Abstract
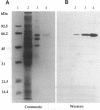
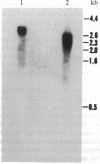
Prolyl 4-hydroxylase (EC 1.14.11.2) is a key enzyme required for the posttranslational hydroxylation of proline residues in collagen. The enzyme is a tetramer composed of two pairs of nonidentical subunits (alpha 2 beta 2). The beta subunit is protein disulfide-isomerase, a ubiquitous enzyme found in the endoplasmic reticulum of many cell types. We report here the amino acid sequence of the alpha subunit. One cDNA clone (alpha 1) was isolated from a chicken embryo cDNA expression library in lambda gt11 by screening with anti-alpha-subunit polyclonal immunoglobulins. This alpha 1 cDNA contains an open reading frame of 1401 base pairs. A comparison of the translation of the nucleotide sequence with protein sequences obtained from the purified chicken alpha-subunit polypeptide verified that alpha 1 cDNA encoded the alpha subunit. Polymerase chain reactions were used to extend the sequence of alpha 1 cDNA toward the 5' end of alpha-subunit mRNA. The mature alpha subunit is composed of 516 amino acids with a calculated molecular mass of 59,373 Da. The compiled amino acid sequence contains two potential glycosylation sites, an observation that agrees with a previous demonstration that the alpha subunit contains two N-linked oligosaccharide chains. Blot hybridization analysis of total chicken embryo RNA detected an mRNA of 3.5 kilobases, a size that closely resembles the size of the cloned cDNA. Since the expression of the alpha subunit is confined to cell types that synthesize and secrete collagens, the regulation of the synthesis of the alpha subunit may play a central role in determining the expression of prolyl 4-hydroxylase activity.
Full text
PDF
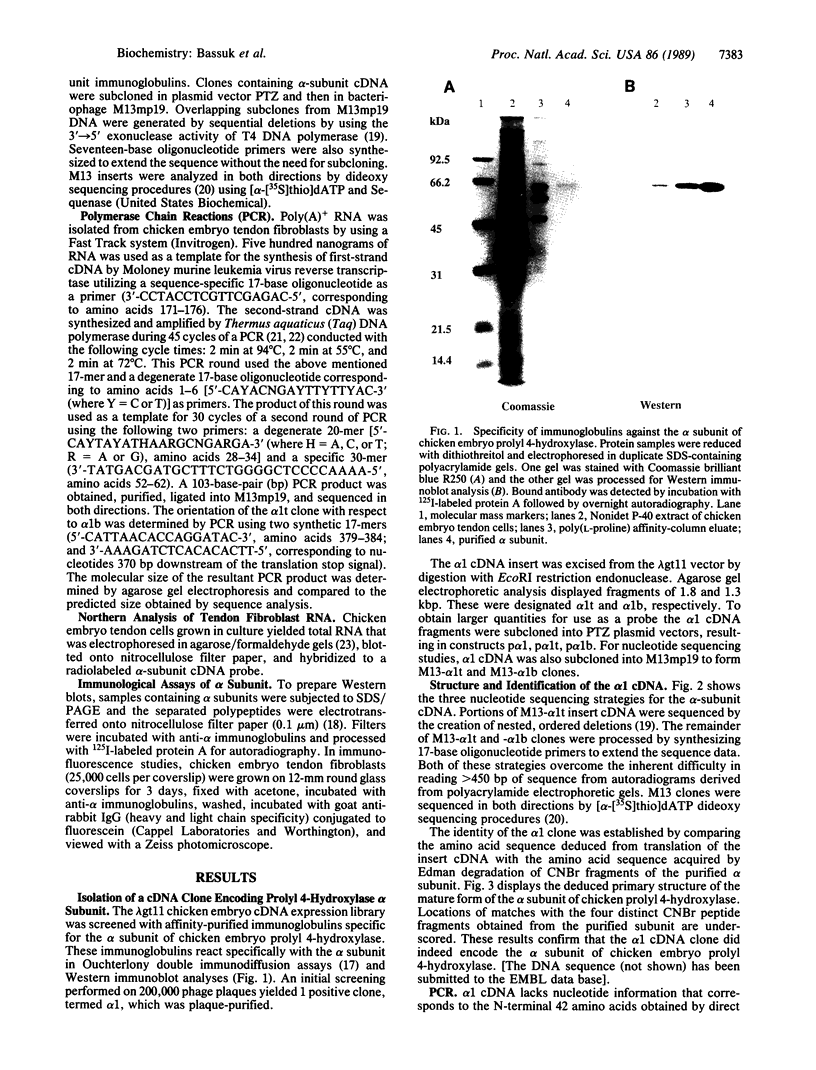



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassuk J. A., Berg R. A. Protein disulphide isomerase, a multifunctional endoplasmic reticulum protein. Matrix. 1989 Jun;9(3):244–258. doi: 10.1016/s0934-8832(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Bassuk J. A., Tsichlis P. N., Sorof S. Liver fatty acid binding protein is the mitosis-associated polypeptide target of a carcinogen in rat hepatocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7547–7551. doi: 10.1073/pnas.84.21.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. F., Balcarek J. M., Varrichio A., Crooke S. T. Molecular cloning and complete amino-acid sequence of form-I phosphoinositide-specific phospholipase C. Nature. 1988 Jul 21;334(6179):268–270. doi: 10.1038/334268a0. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Kao W. W., Kedersha N. L. The assembly of tetrameric prolyl hydroxylase in tendon fibroblasts from newly synthesized alpha-subunits and from preformed cross-reacting protein. Biochem J. 1980 Sep 1;189(3):491–499. doi: 10.1042/bj1890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Kedersha N. L., Guzman N. A. Purification and partial characterization of the two nonidentical subunits of prolyl hydroxylase. J Biol Chem. 1979 Apr 25;254(8):3111–3118. [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. Affinity column purification of protocollagen proline hydroxylase from chick embryos and further characterization of the enzyme. J Biol Chem. 1973 Feb 25;248(4):1175–1182. [PubMed] [Google Scholar]
