Abstract
Background: The optimal lifestyle intervention that reverses diabetes risk factors is not known.
Objective: We examined the effect of a low–glycemic index (GI) diet and exercise intervention on glucose metabolism and insulin secretion in obese, prediabetic individuals.
Design: Twenty-two participants [mean ± SEM age: 66 ± 1 y; body mass index (in kg/m2): 34.4 ± 0.8] underwent a 12-wk exercise-training intervention (1 h/d for 5 d/wk at ≈85% of maximum heart rate) while randomly assigned to receive either a low-GI diet (LoGIX; 40 ± 0.3 units) or a high-GI diet (HiGIX; 80 ± 0.6 units). Body composition (measured by using dual-energy X-ray absorptiometry and computed tomography), insulin sensitivity (measured with a hyperinsulinemic euglycemic clamp with [6,6-2H2]-glucose), and oral glucose–induced insulin and incretin hormone secretion were examined.
Results: Both groups lost equal amounts of body weight (−8.8 ± 0.9%) and adiposity and showed similar improvements in peripheral tissue (+76.2 ± 14.9%) and hepatic insulin sensitivity (+27.1 ± 7.1%) (all P < 0.05). However, oral glucose–induced insulin secretion was reduced only in the LoGIX group (6.59 ± 0.86 nmol in the prestudy compared with 4.70 ± 0.67 nmol in the poststudy, P < 0.05), which was a change related to the suppressed postprandial response of glucose-dependent insulinotropic polypeptide. When corrected for changes in β cell glucose exposure, changes in insulin secretion were attenuated in the LoGIX group but became significantly elevated in the HiGIX group.
Conclusions: Although lifestyle-induced weight loss improves insulin resistance in prediabetic individuals, postprandial hyperinsulinemia is reduced only when a low-GI diet is consumed. In contrast, a high-GI diet impairs pancreatic β cell and intestinal K cell function despite significant weight loss. These findings highlight the important role of the gut in mediating the effects of a low-GI diet on type 2 diabetes risk reduction.
INTRODUCTION
The reversal of progressive pancreatic β cell dysfunction and oral glucose intolerance is a primary goal for prediabetic individuals (1). However, solely treating glucose tolerance and peripheral and hepatic insulin resistance (IR) in overweight and obese individuals may delay, but is unlikely to prevent, the future onset of type 2 diabetes (T2D). Indeed, evidence suggests that preserving β cell function in these at-risk populations is a critical factor in the prevention of T2D onset (2). Therefore, therapeutic management of such individuals should consider all processes involved in maintaining glucose homeostasis.
Although the recent Diabetes Prevention Program Outcomes Study underlined the large effect that intensive lifestyle intervention can have on the prevention of diabetes onset in overweight individuals (3), the mechanisms by which lifestyle intervention prevents the development of glucose intolerance are not fully understood. Exercise training interventions can successfully improve glucose tolerance (4, 5), and several groups have shown that exercise can reverse peripheral tissue and hepatic IR (6–9). In addition, it has been shown that exercise training can alter insulin secretion and improve β cell function in obese humans (10–13). Caloric restriction has also been shown to improve these components of glucose tolerance (8, 14). However, certain nutrients (high-fat feeding) and elevated glycemic concentrations can induce IR and impair β cell function (15–17), whereas a high-carbohydrate and high-fiber diet can improve peripheral insulin sensitivity (18). Thus, dietary composition may play a critical role in determining the ultimate success of such interventions.
The concept of a dietary glycemic index (GI) has received much attention: the consumption of high-GI diets may increase diabetes risk (19), whereas the consumption of low-GI diets may promote weight loss and glycemic control (20, 21). However, the combination of exercise training with diets that vary only by GI and have identical macronutrient and fiber intakes has not been addressed to our knowledge. Indeed, a low-GI diet may complement exercise training because of the partitioning of fuel metabolism toward fat use (22–24); however, Solomon et al (25) recently showed that improvements in IR after 7 d of exercise training were not influenced by dietary GI, a finding that has been confirmed by a nonexercise GI study (26). It is possible that a longer-term intervention is required to see the beneficial effects of a low-GI diet on IR. However, it is possible that dietary GI does not affect insulin sensitivity per se, and perhaps the benefits accrue at the level of the pancreatic β cell and insulin secretion. Such a hypothesis is supported by data that show that prolonged hyperglycemia, such as that seen after high-GI consumption may impair β cell responsiveness to acute glycemia (16, 17) and affect the gastrointestinal incretin axis (27, 28).
In this study, we investigated the effects of consumption of a low-GI diet or high-GI diet combined with supervised exercise training for 12 wk on oral glucose tolerance in older, obese individuals. We examined physiologic changes in whole-body insulin sensitivity and insulin and glucose-dependent insulinotropic polypeptide (GIP) hormone secretion, which are components that are central to glucose clearance in the postprandial period. We hypothesized that a low-GI diet in combination with exercise would elicit greater improvements in glucose metabolism than would a high-GI diet in combination with exercise via larger alterations in insulin action and secretion and improved GIP secretion.
SUBJECTS AND METHODS
Participants
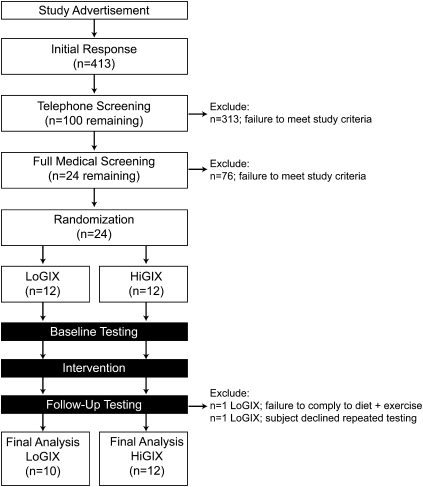
Twenty-two older, obese patients with prediabetes [mean ± SEM age: 66 ± 1 y; body mass index (in kg/m2): 34.4 ± 2.8] volunteered to participate in a 12-wk exercise-training intervention while consuming either a low-GI or high-GI diet. The study was conducted by using a randomized, controlled, parallel-group, repeated-measures design (Figure 1). Medical screenings excluded individuals with heart, kidney, liver, thyroid, intestinal, and pulmonary diseases or individuals taking medications known to affect the outcome variables of the study. Resting 12-lead electrocardiograms and submaximal exercise stress tests excluded individuals with any contraindication to increments in physical activity. All women were postmenopausal and not using hormone replacement therapy. Participants had also been weight stable for at least the previous 6 mo. Measurements of resting metabolic rates via indirect calorimetry were used to ascertain individual caloric requirements. The study was approved by the Cleveland Clinic Institutional Review Board, and all subjects provided informed written consent in accordance with our guidelines for the protection of human subjects.
FIGURE 1.
Study flowchart. A total of 413 men and women responded to the study advertisements. After review of our inclusion and exclusion criteria, 313 individuals were ineligible to partake in the study. After a full medical screening, a further 78 potential subjects were excluded from further participation. Twenty-four individuals were randomly assigned to the 12-wk exercise-training program combined with either a low–glycemic index diet (LoGIX; n = 12) or a high–glycemic index diet (HiGIX; n = 12). Two subjects were excluded from the LoGIX intervention (one subject failed to comply with the intervention, and the second subject declined to complete the postintervention testing). No adverse events were associated with our testing procedures or the intervention. Final statistical analyses were performed in 22 participants (LoGIX, n = 10; HiGIX, n = 12).
