hohenschutz LD, Bell EA, Jewess PJ, Leworthy DP, Pryce RJ, Arnold E, Clardy J. Phytochemistry. 1981;4:811. [Google Scholar]; (b) Nash RJ, Fellows LE, Dring JV, Stirton CH, Carter D, Hegarty MP, Bell EA. Phytochemistry. 1988;5:1403. [Google Scholar]
- 2.(a) Saul R, Chambers JP, Molyneux RJ, Elbein AD. Arch. Biochem. Biophys. 1983;221:593. doi: 10.1016/0003-9861(83)90181-9. (β-glucosidase, β-glucocerebrosidase, β-xylosidase); [DOI] [PubMed] [Google Scholar]; (b) Trugnan G, Rousset M, Zweibaum A. Febs Lett. 1986;195:28. doi: 10.1016/0014-5793(86)80123-5. (sucrase); [DOI] [PubMed] [Google Scholar]; (c) Campbell BC, Molyneux RJ, Jones KC. J. Chem. Ecol. 1987;13:1759. doi: 10.1007/BF00980216. (disaccharidases); [DOI] [PubMed] [Google Scholar]; (h) Scofield AM, Rossiter JT, Witham P, Kite GC, Nash RJ, Fellows LE. Phytochemistry. 1990;29:107. (thioglucosidase); [Google Scholar]; (i) Valaitis AP, Bowers DF. Insect Biochem. Mol. Biol. 1993;23:599. doi: 10.1016/0965-1748(93)90033-o. (trehalase). [DOI] [PubMed] [Google Scholar]
- 3.(a) Gloster T, Meloncelli P, Stick R, Zechel D, Vasella A, Davies G. J. Am. Chem. Soc. 2007;129:2345. doi: 10.1021/ja066961g. [DOI] [PubMed] [Google Scholar]; (b) Pan YT, Hori H, Saul R, Sanford BA, Molyneux RJ, Elbein AD. Biochemistry. 1983;22:3975. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]; (c) Sasak VW, Ordovas JM, Elbein AD, Berninger RW. Biochem. J. 1985;232:759. doi: 10.1042/bj2320759. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ellmers BR, Rhinehart BL, Robinson KM. Biochem. Pharmacol. 1987;36:2381. doi: 10.1016/0006-2952(87)90607-1. [DOI] [PubMed] [Google Scholar]; (e) Foddy L, Hughes RC. Eur. J. Pharmacol. 1988;175:291. doi: 10.1111/j.1432-1033.1988.tb14196.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gloster TM, Davies GJ. Org. Biomol. Chem. 2010;8:305. doi: 10.1039/b915870g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Winchester BG. Tetrahedron: Asymmetry. 2009;20:645. [Google Scholar]
- 5.(a) Durantel D. Curr. Opin. Invest. Drugs. 2009;10:860. (HCV). [PubMed] [Google Scholar]; (b) Tanaka Y, Kato J, Kohara M, Galinski M. Antiviral Res. 2006;72:1. doi: 10.1016/j.antiviral.2006.03.016. (parainfluenza). [DOI] [PubMed] [Google Scholar]; (c) Whitby K, Pierson T, Geiss B, Lane K, Engle M, Zhou Y, Doms R, Diamond M. J. Virol. 2005;79:8698. doi: 10.1128/JVI.79.14.8698-8706.2005. (dengue virus). [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Whitby K, Taylor D, Patel D, Ahmed P, Tyms A. Antivir. Chem. Chemother. 2004;15:141. doi: 10.1177/095632020401500304. (bovine viral diarrhoea virus/HCV). [DOI] [PubMed] [Google Scholar]; (e) Ahmed SP, Nash R, Bridges C, Taylor D, Kang M, Porter E, Tyms A. Biochem. Biophys. Res. Commun. 1995;208:267. doi: 10.1006/bbrc.1995.1333. (HSV-2). [DOI] [PubMed] [Google Scholar]; (f) Bridges C, Brennan T, Taylor D, McPherson M, Tyms A. Antiviral Res. 1994;25:169. doi: 10.1016/0166-3542(94)90105-8. (HIV-1). [DOI] [PubMed] [Google Scholar]
