Abstract
Resting-state functional connectivity (RSFC) approaches offer a novel tool to delineate distinct functional networks in the brain. In the present functional magnetic resonance imaging (fMRI) study, we elucidated patterns of RSFC associated with 6 regions of interest selected primarily from a meta-analysis on word reading (Bolger DJ, Perfetti CA, Schneider W. 2005. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Hum Brain Mapp. 25: 92–104). In 25 native adult readers of English, patterns of positive RSFC were consistent with patterns of task-based activity and functional connectivity associated with word reading. Moreover, conjunction analyses highlighted the posterior left inferior frontal gyrus and the posterior left middle temporal gyrus (post-LMTG) as potentially important loci of functional interaction among 5 of the 6 reading networks. The significance of the post-LMTG has typically been unappreciated in task-based studies on unimpaired readers but is frequently reported to be a locus of hypoactivity in dyslexic readers and exhibits intervention-induced changes of activity in dyslexic children. Finally, patterns of negative RSFC included not only regions of the so-called default mode network but also regions involved in effortful controlled processes, which may not be required once reading becomes automatized. In conclusion, the current study supports the utility of resting-state fMRI for investigating reading networks and has direct relevance for the understanding of reading disorders such as dyslexia.
Keywords: functional connectivity, functional MRI, left middle temporal gyrus, meta-analysis, resting-state, word reading
Introduction
Reading requires dynamic interactions among multiple brain systems. As early as the mid-20th century, Geschwind (1965) claimed that language and reading disorders arise from diminished connectivity between distinct functional systems in the brain. The recent advent of neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have provided researchers with a means of examining connectionist frameworks for reading disorders in the living human brain. For example, using PET, Horwitz et al. (1998) demonstrated that during single-word reading, dyslexic readers, when compared with unimpaired readers, exhibited decreased functional connectivity of the left angular gyrus with the left frontal, temporal, and occipito–temporal areas. This finding was replicated in part by a later fMRI study, in which dyslexic readers showed diminished functional connectivity between the left angular gyrus and the left occipito–temporal areas during tasks that required intensive phonological processing (Pugh et al. 2000).
Despite these initial successes, researchers continue to struggle with the challenge of developing effective probes for examining reading networks, in both nonclinical and clinical populations. In other words, the field lacks consensus regarding the “ideal task” or set of tasks for probing reading networks, yielding inconsistent findings across studies of reading. For example, the specific task (e.g., naming words and semantic categorization) employed in fMRI studies of word reading can produce marked variation in the locations of peak activations (Booth et al. 2006; Nakamura et al. 2007) and in patterns of functional connectivity (Mechelli et al. 2005; Nakamura et al. 2007). Additionally, given that reading abilities are dependent on both age and educational level, researchers face the challenge of devising probe tasks appropriate for a broad range of ages and abilities. Such difficulties are reflected in inconsistent findings from studies of unimpaired (Turkeltaub et al. 2002; Bolger et al. 2005) and dyslexic (Richlan et al. 2009) readers. For example, the importance of the left angular gyrus in reading is suggested by reduced task-related activation in these areas in dyslexic readers (Shaywitz et al. 1998; Richlan et al. 2009), but this region is not reported to be strongly activated during word-reading tasks in unimpaired readers (Turkeltaub et al. 2002; Bolger et al. 2005). As such, task selection can confound the examination of neural mechanisms of reading and reading disorders.