Intervention
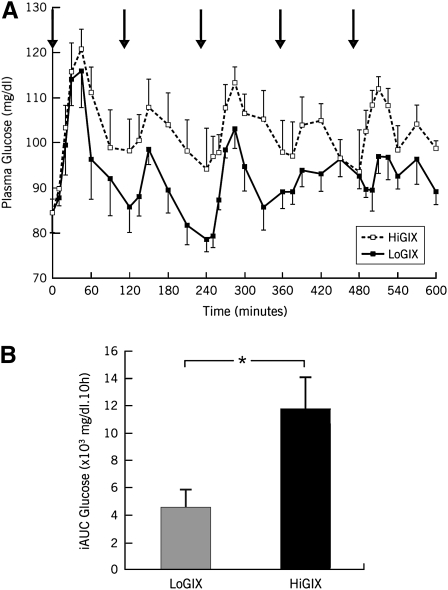
Volunteers were randomly assigned to a low-GI diet plus exercise group (LoGIX group: n = 10; 3 men, 7 women) or a high-GI diet plus exercise group (HiGIX group: n = 12; 5 men, 7 women). All volunteers undertook 60 min of aerobic exercise 5 d/wk for 12 wk (treadmill walking and cycle ergometry) at ≈85% of the maximum heart rate obtained during an incremental maximal aerobic-exercise test [maximal oxygen uptake (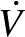 O2max) test; full details of the exercise intervention were previously presented (5)]. Every session was supervised by an exercise physiologist, and all meals, snacks, and beverages for the 12-wk intervention were provided to participants on a daily basis. Diets were carefully formulated by a registered dietitian and were isocaloric to the individual requirements of subjects (screening resting metabolic rate multiplied by a sedentary activity factor of 1.2). The dietary macronutrient composition (including fiber) was matched between groups; however, the diets were designed so that LoGIX subjects received a diet corresponding to a GI of 40 arbitrary units (au), whereas HiGIX subjects were provided an 80-au GI diet. Dietary adherence was ensured via daily food-container weigh backs plus a weekly counseling session with a research dietitian. Daily menus contained a variety of different foods to prevent meal repetition on a 3-wk block rotation [sample menus were shown in Solomon et al (25)]. Dietary analysis was performed with Nutritionist Pro software (Axxya Systems, Stafford, TX). In addition, a subgroup of our participants (n = 8) underwent 1-d diet tests. This subgroup was provided with a sample menu for each diet on 2 separate occasions. Venous blood samples were obtained at 10-min intervals for 10 h on each test day beginning fasted at 0800. The plasma glycemic responses to these tests are presented in Figure 2.
O2max) test; full details of the exercise intervention were previously presented (5)]. Every session was supervised by an exercise physiologist, and all meals, snacks, and beverages for the 12-wk intervention were provided to participants on a daily basis. Diets were carefully formulated by a registered dietitian and were isocaloric to the individual requirements of subjects (screening resting metabolic rate multiplied by a sedentary activity factor of 1.2). The dietary macronutrient composition (including fiber) was matched between groups; however, the diets were designed so that LoGIX subjects received a diet corresponding to a GI of 40 arbitrary units (au), whereas HiGIX subjects were provided an 80-au GI diet. Dietary adherence was ensured via daily food-container weigh backs plus a weekly counseling session with a research dietitian. Daily menus contained a variety of different foods to prevent meal repetition on a 3-wk block rotation [sample menus were shown in Solomon et al (25)]. Dietary analysis was performed with Nutritionist Pro software (Axxya Systems, Stafford, TX). In addition, a subgroup of our participants (n = 8) underwent 1-d diet tests. This subgroup was provided with a sample menu for each diet on 2 separate occasions. Venous blood samples were obtained at 10-min intervals for 10 h on each test day beginning fasted at 0800. The plasma glycemic responses to these tests are presented in Figure 2.
FIGURE 2.
Mean (±SEM) study-diet glycemic responses. A subgroup of individuals (n = 8) were selected to undergo two 1-d diet tests in which they consumed meals (solid black arrows) representative of our low–glycemic index diet (LoGIX) and high–glycemic index diet (HiGIX) study groups in a counterbalanced crossover design. A: Plasma glucose concentrations were measured every 10 min for 10 h. B: The incremental area under the glucose-response curve (iAUC) was calculated over the 10-h diet test. *The HiGIX (black bar) meals elicited a greater glycemic response than did the LoGIX (gray bar), P < 0.05 (paired t test).
Inpatient control period
Pre- and postintervention assessments of body composition, aerobic fitness, insulin sensitivity and secretion, and substrate metabolism were performed during a 3-d inpatient stay in the Clinical Research Unit at the Cleveland Clinic. During this time, participants received a weight-maintenance isocaloric diet (55% carbohydrate, 30% fat, and 15% protein). The postintervention inpatient stay included the final 2 d of the GI dietary provision and exercise prescription so that day 3 of this stay occurred the day after the final exercise session. Metabolic measures were performed on this third day ≈16 h after the last exercise bout.
Body composition
Height and body weight were measured by standard techniques (5). Whole-body adiposity [fat mass (FM) and fat-free mass (FFM)] was measured by dual-energy X-ray absorptiometry (model iDXA; Lunar, Madison, WI) as previously described (25). Computerized tomography scanning was used to quantify subcutaneous and visceral abdominal adiposity with a SOMOTOM Sensation 16 Scanner (Siemens Medical Solutions, Malvern, PA), as previously described (5).
Aerobic fitness
An incremental, graded treadmill exercise test (modified Borg) was used to determine 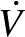 O2max (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA). The test was deemed satisfactory if ≥3 of the following criteria were attained: plateau in oxygen consumption with increasing workloads, volitional fatigue, a heart rate ≤10 beats/min of the age-predicted maximum, or a respiratory exchange ratio >1.10. The maximal heart rate recorded during this test was used to prescribe the correct exercise intensity during training (5).
O2max (Jaeger Oxycon Pro; Viasys, Yorba Linda, CA). The test was deemed satisfactory if ≥3 of the following criteria were attained: plateau in oxygen consumption with increasing workloads, volitional fatigue, a heart rate ≤10 beats/min of the age-predicted maximum, or a respiratory exchange ratio >1.10. The maximal heart rate recorded during this test was used to prescribe the correct exercise intensity during training (5). 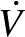 O2max tests were also performed at weeks 4 and 8 to maintain the appropriate exercise intensity corresponding to changes in the aerobic fitness of participants. To control for the acute effects of exercise, preintervention
O2max tests were also performed at weeks 4 and 8 to maintain the appropriate exercise intensity corresponding to changes in the aerobic fitness of participants. To control for the acute effects of exercise, preintervention 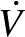 O2max tests were conducted >48 h before metabolic measures.
O2max tests were conducted >48 h before metabolic measures.
Insulin sensitivity
After an overnight fast, at 0800, a 2-h primed (3.28 mg/kg) continuous (0.036 mg · kg−1 · min−1) infusion of [6,6-2H2]-glucose was begun (Cambridge Isotope Laboratories, Andover, MA). At 1000 (t = 0 min), a 40 mU · m−2 · min−1 hyperinsulinemic euglycemic (90 mg/dL) clamp proceeded as previously described (25). Fluctuations in arterialized venous plasma glucose concentrations were titrated via a variable-rate hot glucose (20% dextrose plus [6,6-2H2]-glucose) infusion; adjustments were made every 5 min on the basis of the algorithm of DeFronzo et al (29). Rates of glucose appearance (Ra) and disappearance (Rd) were calculated during basal (t = −30 to 0 min) and insulin-stimulated (t = 90–120 min) conditions by using Steele's single-pool model of glucose kinetics (25). Hepatic glucose production (Ra HGP) was calculated as the difference between the total glucose Ra and the exogenous glucose-infusion rate. A measure of IR was calculated as the reciprocal of glucose Rd divided by the steady-state plasma insulin concentration during hyperinsulinemia.