- 6.Jank T, Ziegler MO, Schulz GE, Aktories K. FEBS Lett. 2008;582:2277. doi: 10.1016/j.febslet.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 7.Lyras D, O'Connor J, Howarth P, Sambol S, Carter G, Phumoonna T, Poon R, Adams V, Vedantam G, Johnson S, Gerding D, Rood J. Nature. 2009;458:1176. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For recent syntheses of castanospermine, see: Liu G, Wu TJ, Ruan YP, Huang PQ. Chem.-Eur. J. 2010;16:5755. doi: 10.1002/chem.200903490. Jensen T, Mikkelsen M, Lauritsen A, Andresen TL, Gotfredsen CH, Madsen R. J. Org. Chem. 2009;74:8886. doi: 10.1021/jo9019495. Ceccon J, Danoun G, Greene AE, Poisson JF. Org. Biomol. Chem. 2009;7:2029. doi: 10.1039/b901488h. Machan T, Davis AS, Liawruangrath B, Pyne SG. Tetrahedron. 2008;64:2725. Karanjule NS, Markad SD, Shinde VS, Dhavale DD. J. Org. Chem. 2006;71:4667. doi: 10.1021/jo0601617. Zhao ZM, Song L, Mariano PS. Tetrahedron. 2005;61:8888. Cronin L, Murphy PV. Org. Lett. 2005;7:2691. doi: 10.1021/ol0508577..
- 9.(a) For a review of recent total syntheses of castanospermine and related α-glucosidase inhibitors, see: Wardrop DJ, Waidyarachchi SL. Nat. Prod. Reports. 2010;27:1413. doi: 10.1039/b914958a.. (b) For a review of earlier syntheses of castanospermine, see: López MD, Cobo J, Nogueras M. Curr. Org. Chem. 2008;12:718..
- 10.For bioactive analogs of castanospermine, see: Louvel J, Botuha C, Chemla F, Demont E, Ferreira F, Pérez-Luna A. Eur. J. Org. Chem. 2010:2921. Pluvinage B, Ghinet MG, Brzezinski R, Boraston AB, Stubbs KA. Org. Biomol. Chem. 2009;7:4169. doi: 10.1039/b913235j. Aguilar-Moncayo M, Mellet CO, Fernández JMG, Garcia-Moreno MI. J. Org. Chem. 2009;74:3595. doi: 10.1021/jo900231b. Aguilar-Moncayo M, Gloster TM, Turkenburg JP, Garcia-Moreno MI, Mellet CO. Davies GJ, Fernández JMG. Org. Biomol. Chem. 2009;7:2738. doi: 10.1039/b906968b. Benltifa M, Garcia Moreno MI, Ortiz Mellet C, Garcia Fernández JM, Wadouachi A. Bioorg. Med. Chem. Lett. 2008;18:2805. doi: 10.1016/j.bmcl.2008.04.004..
- 11.Bowen EG, Wardrop DJ. J. Am. Chem. Soc. 2009;131:6062. doi: 10.1021/ja9005755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.See reference 9a.
- 13.Wardrop DJ, Bowen EG, Forslund RE, Sussman AD, Weerasekera SL. J. Am. Chem. Soc. 2010;132:1188. doi: 10.1021/ja9069997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Rudchenko VF, Ignatov SM, Kostyanovsky RG. Chem. Commun. 1990:261. [Google Scholar]; (b) Vedejs E, Sano H. Tetrahedron Lett. 1992;33:3261. [Google Scholar]; (c) Hoffman RV, Christophe NB. J. Org. Chem. 1988;53:4769. [Google Scholar]
- 15.For regioselective ring opening of a 1-azabicyclo[4.1.0]heptane-based aziridinium ion, see: D'hooghe MD, Vanlangendonck T, Törnroos KW, De Kimpe N. J. Org. Chem. 2006;71:4678. doi: 10.1021/jo060313y.. (b) Reference 13.