A potential solution to this problem is offered by resting-state functional connectivity (RSFC) approaches. In recent years, the application of these approaches to fMRI data has emerged as a powerful and highly efficient method for mapping and assessing the functional architecture of the brain, without task constraints (Biswal et al. 1995). When individuals are scanned at rest, low-frequency (<0.1 Hz) spontaneous fluctuations in the blood oxygen level–dependent (BOLD) signal are temporally synchronized between functionally related brain areas (Biswal et al. 1997; Lowe et al. 1998; Greicius et al. 2003; Damoiseaux et al. 2006; Margulies et al. 2007). Such RSFC patterns are considered to represent intrinsically organized functional networks in the brain (see review by Fox and Raichle 2007). Among identified intrinsic connectivity networks, the so-called default mode network has been most intensively investigated (Raichle et al. 2001; Raichle and Snyder 2007). This network includes the medial prefrontal cortex, posterior cingulate cortex, and precuneus and is typically deactivated during goal-oriented tasks. Spontaneous BOLD fluctuations within the default mode network exhibit negative correlations with fluctuations in brain regions that are typically activated during the performance of attention-demanding tasks (so-called task-positive regions, Fox et al. 2005). Interestingly, alterations in the RSFC of the default mode network have been observed in several clinical populations, such as individuals with Alzheimer's disease (Li et al. 2002; Greicius et al. 2004), attention-deficit hyperactivity disorder (Castellanos et al. 2008; Uddin et al. 2008), and schizophrenia (Liang et al. 2006; Zhou et al. 2007).
Previous studies have successfully applied RSFC approaches to the study of language and reading networks. Hampson et al. (2006) showed that patterns of functional connectivity among reading-related areas are strikingly consistent across reading and rest conditions. In particular, Broca's area (i.e., the left inferior frontal gyrus [IFG]) was significantly correlated with the left angular gyrus (BA39) as well as with the left occipitotemporal cortex (i.e., fusiform gyrus [FFG]). Intriguingly, the strength of functional connectivity between Broca's area and the left angular gyrus during both reading and rest differentiated better readers from worse readers (although none were impaired readers). Most recently, Lohmann et al. (2009) demonstrated that functional connectivity between language areas observed during 4 different language tasks remained even after regressing out specifics of the task paradigms and low-pass filtering (<0.01 Hz). This finding supports the suggestion that patterns of RSFC reflect an intrinsic functional organization underlying cognitive processes. Furthermore, these initial results support the utility of RSFC methods for further characterization of the neural networks underlying reading.
In order to build on the work of Hampson et al. (2006), we examined patterns of RSFC associated with 6 regions of interest (ROIs) that were found to be consistently activated by word reading tasks in 2 recently published meta-analyses (Turkeltaub et al. 2002; Bolger et al. 2005). Given that even subtle differences in the specific paradigms and cognitive constructs examined can produce marked variation in findings across task-based studies, we selected our ROIs on the basis of a meta-analysis rather than on any single study so as to best sample the regions activated by word-reading tasks. We hypothesized that patterns of positive RSFC (positive correlations) associated with the 6 reading-related ROIs would be consistent with previously shown patterns of task-related activation and functional connectivity during word reading. Second, we tested whether patterns of negative RSFC (negative correlations) associated with the 6 reading-related ROIs would coincide with the default mode network. Finally, we aimed to identify potentially important “loci of functional interaction,” defined as regions of overlap among the 6 reading networks, by performing a conjunction analysis. The networks and loci of interaction identified using this approach have clinical relevance in that they provide targets for investigation in reading disorders such as dyslexia.
Materials and Methods
Participants
Twenty-five right-handed healthy adults (mean age: 32.0 ± 8.4 years, 19 males) participated in the current fMRI study. Data from these participants have been included in several previously published studies from our laboratory (e.g., Margulies et al. 2007; Di Martino et al. 2008; Shehzad et al. 2009). All participants were college educated and native speakers (and readers) of English, with no history of neurological, psychiatric, or learning disorders, including dyslexia, as confirmed by both clinical interview and questionnaires. The study was approved by the institutional review boards of the New York University School of Medicine and New York University. Prior written informed consent was obtained from all participants.
fMRI Data Acquisition
MRI data were collected on a Siemens Allegra 3.0-T scanner at the NYU Center for Brain Imaging. Scans were brief (6 min 38 s) and comprised of 197 continuous echo planar imaging functional volumes (time repetition [TR] = 2000 ms; time echo [TE] = 25 ms; flip angle = 90; 39 slices, matrix = 64 × 64; field of view [FOV] = 192 mm; acquisition voxel size = 3 × 3 × 3 mm). During the scan, participants were instructed to relax with their eyes open while the word “Relax” was projected at the center of the display. A high-resolution T1-weighted anatomical image was also acquired using a magnetization prepared gradient echo sequence (TR = 2500 ms; TE = 3.93 ms; inversion time = 900 ms; flip angle = 8; 176 slices; and FOV = 256 mm).