Oral glucosendashinduced insulin and incretin hormone secretion
A 75-g oral-glucose-tolerance test (OGTT) was performed at 0800 after an overnight fast, and blood samples were drawn from an antecubital vein at baseline and every 30 min for 3 h. Prehepatic insulin secretion was calculated by deconvolution analysis of peripheral blood C-peptide concentrations after oral glucose ingestion by using a 2-compartment model of C-peptide kinetics (30). The β cell insulin secretory response to glucose was estimated as the insulin secretion rate divided by the plasma glucose response to the OGTT (ISR/G) (31). In addition, to control for the reductions in pancreatic exposure to glucose because of increases in insulin sensitivity, the β cell insulin secretory response to glucose was divided by the glucose clamp-derived measure of IR (ISR/G ÷ IR, an index of β cell function) (31).
Biochemical analyses
Plasma glucose concentrations were measured with a YSI 2300 STAT Plus analyzer (Yellow Springs Instruments, Yellow Springs, OH), plasma insulin concentrations were measured via radioimmunoassay (Millipore, Billerica, MA), and C-peptide concentrations were measured by using enzyme-linked immunosorbent assay (Millipore). Plasma triglycerides and cholesterol were analyzed by using enzymatic methods with an automated platform (Roche Modular Diagnostics, Indianapolis, IN). Glycated hemoglobin (Hb A1c) was measured via nonporous ion exchange high-performance liquid chromatography (G7 HPLC Analyzer; Tosoh Bioscience, San Francisco, CA). Samples for incretin hormone analysis were collected in EDTA evacuated tubes that contained aprotinin and dipeptidyl peptidase IV inhibitor. Total GIP concentrations were analyzed by enzyme-linked immunosorbent assay (Millipore). For glucose kinetics analyses, plasma glucose underwent derivatization to synthesize a pentacetate-glucose derivative, and glucose enrichment was measured via gas chromatography–mass spectrometry (Hewlett-Packard, Palo Alto, CA) (25).
Statistics
Between-group (LoGIX compared with HiGIX) comparisons were analyzed by using 2-factor (group × time) repeated-measures analysis of variance (ANOVA), and Bonferroni post hoc tests were applied to significant group × time interactions. Baseline values for each variable were compared between groups by using unpaired t tests. In the event of a significant t statistic, baseline values were used as a covariate in the 2-factor repeated-measures ANOVA. Bivariate correlation analyses were used to identify relations between variables. Significance was accepted at P < 0.05. Additional ANCOVA analyses were performed by using sex as a covariate and revealed no sex effects on any of the outcome variables in this intervention. Analyses were carried out with StatView for Windows 5.0.1 (SAS Institute, Cary, NC), and all data are expressed as means ± SEMs.
RESULTS
Diet and exercise
Dietary analyses for the LoGIX and HiGIX groups are shown in Table 1. Group diets were carefully matched with respect to macronutrient composition, including fiber, with GI units of 39.8 ± 0.3 and 80.0 ± 0.6 au, respectively. The significant difference in GIs between our study diets was confirmed by the study diet tests (Figure 1; P < 0.05). Dietary compliance was high (97 ± 1%), and there was a 95 ± 1% attendance at exercise sessions. During the intervention, exercise was performed for 56 ± 5 min/d at 83.4 ± 0.6% of the maximum heart rate, and after the study, there was an equal increment in 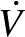 O2max in each group (Table 2). In addition, there were significant improvements in resting blood pressures (Table 2; P < 0.05).
O2max in each group (Table 2). In addition, there were significant improvements in resting blood pressures (Table 2; P < 0.05).
TABLE 1.
Study diets1
| Study diet | LoGIX | HiGIX |
| GI (au) | 39.8 ± 0.3 | 80.0 ± 0.6* |
| GL (au) | 98.5 ± 6.2 | 205.5 ± 9.3* |
| EI (kcal/d) | 1760 ± 109 | 1791 ± 83 |
| Carbohydrate (g/d) | 247 ± 16 | 258 ± 12 |
| Percentage of energy | 54.7 ± 0.1 | 55.6 ± 0.2 |
| Fat (g/d) | 56.7 ± 3.4 | 57.3 ± 2.9 |
| Percentage of energy | 28.3 ± 0.1 | 27.8 ± 0.2 |
| Protein (g/d) | 76.8 ± 4.8 | 76.7 ± 3.5 |
| Percentage of energy | 17.0 ± 0.1 | 16.6 ± 0.1 |
| Fiber (g/d) | 28.5 ± 1.6 | 26.1 ± 1.4 |
| Compliance (%) | 98 ± 1 | 96 ± 1 |
All values are means ± SEMs (n = 22). LoGIX, low–glycemic index diet; HiGIX, high–glycemic index diet; GI, glycemic index; au, arbitrary units; GL, glycemic load; EI, energy intake. *Significant group differences, P < 0.05 (paired t test).
TABLE 2.
Subject characteristics1
| LoGIX |
HiGIX |
P (2-factor ANOVA) |
|||||
| Subject characteristics | Prestudy | Poststudy | Prestudy | Poststudy | Time | Trial | Time × trial |
| n (M, F) | 10 (3, 7) | 10 (3, 7) | 12 (5, 7) | 12 (5, 7) | — | — | — |
| Age (y) | 67 ± 2 | 67 ± 2 | 64 ± 1 | 64 ± 1 | — | — | — |
| Weight (kg) | 97.4 ± 3.8 | 89.6 ± 3.4 | 94.7 ± 4.4 | 85.7 ± 4.1 | <0.0001 | 0.46 | 0.52 |