- 16.Hoffmann RW. Chem. Rev. 1989;89:1841. [Google Scholar]
- 17.For a related conformational analysis made in reference to an intramolecular Huisgen alkene-azide cycloaddition, see: Zhou Y, Murphy PV. Org. Lett. 2008;10:3777. doi: 10.1021/ol8014495..
- 18.López R, Fernández-Mayorala A. J. Org. Chem. 1994;59:737. [Google Scholar]
- 19.(a) Takahashi H, Hitomi Y, Iwai Y, Ikegami S. J. Am. Chem. Soc. 2000;122:2995. [Google Scholar]; (b) Basha A, Lipton M, Weinreb SM. Tetrahedron Lett. 1977;18:4171. [Google Scholar]
- 20.De Luca L, Giacomelli G, Porcheddu A. Org. Lett. 2001;3:3041. doi: 10.1021/ol016501m. [DOI] [PubMed] [Google Scholar]
- 21.Upon standing for 12 h at room temperature, compound 9 underwent cyclization to form a mixture of cyclic carbinolamines diastereomers.
- 22.Glebocka A, Sicinski R, Plum L, Clagett-Dame M, Deluca H. J. Med. Chem. 2006;49:2909. doi: 10.1021/jm051082a. [DOI] [PubMed] [Google Scholar]
- 23.Kikugawa Y, Shimado M, Matsumoto K. Heterocycles. 1994;37:293. [Google Scholar]
- 24.Prepared in two steps, from d-xylose: Rosenberg H, Riley A, Marwood R, Correa V, Taylor C, Potter B. Carbohydr. Res. 2001;332:53. doi: 10.1016/s0008-6215(01)00067-2..
- 25.Hatakeyama S, Mori H, Kitano K, Yamada H, Nishizawa M. Tetrahedron Lett. 1994;35:4367. [Google Scholar]
- 26.(a) Boons G, Burton A, Isles S. Chem. Commun. 1996;141 [Google Scholar]; (b) Uma R, Davies M, Crévisy C, Grée R. Eur. J. Org. Chem. 2001:3141. [Google Scholar]; (c) Corey EJ, Suggs JW. J. Org. Chem. 1973;38:3224. [Google Scholar]
- 27.Albright JD, Goldman L. J. Am. Chem. Soc. 1965;18:4214. [Google Scholar]
- 28.For details of preparation, see: Supporting Information and Clayden J, Knowles FE, Baldwin IR. J. Am. Chem. Soc. 2005;127:2412. doi: 10.1021/ja042415g..
- 29.Oxidation of O-alkyl hydroxamates can also lead to the formation of thermally unstable N,N'-dialkoxy-N,N'-diacylhydrazines:. De Almeida MV, Barton DHR, Bytheway I, Ferreira JA, Hall MB, Liu W, Taylor DK, Thomson L. J. Am. Chem. Soc. 1995;117:4870. Cooley JH, Mosher MW, Khan MA. J. Am. Chem. Soc. 1968;90:1867. Crawford RJ, Raap R. J. Org. Chem. 1963;28:2419..
- 30.(a) Glover SA. Adv. Phys. Org. Chem. 2008;42:35. [Google Scholar]; (b) Glover SA, Rauk A. J. Chem. Soc., Perkin Trans. 2002;2:1740. [Google Scholar]; (c) Glover SA, Hammond GP. J. Org. Chem. 1998;63:9684. [Google Scholar]
- 31.Boudreault N, Ball RG, Bayly C, Bernstein MA, Leblanc Y. Tetrahedron. 1994;50:7947. [Google Scholar]
- 32.Cicchi S, Goti A, Brandi A, Guarna A, De Sarlo F. Tetrahedron Letters. 1990;31:3351. [Google Scholar]
- 33.Appel R. Angew. Chem. Int. Ed. 1975;14:801. [Google Scholar]
- 34.Scheider MJ, Ungemach FS, Broquist HP, Harris TM. Tetrahedron. 1983;39:29. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.