Preprocessing
Preprocessing steps for slice timing correction, motion correction, and despiking (detection and reduction of extreme time series outliers) were performed using analysis of functional neuroimages (AFNI; Cox 1996). Further preprocessing steps were performed using FMRIB Software Library (FSL) (Smith et al. 2004) and comprised spatial smoothing (using a Gaussian kernel of full width half maximum 6 mm), mean-based intensity normalization of all volumes by the same factor, temporal bandpass filtering (0.01–0.1 Hz), and prewhitening (correction for time series autocorrelation). Finally, each individual's time series was spatially normalized by a 12-degrees-of-freedom affine registration to the MNI152 standard brain template (Montreal Neurological Institute), with 2-mm3 resolution.
Nuisance Signal Regression
To control for the effects of physiological processes (such as fluctuations related to cardiac and respiratory cycles) and motion, we removed signal associated with several nuisance covariates. Specifically, we regressed each subject's preprocessed 4-D volume on 9 predictors that modeled nuisance signals from white matter, cerebrospinal fluid, the global signal, and 6 motion parameters, as detailed elsewhere (Kelly, de Zubicaray, et al. 2009; Kelly, Di Martino, et al. 2009).
ROI Selection and Seed Generation
We selected a total of 6 ROIs on the basis of 2 meta-analyses of single word reading in alphabetic languages (Turkeltaub et al. 2002; Bolger et al. 2005). These meta-analyses used an activation likelihood estimate approach to identify several foci reliably activated across studies of single word reading. First, we identified the 5 brain areas that were identified as being colocated in regions defined by the Harvard–Oxford Cortical Structural Probabilistic Atlas (Kennedy et al. 1998; Makris et al. 1999) in both meta-analyses (the left FFG, the left superior temporal gyrus (STG), the left temporoparietal junction (TPJ), the left precentral gyrus (PCG), and the left IFG). Because the peak coordinates between the 2 meta-analyses differed by 17 mm for the left FFG and 14 mm for the left IFG, we used the coordinates reported by the more recent Bolger et al. (2005) meta-analysis, which included more contrasts (i.e., 38 contrasts from 25 studies) than Turkeltaub et al. (2002), which contained 16 contrasts from 11 studies. Limiting coordinate selection to 1 meta-analysis also avoided potentially over representing regions. Of note, the 5 regions selected have also been reported to be hypoactivated in dyslexic readers compared with unimpaired readers (Richlan et al. 2009).
Second, we added a sixth ROI located in the posterior part of the left inferior occipital gyrus. This cortical area was reported only in Bolger et al.'s (2005) meta-analysis but was included in light of the clinical observation that damage to the posterior occipital area, particularly in the left hemisphere, can result in pure alexia characterized by letter-by-letter reading (Behrmann et al. 1998; Sakurai et al. 2001). We verified that all ROIs were at least 12 mm apart in Euclidean distance to ensure that the regions were spatially distinct. For each ROI, we converted the reported Talairach coordinates into MNI coordinates (Brett et al. 2001) and created a spherical seed centered on these coordinates in 2 × 2 × 2-mm space, with a radius of 6 mm.