| BMI (kg/m2) | 34.9 ± 1.1 | 32.1 ± 1.3 | 34.1 ± 1.1 | 30.9 ± 1.2 | <0.0001 | 0.40 | 0.43 |
| FM (kg) | 46.8 ± 2.0 | 40.0 ± 2.2 | 42.0 ± 2.2 | 36.1 ± 2.9 | <0.0001 | 0.33 | 0.49 |
| FFM (kg) | 49.9 ± 2.9 | 49.7 ± 2.8 | 55.2 ± 3.6 | 53.9 ± 3.5 | 0.02 | 0.08 | 0.08 |
| TAAT (cm2) | 620.4 ± 31.3 | 535.3 ± 33.2 | 553.4 ± 44.9 | 432.0 ± 48.9 | <0.0001 | 0.25 | 0.12 |
| Super SAT (cm2) | 264.0 ± 23.7 | 262.4 ± 27.6 | 218.9 ± 20.2 | 202.0 ± 27.1 | 0.26 | 0.27 | 0.35 |
| Deep SAT (cm2) | 249.5 ± 27.4 | 194.2 ± 16.8 | 217.0 ± 22.5 | 156.9 ± 18.2 | <0.0001 | 0.50 | 0.83 |
| VAT (cm2) | 106.9 ± 12.7 | 78.7 ± 12.1 | 117.5 ± 26.3 | 73.0 ± 18.5 | <0.0001 | 0.17 | 0.27 |
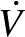 O2max (L/min) O2max (L/min) |
2.03 ± 0.15 | 2.32 ± 0.22 | 2.19 ± 0.14 | 2.45 ± 0.18 | <0.0001 | 0.52 | 0.81 |
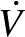 O2max (mL · kg−1 · min−1) O2max (mL · kg−1 · min−1) |
20.4 ± 1.1 | 25.6 ± 2.1 | 23.2 ± 1.9 | 28.8 ± 1.8 | <0.0001 | 0.67 | 0.78 |
| SBP (mm Hg) | 127 ± 3 | 118 ± 2 | 133 ± 5 | 119 ± 4 | 0.0002 | 0.42 | 0.30 |
| DBP (mm Hg) | 76 ± 3 | 73 ± 2 | 79 ± 3 | 71 ± 3 | 0.02 | 0.27 | 0.26 |
| Hb A1c (%) | 5.66 ± 0.14 | 5.44 ± 0.16 | 5.48 ± 0.15 | 5.53 ± 0.13 | 0.57 | 0.44 | 0.27 |
| FPG (mg/dL) | 101.1 ± 2.3 | 97.6 ± 1.5 | 96.7 ± 2.0 | 90.8 ± 1.7 | 0.02 | 0.71 | 0.92 |
| FPI (μU/mL) | 24.6 ± 4.8 | 14.2 ± 2.4 | 20.9 ± 6.4 | 9.2 ± 1.2 | <0.0001 | 0.06 | 0.04 |
| FC-pep (ng/mL) | 3.18 ± 0.58 | 2.53 ± 0.34 | 2.11 ± 0.27 | 1.88 ± 0.16 | 0.0008 | 0.09 | 0.13 |
| TG (mg/dL) | 164.2 ± 26.1 | 110.9 ± 12.2 | 129.8 ± 18.9 | 89.0 ± 14.2 | 0.0004 | 0.49 | 0.58 |
| Cholesterol (mg/dL) | 214.6 ± 11.3 | 183.5 ± 9.6 | 207.3 ± 8.3 | 181.2 ± 9.2 | <0.0001 | 0.35 | 0.60 |
| HDL cholesterol (mg/dL) | 53.3 ± 3.5 | 50.3 ± 3.9 | 49.8 ± 4.1 | 47.0 ± 3.6 | 0.06 | 0.46 | 0.93 |
| VLDL cholesterol (mg/dL) | 32.8 ± 5.3 | 22.1 ± 2.4 | 25.9 ± 3.8 | 17.8 ± 2.8 | 0.0004 | 0.80 | 0.62 |
| LDL cholesterol (mg/dL) | 128.5 ± 9.1 | 111.1 ± 9.7 | 131.6 ± 6.7 | 116.4 ± 7.8 | 0.001 | 0.53 | 0.80 |
All values are means ± SEMs (n = 22). LoGIX, low–glycemic index diet; HiGIX, high–glycemic index diet; FM, fat mass; FFM, fat-free mass; TAAT, total abdominal adipose tissue; Super, superficial; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; 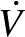 O2max, maximal oxygen uptake during exhaustive aerobic exercise; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hb A1c, glycated hemoglobin; FPG, fasting plasma glucose; FPI, fasting plasma insulin; FC-pep, fasting C-peptide; TG, triglycerides. After the intervention, both groups showed significant improvements in body composition, aerobic fitness, fasting glycemia, and lipemia (P < 0.05, 2-factor ANOVA). No between-group prestudy differences were observed (all P > 0.05, unpaired t test).
O2max, maximal oxygen uptake during exhaustive aerobic exercise; SBP, systolic blood pressure; DBP, diastolic blood pressure; Hb A1c, glycated hemoglobin; FPG, fasting plasma glucose; FPI, fasting plasma insulin; FC-pep, fasting C-peptide; TG, triglycerides. After the intervention, both groups showed significant improvements in body composition, aerobic fitness, fasting glycemia, and lipemia (P < 0.05, 2-factor ANOVA). No between-group prestudy differences were observed (all P > 0.05, unpaired t test).
Body composition
Similar weight loss was achieved after both interventions (Table 2; −8.8 ± 0.9%; P < 0.05). This improvement was driven by decreases in whole-body fat mass and deep subcutaneous and visceral abdominal adipose tissue (Table 2; P < 0.05). Fat-free mass showed a significant decrease; however, this change was clinically insignificant (−1.4 ± 0.6%). No prestudy group differences in body composition were observed (P > 0.05).
Blood biochemistry
The lifestyle intervention reduced fasting plasma glucose, triglyceride, and cholesterol concentrations equally in the LoGIX and HiGIX groups (Table 2; P < 0.05). No prestudy group differences in these blood biochemistry measures were observed (P > 0.05). Fasting plasma insulin and C-peptide concentrations were also reduced in both groups (Table 2; both P < 0.05). No changes in Hb A1c were identified after the study (Table 2; P > 0.05)
Insulin sensitivity
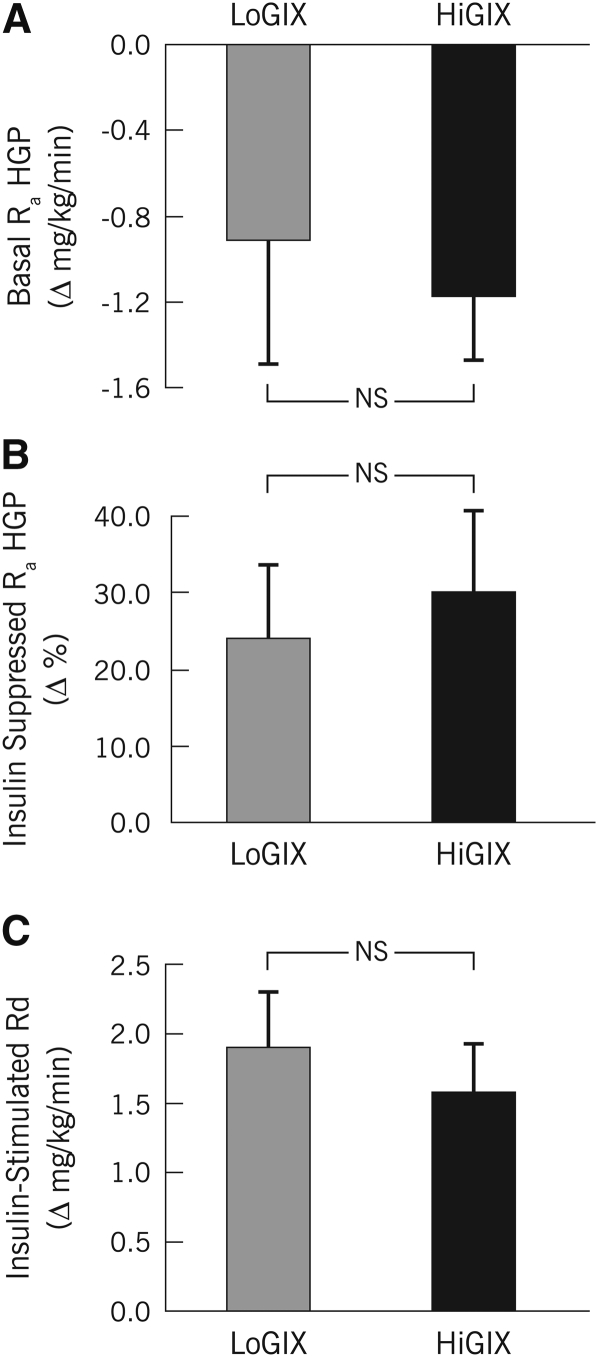
During hyperinsulinemia, steady-state euglycemia was achieved [88.7 ± 0.6 mg/dL prestudy (CV = 4.2 ± 0.5%) and 88.7 ± 0.4 mg/dL poststudy (CV = 3.4 ± 0.4%)]. Basal glucose Ra HGP was significantly reduced after the intervention (Table 3, Figure 3A; −31.1 ± 6.7%; P < 0.05), whereas insulin suppression of Ra HGP (Table 3, Figure 3B; +27.1 ± 7.1%; P < 0.05) and insulin-stimulated glucose Rd increased (Table 3, Figure 3C; +76.2 ± 14.9%; P < 0.05). These changes were similar between groups (Figure 2; LoGIX compared with HiGIX; 2-factor ANOVA interaction, P = NS), and no prestudy differences were evident (P > 0.05).
TABLE 3.