- Berg R. A., Prockop D. J. The thermal transition of a non-hydroxylated form of collagen. Evidence for a role for hydroxyproline in stabilizing the triple-helix of collagen. Biochem Biophys Res Commun. 1973 May 1;52(1):115–120. doi: 10.1016/0006-291x(73)90961-3. [DOI] [PubMed] [Google Scholar]
- Dale R. M., Arrow A. A rapid single-stranded cloning, sequencing, insertion, and deletion strategy. Methods Enzymol. 1987;155:204–214. doi: 10.1016/0076-6879(87)55017-0. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Helaakoski T., Vuori K., Myllylä R., Kivirikko K. I., Pihlajaniemi T. Molecular cloning of the alpha-subunit of human prolyl 4-hydroxylase: the complete cDNA-derived amino acid sequence and evidence for alternative splicing of RNA transcripts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4392–4396. doi: 10.1073/pnas.86.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao W. W., Nakazawa M., Aida T., Everson W. V., Kao C. W., Seyer J. M., Hughes S. H. Isolation of cDNA clones and genomic DNA clones of beta-subunit of chicken prolyl 4-hydroxylase. Connect Tissue Res. 1988;18(3):157–174. doi: 10.3109/03008208809016805. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Berg R. A. An improved method for the purification of vertebrate prolyl hydroxylase by affinity chromatography. Coll Relat Res. 1981 Jul;1(4):345–353. doi: 10.1016/s0174-173x(81)80011-8. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Biosynthesis of prolyl hydroxylase: evidence for two separate dolichol-media pathways of glycosylation. Biochemistry. 1985 Oct 8;24(21):5960–5967. doi: 10.1021/bi00342a041. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Characterization of the oligosaccharides of prolyl hydroxylase, a microsomal glycoprotein. Biochemistry. 1985 Oct 8;24(21):5952–5960. doi: 10.1021/bi00342a040. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R., Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J. 1989 Mar;3(5):1609–1617. [PubMed] [Google Scholar]
- Koivu J., Myllylä R., Helaakoski T., Pihlajaniemi T., Tasanen K., Kivirikko K. I. A single polypeptide acts both as the beta subunit of prolyl 4-hydroxylase and as a protein disulfide-isomerase. J Biol Chem. 1987 May 15;262(14):6447–6449. [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Olsen B. R., Berg R. A., Kishida Y., Prockop D. J. Collagen synthesis: localization of prolyl hydroxylase in tendon cells detected with ferritin-labeled antibodies. Science. 1973 Nov 23;182(4114):825–827. doi: 10.1126/science.182.4114.825. [DOI] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L. Processive action of the two peptide binding sites of prolyl 4-hydroxylase in the hydroxylation of procollagen. Biochemistry. 1988 Jan 12;27(1):150–155. doi: 10.1021/bi00401a023. [DOI] [PubMed] [Google Scholar]