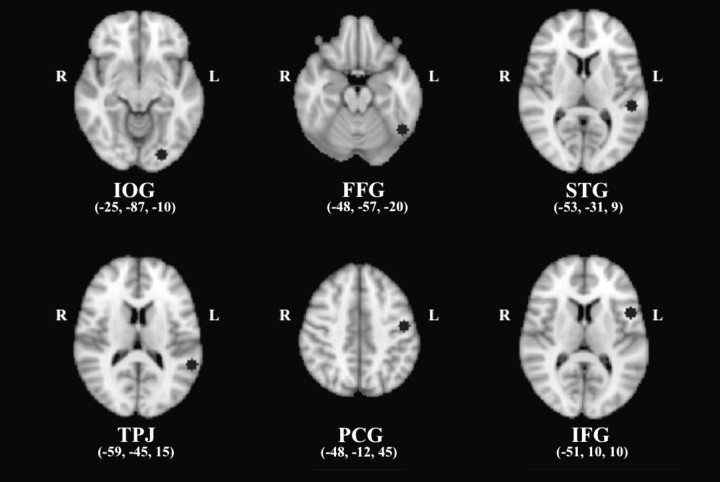
The 6 seed ROIs were spatially distributed in the left hemisphere (Fig. 1) and labeled according to the Harvard–Oxford Cortical Structural Atlas (Kennedy et al. 1998; Makris et al. 1999); 1) inferior occipital gyrus (IOG) (x = −25, y = −87, z = −10), in the medial posterior part of the left inferior occipital gyrus, part of the visual ventral pathway; 2) FFG (x = −48, y = −57, z = −20), in the posterior part of the left FFG, at a location coinciding with the “Visual Word Form Area” that is responsive to word and word-like orthography (Cohen et al. 2000; Dehaene et al. 2002); 3) STG (x = −53, y = −31, z = 9), in the posterior part of the left STG, which is included in Wernicke's area and involved in speech perception (Scott and Wise 2004); 4) TPJ (x = −59, y = −45, z = 15), in the left TPJ, where the ventral posterior part of the left supramarginal gyrus meets the posterior part of STG. This region's activation is often observed in association with grapheme-phoneme conversion (phonological decoding, Fiez and Petersen 1998; Turkeltaub et al. 2003); 5) PCG (x = −48, y = −12, z = 45), in the dorsal part of the left PCG, part of the primary motor cortex; and 6) IFG (x = −51, y = 10, z = 10), in the pars opercularis of the left IFG, which is included in Broca's area and involved in speech articulation (Wise et al. 1999; Nixon et al. 2004; Owen et al. 2004). The location of each seed is shown in MNI space in Figure 1. For each participant, we calculated the mean time series of each seed by averaging across all voxels within the seed.
Figure 1.
Six seed regions (black circles) were selected based primarily on a meta-analysis of single word reading (Bolger et al. 2005). All the seed regions are indicated in MNI coordinates; IOG = inferior occipital gyrus (posterior), FFG = fusiform gyrus (posterior), STG = superior temporal gyrus (posterior), TPJ = temporoparietal junction, PCG = precentral gyrus (dorsal), and IFG = inferior frontal gyrus (opercularis).
We note that our set of ROIs did not include BA39, the activation level and connectivity strength of which are diminished in dyslexic readers (Horwitz et al. 1998; Shaywitz et al. 1998; Pugh et al. 2000). This was because neither of the recent meta-analyses of unimpaired reading (Turkeltaub et al. 2002; Bolger et al. 2005) highlighted the left angular gyrus as a locus of consistent activation for word reading. The exclusion of the left angular gyrus can be further rationalized given that this region's functional significance in reading appears to be specific to less experienced readers (children; Church et al. 2008) and individuals with reading disorders (Dejerine 1891).
Subject- and Group-Level RSFC Maps
For each participant, we performed a multiple regression analysis (using the general linear model implemented in the FSL program FEAT) for each seed. The mean time series of each seed was calculated by averaging across all voxels within the seed for each subject, using the AFNI program 3dmaskave. This analysis produced individual subject-level maps of all voxels that were positively and negatively correlated with the seed-time series. Group-level analyses were carried out using a mixed-effects model as implemented in the FSL program FLAME. Corrections for multiple comparisons were conducted at the cluster level for each RSFC map (Z > 2.3; P < 0.05, corrected). The group analysis created 6 positive and 6 negative thresholded Z-statistic maps (RSFC maps).
Conjunction Analysis
To identify loci of functional interactions among intrinsic reading networks, the 6 group-level thresholded maps of positive RSFC were each binarized and then summed to create a conjunction map. The resultant map was then thresholded to identify areas that are common to all 6 RSFC maps and to 5, 4, 3, and 2 of the 6 RSFC maps. Similarly, we conducted a conjunction analyses for the 6 maps of negative RSFC. To reduce the rate of false positives, areas of overlap were required to comprise >10 voxels.