Glucose metabolism, insulin secretion, and β cell function1
| Glucose metabolism and insulin kinetics | LoGIX |
HiGIX |
P (2-factor ANOVA) |
||||
| Prestudy | Poststudy | Prestudy | Poststudy | Time | Trial | Time × trial | |
| Basal Ra (mg · kg−1 · min−1) | 2.43 ± 0.57 | 1.52 ± 0.07 | 2.60 ± 0.27 | 1.43 ± 0.12 | 0.005 | 0.51 | 0.69 |
| Insulin Ra (percentage suppression) | 52.7 ± 5.9 | 76.6 ± 7.2 | 33.6 ± 9.0 | 63.5 ± 11.4 | 0.002 | 0.22 | 0.68 |
| Insulin Rd (mg · kg−1 · min−1) | 2.22 ± 0.26 | 4.12 ± 0.40 | 3.12 ± 0.40 | 4.60 ± 0.59 | <0.0001 | 0.31 | 0.42 |
| Insulin Rd (mg · kg−1 FFM · min−1) | 4.41 ± 0.56 | 7.48 ± 0.80 | 5.48 ± 0.75 | 7.75 ± 0.96 | <0.0001 | 0.31 | 0.39 |
| Basal ISR (nmol/min) | 2.86 ± 0.49 | 1.87 ± 0.31 | 1.80 ± 0.23 | 1.45 ± 0.15* | 0.0004 | 0.02 | 0.05 |
| OGTT ISRauc (nmol) | 6.59 ± 0.86 | 4.70 ± 0.67 | 4.40 ± 0.55 | 4.25 ± 0.66 | 0.01 | 0.02 | 0.02 |
| ISR/G (au) | 8.28 ± 0.91 | 6.41 ± 0.89 | 6.01 ± 0.72 | 5.65 ± 0.64* | 0.02 | 0.07 | 0.09 |
| ISR/G ÷ IR (au) | 31.5 ± 4.1 | 32.6 ± 4.3 | 26.8 ± 3.0 | 37.3 ± 3.3* | 0.01 | 0.03 | 0.04 |
All values are means ± SEMs (n = 22). LoGIX, low–glycemic index diet; HiGIX, high–glycemic index diet; Basal Ra, rate of appearance of plasma glucose during fasting conditions; Insulin Ra, insulin-stimulated rate of appearance of plasma glucose; Rd, insulin-stimulated rate of disappearance of plasma glucose; FFM, fat-free mass; Basal ISR, insulin secretion rate during fasting conditions; OGTT ISRauc, total area under the insulin secretion rate curve after ingestion of 75 g glucose (oral-glucose-tolerance test ); ISR/G, insulin secretory response to OGTT divided by the plasma glucose response; ISR/G ÷ IR, insulin secretory response to OGTT corrected for changes in insulin resistance; au, arbitrary units. After the study, both groups showed significant improvements in insulin sensitivity, whereas only the LoGIX group showed improvement in insulin secretion. *Group differences between poststudy values, P < 0.05. With the exception of basal ISR (P = 0.05), no between-group prestudy differences were observed (all P > 0.05, unpaired t test).
FIGURE 3.
Mean (±SEM) insulin sensitivity (n = 22). After 12 wk of aerobic exercise combined with either a low–glycemic index diet (LoGIX; gray bars) or a high–glycemic index diet (HiGIX; black bars), changes in glucose kinetics were measured during 40 mU · m−2 · min−1 hyperinsulinemic euglycemic clamps. Values represent poststudy minus prestudy changes (Δ) in glucose kinetics. Basal rates of glucose appearance (Ra) decreased significantly (A) [basal Ra of hepatic glucose production (Basal Ra HGP); main effect of time, P < 0.05 (2-factor ANOVA)], whereas insulin-suppression of HGP (Insulin-Suppressed Ra HGP) increased after the intervention (B) (P < 0.05). Rates of glucose disappearance during hyperinsulinemia (Insulin-Stimulated Rd) increased significantly (C) (P < 0.05). No significant differences between LoGIX and HiGIX groups were observed (P = NS).
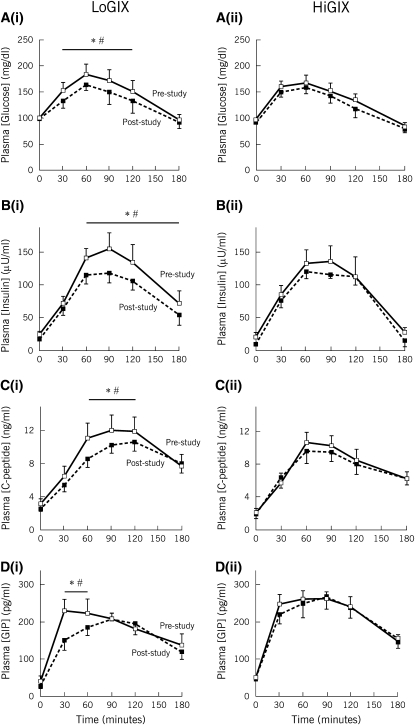
Oral glucosendashinduced metabolic responses
The glucose (Figure 4A), insulin (Figure 4B), C-peptide (Figure 4C), and GIP (Figure 4D) responses to oral glucose ingestion, before and after the intervention, are shown in Figure 4. The area under the glucose response curve improved in the LoGIX group only; however, these data did not reach significance (main effect of time, P = 0.10; interaction, P = 0.16; LoGIX: 1.38 ± 0.07 compared with 1.30 ± 0.04 mol · L/min for pre- and poststudy, respectively; HiGIX: 1.32 ± 0.06 compared with 1.31 ± 0.06 mol · L/min for pre- and poststudy, respectively). Insulin, C-peptide, and GIP responses to the OGTT were significantly attenuated after the intervention in the LoGIX group only (P < 0.05; Figure 4) and showed no changes in the HiGIX group. The area under the GIP response curve (0–180 min) and the early phase GIP response (Δ0–30 min) were reduced in the LoGIX group (both P < 0.05).
FIGURE 4.
Mean (±SEM) oral glucose–induced metabolic responses (n = 22). After the 12-wk intervention, changes in the glucose [A(i) and A(ii) (low–glycemic index diet [LoGIX] and high–glycemic index diet [HiGIX], respectively)], insulin [B(i) and B(ii)], C-peptide [C(i) and C(ii)], and glucose-dependent insulinotropic polypeptide (GIP) [D(i) and D(ii)] secretory responses to oral glucose were assessed. Each panel represents the metabolic response after glucose ingestion (oral-glucose-tolerance test) at t = 0 min. Solid lines represent prestudy values; dotted lines represent poststudy values. Glucose, insulin, C-peptide, and GIP responses to oral glucose ingestion were attenuated in the LoGIX group only after the intervention. *Significant prestudy compared with poststudy differences, P < 0.05 (2-factor ANOVA). The black bar is positioned above prestudy and poststudy time points that were significantly different. #Significant differences between groups (LoGIX compared with HiGIX) with regard to the pre- to postintervention changes at specific time points, P < 0.05.