Results
Positive Connectivity
For each of 6 brain regions previously implicated in reading (Bolger et al. 2005), seed-based correlation analyses were employed to characterize their associated functional systems during rest. Figure 2 depicts positive RSFC for each of the 6 reading-related seeds (second to seventh columns). Detailed descriptions of each RSFC pattern and peak coordinates of significant positive correlations are found in Supplementary Document 1 and Supplementary Table 1, respectively.
Figure 2.
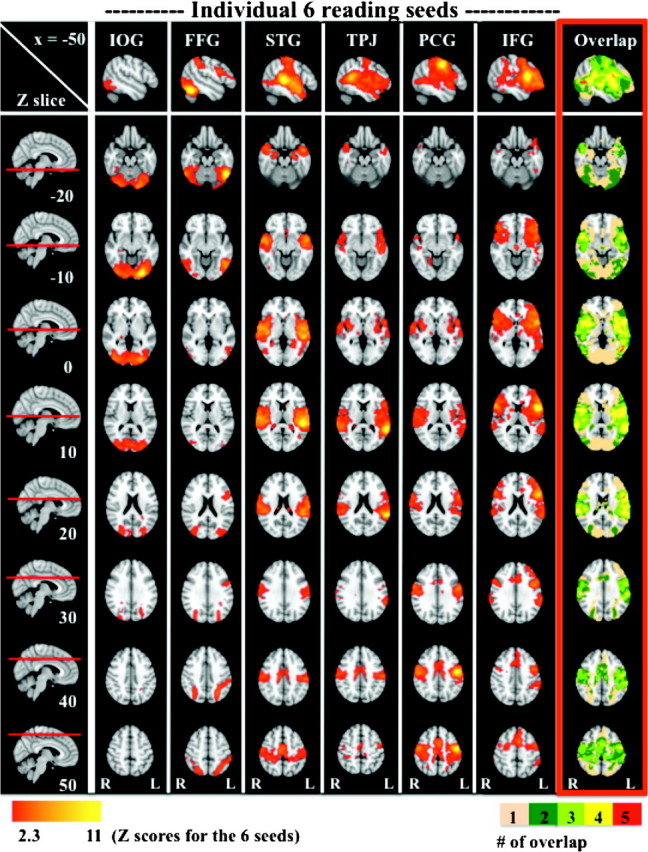
Positive RSFC maps associated with the 6 reading-related seeds. The top row displays RSFC patterns on the left hemisphere (x = −50). The first column presents each z-dimension where correlation maps are superimposed. Each RSFC map is illustrated from the second column until the seventh column (Z > 2.3, P < 0.05, corrected). The last column framed in red depicts “overlap” between RSFC maps. Loci of overlap among 1, 2, 3, 4, and 5 RSFC maps are colored in beige, dark green, light green, yellow, and red, respectively. There were no clusters (>10 voxels) exhibiting significant positive correlations with all 6 seeds; IOG = inferior occipital gyrus (posterior), FFG = fusiform gyrus (posterior), STG = superior temporal gyrus (posterior), TPJ = temporoparietal junction, PCG = precentral gyrus (dorsal), and IFG = inferior frontal gyrus (opercularis).
Overall, the patterns of RSFC associated with each of our 6 seed ROIs were consistent with reading networks previously identified using task-based approaches. Most notably, RSFC between the TPJ seed and left frontal and temporal areas showed remarkable similarities to previously shown functional connectivity patterns of the left angular gyrus (in the vicinity of the TPJ seed used in the present study) during reading tasks (Horwitz et al. 1998; Pugh et al. 2000) as well as during rest (Hampson et al. 2006). In addition, the FFG seed exhibited long-range RSFC with the left IFG extending into the PCG. This was exactly mirrored by long-range RSFC of the IFG seed with the left FFG. Such long-range connectivity patterns between occipito-temporal and frontal areas have been robustly demonstrated by task-based fMRI studies on reading (Mechelli et al. 2005; Bitan et al. 2006). Of note, RSFC patterns for the IFG seed also were comparable with those demonstrated by Hampson et al. (2006). In sum, even though no reading tasks or word stimuli were included, our findings were highly consistent with those previously reported in the task-based reading literature.
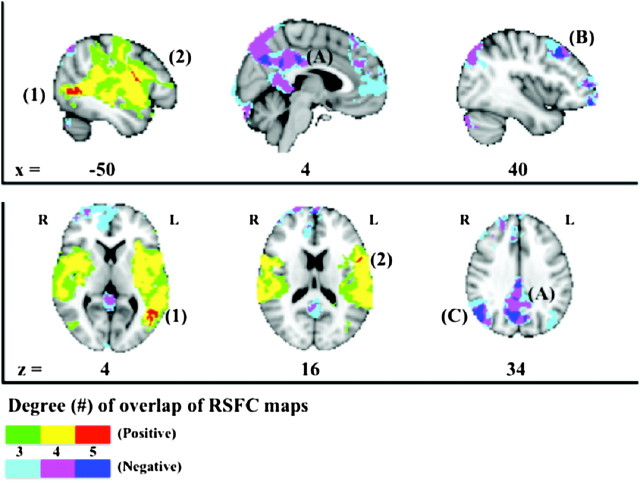
As shown in the red-framed column (“overlap”) of Figure 2, a broad array of brain areas covering much of the cerebral cortex was found to be common to one or more of the reading-related seeds. In order to identify “loci of functional interaction” among the 6 RSFC networks, we carried out a series of conjunction analyses. Although no voxels exhibited significant positive correlations with all 6 seeds, 2 loci in the left hemisphere were common to 5 of the 6 RSFC maps (Fig. 3, shown in red). The first was located within the posterior part of the left middle temporal gyrus (post-LMTG; center of gravity in MNI space: x = −48, y = −62, z = 4; cluster extent = 82 voxels). The second region of overlap was located in the posterior boundary of the left IFG (post-LIFG; center of gravity in MNI space: x = −54, y = 6, z = 18; cluster extent = 47 voxels). Of note, inspection of the data revealed that the IOG seed was the only seed that did not exhibit connectivity with these 2 loci. Finally, Figure 3 shows that overlap between 3 (in green) and 4 (in yellow) of the 6 correlation maps was evident in multiple brain areas, distributed across frontal, motor, insula, temporal, and parietal regions, bilaterally.
Figure 3.
Loci of overlap among RSFC maps. Green, yellow, and red represent regions of overlap among 3, 4, and 5 positive RSFC maps, respectively. Light blue, purple, and dark blue represent regions of overlap among 3, 4, and 5 negative RSFC maps, respectively. Loci of overlap among the 5 positive maps (red) are identified in 1) the posterior part of the left middle temporal gyrus and 2) the posterior part of the IFG. In contrast, loci of overlap among the 5 negative maps (dark blue) are found in (A) the posterior cingulate cortex extending into the precuneus, (B) the right middle frontal gyrus, and (C) the right angular gyrus.
Negative Connectivity
Brain areas that exhibited significant negative connectivity with one or more of the 6 seeds included the medial prefrontal cortex, dorsolateral prefrontal cortex, anterior cingulate cortex, superior lateral parietal cortex, lateral temporal cortex, posterior cingulate cortex, and adjacent precuneus (Fig. 3). Many, but not all, of these negatively connected brain areas are topographically consistent with the default mode network, which is typically deactivated during tasks and negatively correlated with task-active networks (Raichle et al. 2001; Fox et al. 2005; Toro et al. 2008). Similar to the positive connectivity maps, although no voxels exhibited significant negative correlations with all 6 seeds, there was overlap among 5 of the 6 negative RSFC maps (again, excluding the map associated with the IOG seed) in several cortical areas. These included the posterior cingulate cortex extending into the precuneus, the dorsolateral prefrontal cortex (i.e., the right middle frontal gyrus), and the superior lateral parietal cortex (i.e., the right angular gyrus; Fig. 3 in dark blue). In addition, Figure 3 illustrates loci of overlap among 3 (in light blue) and 4 (in purple) of the 6 negative RSFC maps, which extended outward from the aforementioned loci of overlap to comprise much of the classically defined default mode network.