Oral glucosendashinduced insulin secretion
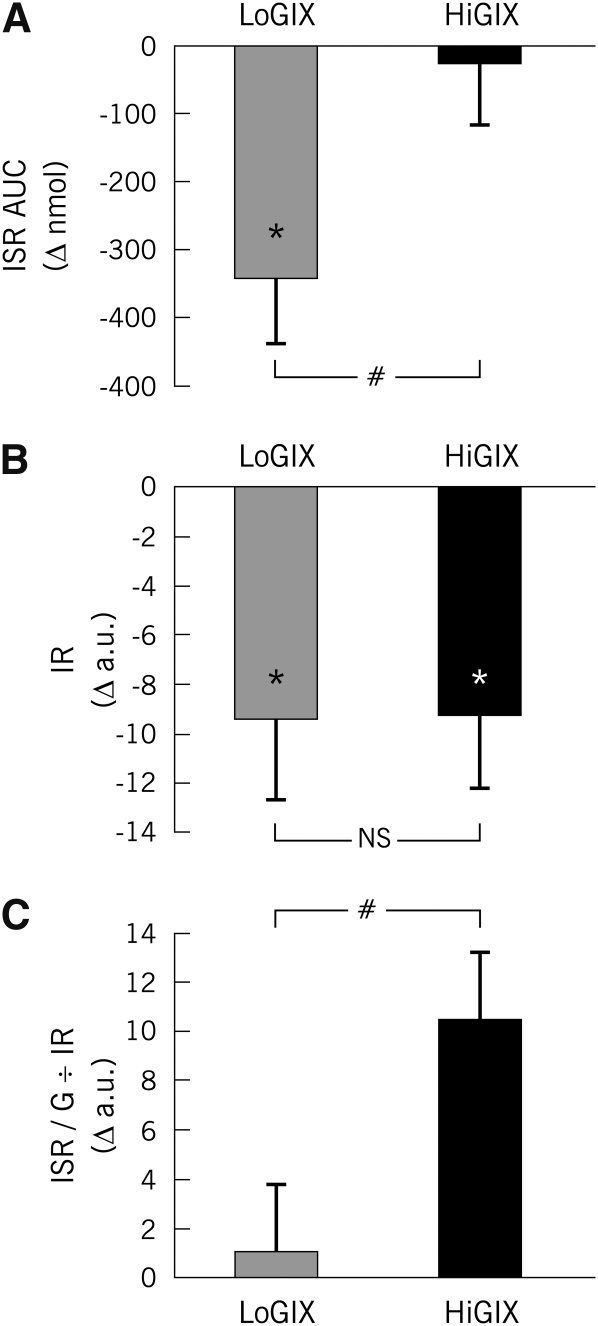
Basal ISR was significantly decreased in both groups after the intervention, with larger changes seen in the LoGIX group (Table 3). Oral glucose–induced ISR was decreased only in the LoGIX group after the lifestyle intervention (Figure 5A) despite the finding that IR (Figure 5B) was attenuated to a similar extent in both LoGIX and HiGIX groups (main effect of time, P < 0.05; LoGIX compared with HiGIX; P = NS). When corrected for changes in IR, oral glucose–induced ISR became elevated in the HiGIX group, whereas there were no intervention-induced effects in the LoGIX group (Figure 5C). Correction of ISR for changes in IR was appropriate as indicated by the strong relation between the 2 variables (r = 0.59, P = 0.002). Prestudy baseline variables showed a trend toward ISR being higher in the LoGIX group; however, of these variables, only the basal ISR reached significance (P < 0.05).
FIGURE 5.
Mean (±SEM) oral glucose–induced insulin secretion (n = 22). After 12 wk of aerobic exercise combined with either a low–glycemic index diet (LoGIX; gray bars) or a high–glycemic index diet (HiGIX; black bars), changes in oral glucose–induced insulin secretion were assessed via peripheral blood C-peptide deconvolution. Values represent poststudy minus prestudy changes (Δ). A: The oral glucose–induced insulin secretion rate area under the curve (ISR AUC) was significantly reduced only in the LoGIX group (#LoGIX compared with HiGIX: P < 0.05, 2-factor ANOVA). B: Insulin resistance (IR; defined as the reciprocal of the glucose rate of disappearance divided by the steady state plasma insulin concentration) was equally reduced in both groups (LoGIX compared with HiGIX, P = NS). C: Oral glucose–induced insulin secretion corrected for changes in insulin resistance [the insulin secretory response to an oral-glucose-tolerance test corrected for changes in IR (ISR/G ÷ IR)] was unchanged in the LoGIX group but increased in the HiGIX group (#LoGIX compared with HiGIX, P < 0.05). *Significant prestudy compared with poststudy differences, P < 0.05. a.u., arbitrary units.
Correlation analyses
Intervention-induced changes in the ISR were not related to changes in body weight (r = 0.16, P = 0.47) or fat mass (r = 0.09, P = 0.69); however, the early phase insulin response (ISR: 0–30 min) was correlated with the oral glucose–induced GIP response (0–30 min; r = 0.44, P = 0.03).
DISCUSSION
The key finding in this study is that, when older, obese, prediabetic individuals underwent a 12-wk diet and exercise-based lifestyle intervention, reductions in compensatory hyperinsulinemia were only seen when individuals consumed a low-GI diet. It was also observed that a high-GI diet increased the β cell insulin secretory response to glucose when correcting the changes in oral glucose–induced insulin secretion for changes in the underlying IR, which was indicative of β cell dysfunction. These, to our knowledge, novel findings were shown to be independent of the ≈9% decrease in body weight and fat mass but were related to changes in the gastrointestinal incretin peptide, GIP, the postprandial response of which was decreased only in subjects who consumed a low-GI diet.
It was previously shown that weight loss via caloric restriction and/or exercise reduced IR in obese men and women (6–8, 14). Such improvements in peripheral tissue glucose disposal or suppression of hepatic glucose production, as seen in this study, reduce hyperglycemia. This alone reduces glucose exposure of the pancreatic β cells and thus reduces insulin secretion; however, this does not reflect an alteration in β cell function per se. In this study, reductions in hyperinsulinemia and postprandial insulin secretion were only seen in the LoGIX group. Because changes in β cell glucose exposure (ΔIR) were identical in both dietary groups, it was surprising that the HiGIX group showed no change in postprandial ISR. These observations suggest that it is appropriate to control for β cell glucose exposure (eg, ISR/G ÷ IR) when assessing insulin secretion. As such, no intervention-induced changes in β cell function per se were evident in the LoGIX group, whereas there was an elevation in this index in the HiGIX group. These data indicate that when a low-GI diet is combined with exercise training in hyperinsulinemic individuals, the reduction in insulin secretion is driven by reduced β cell glucose exposure, but that there is an inappropriate increase in the β cell response to glucose, and compensatory hyperinsulinemia is not corrected, when a high-GI diet is consumed, which indicates evidence of β cell dysfunction. These findings are clinically significant with regards to the pharmacologic management of individuals at risk of developing T2D. Current evidence indicates that the treatment of hyperglycemia in prediabetic individuals must target insulin action and secretion to prevent the onset of T2D (ie, treating IR alone will merely delay the onset of diabetes) (2). In the context of our current findings, it is clear that, although diet and exercise-induced weight loss will reverse peripheral and hepatic IR, only a diet that elicits lower postprandial glycemic responses will reduce compensatory hyperinsulinemia, whereas a high-GI diet appears to exacerbate β cell dysfunction. These data suggest that a high-GI diet, when combined with exercise, may delay the onset of T2D in at-risk individuals, but only a low-GI treatment method will prevent diabetes onset.
To the best of our knowledge, this is the first investigation to prescribe and provide isocaloric macronutrient-matched meals with low or high GI values during a long-term lifestyle intervention in older, obese men and women at risk of developing diabetes. To our knowledge, this is also the first dietary intervention to use identical recipes and meal patterns in each of the 2 interventions by substituting only low-GI compared with high-GI foods. The glucose responses during our study diets (Figure 2) confirmed the GI differences between the LoGIX (GI = 40) and HiGIX (GI = 80) groups. A comprehensive evaluation of the effects of GI on oral glucose tolerance has not previously been published. The mechanistic advantage of the OGTT is the ability to study incretin-dependent insulin secretion. Although we showed no group differences in IR (or body composition) for the 2 diets, the beneficial reduction in insulin secretion in the LoGIX group was related to reduced postprandial GIP responses. This opens up a novel paradigm whereby the LoGIX-induced reductions in insulin secretion appear not to be due to changes in β cell function when accounting for β cell glucose exposure but instead are related to an incretin-mediated effect on the β cell. The incretin axis may be critical for appropriate β cell function (32) as GIP (and glucagon-like peptide 1) secretion is reduced in T2D (33), pancreatic β cells are insensitive to incretin action in insulin resistant and T2D patients (34, 35), whereas β cell GIP insensitivity has been reported in human aging (36). Thus, the absence of an intervention effect on the incretin axis in the HiGIX group may partly explain the ensuing β cell dysfunction. Kashyap et al (37) recently showed that the Roux-en-Y gastric bypass alters the incretin axis in line with beneficial changes in insulin secretion; the current study builds on these observations and adds some mechanistic insight into the effect of a nonsurgical lifestyle intervention on the incretin axis. Chronic hyperglycemia impairs β cell function in humans (16) and isolated pancreatic islets (17). Our high-GI diets elicited significantly higher day-long plasma glycemia (Figure 1); therefore, it is possible that 12 wk of a high-GI diet exposed intestinal incretin-secreting cells and pancreatic β cells to higher glucose concentrations. Glucotoxicity is a phenomenon related to β cell dysfunction (38); thus, one may speculate that a similar stressor acts on the incretin-releasing K cells (GIP) of the intestinal tract, causing impaired function that prevents the exercise and diet-induced reduction in incretin secretion. To support this hypothesis, hyperglycemia has been shown to down-regulate GIP receptor expression (27, 39), alter the postprandial incretin response (28), and to increase GIP glycation (40). However, the precise mechanisms for the role of incretin peptides and intestinal K cell function in hyperglycemia-related disease require further investigation.
Previous data have shown marked attenuation of peripheral tissue and hepatic IR and improved oral glucose tolerance after exercise training or weight loss via exercise and/or caloric restriction (4–9). Furthermore, a reversal of hyperinsulinemia has been shown in overweight and obese men and women (11–13), whereas improvements in β cell function have been identified in T2D patients (10, 41). Experiments in our laboratory have shown that the reduction in compensatory hyperinsulinemia in obese nondiabetic individuals after 12 wk of lifestyle-induced weight loss is driven by reduced β cell glucose exposure rather than an intrinsic alteration in β cell function (41). Previous work by Solomon et al (41) and Kelly et al (42) has also shown that lifestyle-induced changes in insulin secretion are related to changes in GIP. The current data further highlight the importance of incretin-mediated effects on the β cell after weight loss, which are independent of β cell glucose exposure. While bariatric surgery and pharmaceutical intervention has identified the incretin axis as a key mediator of insulin secretion, these current findings highlight the value of lifestyle intervention for the treatment of incretin-axis dysfunction and hyperglycemia-related disease.
An optimal dietary prescription for individuals at risk of developing T2D is important in order to maintain glycemic control. The concept of dietary GI has been well studied. Reports have indicated that, when compared with a high-GI diet, low-GI dietary intake may be advantageous for promoting weight loss (21) and glycemic control (20) and for reducing IR (43) and risk of cardiovascular disease (44). However, there have also been equivocal findings with regard to weight loss (45, 46), disease risk (47, 48), and effects on Hb A1c (49). Previously, Solomon et al (25) reported that a 7-d LoGIX compared with a HiGIX intervention improved, but had no divergent effects on, IR. Herein, we showed that extending the duration of the intervention to 12-wk produced a similar result concerning peripheral insulin sensitivity. Although Kirwan et al (50) previously showed that dietary switching from a high-GI to a low-GI diet may augment insulin sensitivity, to our knowledge, only the First Step First Bite program group has previously examined a combined exercise and low-GI approach (51). Their findings indicated no increased benefit of adding low-GI foods to a low-intensity walking program on metabolic or anthropometric variables, including Hb A1c. However, in contrast to their diet/exercise counseling, our current study program offers a more robust and highly controlled dietary provision and daily exercise supervision and adds strong evidence to the hypothesis that a low-GI diet may be advantageous for reducing diabetes risk.
In conclusion, this study showed that although diet and exercise-induced improvements in body composition and IR in obese patients with prediabetes were not influenced by dietary GI, the consumption of a high-GI diet during such therapy impaired pancreatic β cell function and prevented the improvement in intestinal K cell function. In contrast, a low-GI diet decreased both hyperglycemia and hyperinsulinemia and thus relieved β cell stress, therefore potentially preventing diabetes onset. These findings support an important role for gut hormones in mediating the metabolic effects of a low-GI diet in obese, prediabetic patients.
Acknowledgments
We thank the research volunteers for their outstanding dedication and effort and the nursing staff of the Clinical Research Unit and the staff and students who helped with the implementation of the study and assisted with data collection. We also thank the dietary staff in the Bionutrition Unit of the CTSC for their assistance with preparing meals for this study.
The authors' responsibilities were as follows—JPK and HB: conception and design; TPJS, JMH, KRK, MDC, JF, MR, SRK, HB, and JPK: data acquisition; TPJS, JMH, KRK, SRK, RMW, HB, and JPK: analysis and interpretation of data; TPJS: drafting of the manuscript; JMH, SRK, RMW, HB, and JPK: critical revision of the manuscript; TPJS: statistical analyses; JPK: obtained funding; MDC and JF: technical support; HB: dietary support; MR and SRK: clinical support; and HB and JPK: supervision. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Jallut D, Golay A, Munger R, et al. Impaired glucose tolerance and diabetes in obesity: a 6-year follow-up study of glucose metabolism. Metabolism 1990;39:1068–75 [DOI] [PubMed] [Google Scholar]
- 2.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowler W, Fowler S, Hamman R, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009;374:1677–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rogers MA, Yamamoto C, King DS, Hagberg JM, Ehsani AA, Holloszy JO. Improvement in glucose tolerance after 1 wk of exercise in patients with mild NIDDM. Diabetes Care 1988;11:613–8 [DOI] [PubMed] [Google Scholar]
- 5.O'Leary VB, Marchetti CM, Krishnan RK, Stetzer BP, Gonzalez F, Kirwan JP. Exercise-induced reversal of insulin resistance in obese elderly is associated with reduced visceral fat. J Appl Physiol 2006;100:1584–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haus JM, Solomon TP, Marchetti CM, Edmison JM, Gonzalez F, Kirwan JP. Free fatty acid-induced hepatic insulin resistance is attenuated following lifestyle intervention in obese individuals with impaired glucose tolerance. J Clin Endocrinol Metab 2010;95:323–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon TP, Sistrun SN, Krishnan RK, et al. Exercise and diet enhance fat oxidation and reduce insulin resistance in older obese adults. J Appl Physiol 2008;104:1313–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodpaster BH, Katsiaras A, Kelley DE. Enhanced fat oxidation through physical activity is associated with improvements in insulin sensitivity in obesity. Diabetes 2003;52:2191–7 [DOI] [PubMed] [Google Scholar]
- 9.Coker RH, Williams RH, Yeo SE, et al. The impact of exercise training compared to caloric restriction on hepatic and peripheral insulin resistance in obesity. J Clin Endocrinol Metab 2009;94:4258–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E1024–31 [DOI] [PubMed] [Google Scholar]
- 11.Slentz CA, Tanner CJ, Bateman LA, et al. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009;32:1807–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn SE, Larson VG, Beard JC, et al. Effect of exercise on insulin action, glucose tolerance, and insulin secretion in aging. Am J Physiol 1990;258:E937–43 [DOI] [PubMed] [Google Scholar]
- 13.Kirwan JP, Kohrt WM, Wojta DM, Bourey RE, Holloszy JO. Endurance exercise training reduces glucose-stimulated insulin levels in 60- to 70-year-old men and women. J Gerontol 1993;48:M84–90 [DOI] [PubMed] [Google Scholar]
- 14.Utzschneider KM, Carr DB, Barsness SM, Kahn SE, Schwartz RS. Diet-induced weight loss is associated with an improvement in beta-cell function in older men. J Clin Endocrinol Metab 2004;89:2704–10 [DOI] [PubMed] [Google Scholar]
- 15.Brons C, Jensen CB, Storgaard H, et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J Physiol 2009;587:2387–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Prato S, Leonetti F, Simonson DC, Sheehan P, Matsuda M, DeFronzo RA. Effect of sustained physiologic hyperinsulinaemia and hyperglycaemia on insulin secretion and insulin sensitivity in man. Diabetologia 1994;37:1025–35 [DOI] [PubMed] [Google Scholar]