Secondary Analysis: Post-LMTG
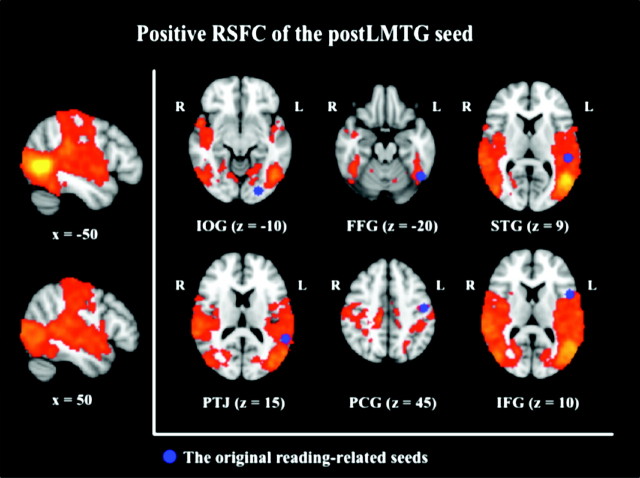
In order to confirm the importance of the post-LMTG as a locus of putative functional interaction for reading, we used the post-LMTG region that exhibited overlap among 5 of the 6 maps of positive RSFC as a seed in a secondary RSFC analysis (Fig. 4). This analysis allowed us to determine the extent to which the pattern of RSFC associated with this region overlapped with those associated with the 6 seed regions. The post-LMTG seed was positively connected with a range of cortical areas in both hemispheres, including the lateral occipital cortex, the lingual gyrus, the temporal cortices, the parietal cortices, the insula, and the precentral/postcentral gyri extending into the posterior boundary of the IFG. This map of positive RSFC comprised the locations of 5 of the 6 reading seeds, with the one exception being the IOG (Fig. 4). This confirms the observed result of the conjunction analysis, which suggested that the post-LMTG region is a locus of functional interaction among the 5 intrinsic reading networks. Notably, the post-LMTG seed was not positively connected with areas of the prefrontal cortex nor the posterior–medial part of the occipital gyrus, where the IOG seed was located.
Figure 4.
Positive RSFC of the posterior part of the left middle temporal gyrus (post-LMTG) seed as a locus of functional interaction among reading networks. The original 6 seed regions are presented in blue. The post-LMTG was positively connected with all the seed regions except for the IOG seed, reconfirming the results of the conjunction analysis (Z > 2.3, P < 0.05, corrected); IOG = inferior occipital gyrus (posterior), FFG = fusiform gyrus (posterior), STG = superior temporal gyrus (posterior), TPJ = temporoparietal junction, PCG = precentral gyrus (dorsal), and IFG = inferior frontal gyrus (opercularis).
In short, our resting-state fMRI results successfully replicated previous patterns of task-based activity and functional connectivity associated with word reading, even in the absence of reading tasks or word stimuli. Moreover, the results highlighted the post-LIFG and post-LMTG as loci of functional interaction among 5 of the 6 reading networks we examined.
Discussion
We examined patterns of RSFC associated with each of 6 reading-related ROIs. We selected these ROIs on the basis of recent meta-analyses of single word reading (Turkeltaub et al. 2002; Bolger et al. 2005), rather than deriving them from a single study or on the basis of prior theoretical knowledge about language networks. There were 3 main findings. First, the observed patterns of positive RSFC largely replicated patterns of task-based activity and functional connectivity previously demonstrated during word reading, despite the absence of reading tasks or word stimuli. Second, patterns of negative RSFC included both the default mode network as well as several executive regions involved in effortful or controlled processing (e.g., dorsolateral prefrontal and superior parietal cortices; Cazalis et al. 2003; MacDonald 2008). Third, and most importantly, conjunction analyses highlighted the post-LIFG and the post-LMTG as potentially important loci of functional interaction among the reading networks. The post-LMTG was not highlighted in the recently published meta-analyses on word reading in unimpaired readers (Turkeltaub et al. 2002; Bolger et al. 2005) but has been reported as a region of dysfunction in dyslexic readers (Richlan et al. 2009). Our findings suggest the utility of RSFC approaches for studying the functional organization of the brain underlying reading and reading disorders.