- 17.Hribal ML, Perego L, Lovari S, et al. Hyperglycemia impairs insulin secretion by affecting insulin receptor expression, splicing, and signaling in RIN beta cell line and human islets of Langerhans. FASEB J 2003;17:1340–2 [DOI] [PubMed] [Google Scholar]
- 18.Fukagawa NK, Anderson JW, Hageman G, Young VR, Minaker KL. High-carbohydrate, high-fiber diets increase peripheral insulin sensitivity in healthy young and old adults. Am J Clin Nutr 1990;52:524–8 [DOI] [PubMed] [Google Scholar]
- 19.Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997;277:472–7 [DOI] [PubMed] [Google Scholar]
- 20.Rizkalla SW, Taghrid L, Laromiguiere M, et al. Improved plasma glucose control, whole-body glucose utilization, and lipid profile on a low-glycemic index diet in type 2 diabetic men: a randomized controlled trial. Diabetes Care 2004;27:1866–72 [DOI] [PubMed] [Google Scholar]
- 21.Pittas AG, Das SK, Hajduk CL, et al. A low-glycemic load diet facilitates greater weight loss in overweight adults with high insulin secretion but not in overweight adults with low insulin secretion in the CALERIE Trial. Diabetes Care 2005;28:2939–41 [DOI] [PubMed] [Google Scholar]
- 22.Kirwan JP, O'Gorman D, Evans WJ. A moderate glycemic meal before endurance exercise can enhance performance. J Appl Physiol 1998;84:53–9 [DOI] [PubMed] [Google Scholar]
- 23.Febbraio MA, Keenan J, Angus DJ, Campbell SE, Garnham AP. Preexercise carbohydrate ingestion, glucose kinetics, and muscle glycogen use: effect of the glycemic index. J Appl Physiol 2000;89:1845–51 [DOI] [PubMed] [Google Scholar]
- 24.Stevenson EJ, Thelwall PE, Thomas K, Smith F, Brand-Miller J, Trenell MI. Dietary glycemic index influences lipid oxidation but not muscle or liver glycogen oxidation during exercise. Am J Physiol Endocrinol Metab 2009;296:E1140–7 [DOI] [PubMed] [Google Scholar]
- 25.Solomon TP, Haus JM, Kelly KR, et al. Randomized trial on the effects of a 7-d low-glycemic diet and exercise intervention on insulin resistance in older obese humans. Am J Clin Nutr 2009;90:1222–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Botero D, Ebbeling CB, Blumberg JB, et al. Acute effects of dietary glycemic index on antioxidant capacity in a nutrient-controlled feeding study. Obesity (Silver Spring) 2009;17:1664–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J, Livak MF, Bernier M, et al. Ubiquitination is involved in glucose-mediated downregulation of GIP receptors in islets. Am J Physiol Endocrinol Metab 2007;293:E538–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vollmer K, Gardiwal H, Menge BA, et al. Hyperglycemia acutely lowers the postprandial excursions of glucagon-like Peptide-1 and gastric inhibitory polypeptide in humans. J Clin Endocrinol Metab 2009;94:1379–85 [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–23 [DOI] [PubMed] [Google Scholar]
- 30.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: Kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 1980;51:520–8 [DOI] [PubMed] [Google Scholar]
- 31.Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Thiazolidinediones improve beta-cell function in type 2 diabetic patients. Am J Physiol Endocrinol Metab 2007;292:E871–83 [DOI] [PubMed] [Google Scholar]
- 32.Holst JJ, Vilsboll T, Deacon CF. The incretin system and its role in type 2 diabetes mellitus. Mol Cell Endocrinol 2009;297:127–36 [DOI] [PubMed] [Google Scholar]
- 33.Meier JJ, Hucking K, Holst JJ, Deacon CF, Schmiegel WH, Nauck MA. Reduced insulinotropic effect of gastric inhibitory polypeptide in first-degree relatives of patients with type 2 diabetes. Diabetes 2001;50:2497–504 [DOI] [PubMed] [Google Scholar]
- 34.Fritsche A, Stefan N, Hardt E, Haring H, Stumvoll M. Characterisation of beta-cell dysfunction of impaired glucose tolerance: evidence for impairment of incretin-induced insulin secretion. Diabetologia 2000;43:852–8 [DOI] [PubMed] [Google Scholar]
- 35.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes 2003;52:380–6 [DOI] [PubMed] [Google Scholar]
- 36.Meneilly GS, Ryan AS, Minaker KL, Elahi D. The effect of age and glycemic level on the response of the beta-cell to glucose-dependent insulinotropic polypeptide and peripheral tissue sensitivity to endogenously released insulin. J Clin Endocrinol Metab 1998;83:2925–32 [DOI] [PubMed] [Google Scholar]
- 37.Kashyap SR, Daud S, Kelly KR, et al. Acute effects of gastric bypass versus gastric restrictive surgery on beta-cell function and insulinotropic hormones in severely obese patients with type 2 diabetes. Int J Obes (Lond) 2010;34:462–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 2008;29:351–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu G, Kaneto H, Laybutt DR, et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes 2007;56:1551–8 [DOI] [PubMed] [Google Scholar]
- 40.Mooney MH, Abdel-Wahab YH, Morgan LM, O'Harte FP, Flatt PR. Detection of glycated gastric inhibitory polypeptide within the intestines of diabetic obese (ob/ob) mice. Endocrine 2001;16:167–71 [DOI] [PubMed] [Google Scholar]
- 41.Solomon TP, Haus JM, Kelly KR, Rocco M, Kashyap SR, Kirwan JP. Improved pancreatic β-cell function in type 2 diabetics following lifestyle-induced weight loss is related to glucose-dependent insulinotropic polypeptide. Diabetes Care 2010;33:1561–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly KR, Brooks LM, Solomon TP, Kashyap SR, O'Leary VB, Kirwan JP. The glucose-dependent insulinotropic polypeptide and glucose-stimulated insulin response to exercise training and diet in obesity. Am J Physiol Endocrinol Metab 2009;296:E1269–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira MA, Swain J, Goldfine AB, Rifai N, Ludwig DS. Effects of a low-glycemic load diet on resting energy expenditure and heart disease risk factors during weight loss. JAMA 2004;292:2482–90 [DOI] [PubMed] [Google Scholar]
- 44.McKeown NM, Meigs JB, Liu S, et al. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr 2009;28:150–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr 2007;85:1023–30 [DOI] [PubMed] [Google Scholar]
- 46.Bouche C, Rizkalla SW, Luo J, et al. Five-week, low-glycemic index diet decreases total fat mass and improves plasma lipid profile in moderately overweight nondiabetic men. Diabetes Care 2002;25:822–8 [DOI] [PubMed] [Google Scholar]
- 47.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity (Silver Spring) 2006;14:2200–9 [DOI] [PubMed] [Google Scholar]
- 48.Levitan EB, Mittleman MA, Wolk A. Dietary glycemic index, dietary glycemic load and mortality among men with established cardiovascular disease. Eur J Clin Nutr 2009;63:552–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114–25 [DOI] [PubMed] [Google Scholar]
- 50.Kirwan JP, Barkoukis H, Brooks LM, Marchetti CM, Stetzer BP, Gonzalez F. Exercise training and dietary glycemic load may have synergistic effects on insulin resistance in older obese adults. Ann Nutr Metab 2009;55:326–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheong SH, McCargar LJ, Paty BW, Tudor-Locke C, Bell RC. The First Step First Bite Program: guidance to increase physical activity and daily intake of low-glycemic index foods. J Am Diet Assoc 2009;109:1411–6 [DOI] [PubMed] [Google Scholar]