Reading Networks at Rest
The first finding of the current study is that reading networks are intrinsically represented in spontaneous BOLD signal fluctuations, at least for experienced adult readers. For example, the observed RSFC between the left temporoparietal area (i.e., the TPJ seed) with temporal areas as well as with frontal areas in the left hemisphere has been robustly demonstrated during nonword reading in unimpaired readers (Horwitz et al. 1998; Pugh et al. 2000; Hampson et al. 2006). Functional connectivity in these circuits was diminished in individuals with dyslexia, suggesting the importance of its integrity to reading ability (Horwitz et al. 1998). Similarly, long-range patterns of left-hemisphere RSFC between frontal (i.e., the IFG seed) and posterior parietal areas as well as those between occipito–temporal (i.e., the FFG seed) and frontal areas are consistent with reading networks identified in task-based fMRI studies (Mechelli et al. 2005; Nakamura et al. 2007) and with reading networks identified in a previous resting-state fMRI study (Hampson et al. 2006). The observed long-range RSFC between the IFG and the FFG in the left hemisphere is also consistent with a recent magnetoencephalography study, in which these 2 regions showed temporal coactivation at the early stage (130–140 ms) of visual word recognition (Cornelissen et al. 2009).
The observed intrinsic representations for reading are likely to result from long-term experience of reading in our participants. RSFC patterns undergo developmental changes (Fair et al. 2007; 2008; Kelly, de Zubicaray, et al. 2009; Kelly, Di Martino, et al. 2009; Supekar et al. 2009), which may reflect experience-dependent (e.g., sensory and cognitive learning) changes in regional coactivation patterns (Dosenbach et al. 2007; Pinsk and Kastner 2007). In other words, experience likely modifies functional connectivity throughout development. Such experiential effects on brain networks are not limited to the highly plastic developing brain (Johnston 2009). Even in adults, learning experiences (e.g., motor learning) can alter RSFC patterns (Albert et al. 2009; Xiong et al. 2009). To test experience-dependent formation of RSFC associated with reading, future research should investigate developmental trajectories of intrinsic representations of reading in children and adolescents in relation to proficiency.
Bilaterality of RSFC
The observed RSFC patterns for reading were largely bilateral, rather than left lateralized, as expected for regions supporting language functions (including reading) in the human brain (Frost et al. 1999; Pujol et al. 1999; Springer et al. 1999; Powell et al. 2006). Findings of largely bilateral patterns of RSFC are not surprising, as the robust homotopic RSFC for the majority of brain regions has been documented (Salvador et al. 2005; Stark et al. 2008). With that said, Stark et al. (2008) noted that primary sensory/motor regions exhibit the highest degree of homotopic connectivity and that higher-order heteromodal association areas exhibit weaker homotopic connectivity. This observation of weaker homotopic connectivity among higher-order heteromodal association areas may reflect hemispheric specialization for higher cognitive functions, such as language. In this regard, we did observe some left-lateralized RSFC patterns, for example, between the left IFG and the left FFG (see Fig. 2).
Alternatively, task-free RSFC may not be sensitive enough to detect lateralization for reading, given that the degree of left lateralization is highly dependent on the nature of tasks (Spironelli and Angrilli 2006; Yang et al. 2009). Although the present study did not focus on the laterality of reading, investigating relationships between asymmetric RSFC patterns and reading performance remains of interest in future studies.
Interpretations of Negative Connectivity
We found evidence of negative connectivity between reading-related regions and 2 key sets of regions. First, we observed negative RSFC between reading-related regions and the posterior cingulate cortex extending into the precuneus, areas commonly included in the default mode network. This result is consistent with previous studies that note strong negative connectivity between task-positive networks and the default mode network (Raichle et al. 2001; Fox et al. 2005; Fransson 2005). The strength of such negative connectivity has been related to behavioral variability (e.g., reaction time in an attentional task; Kelly et al. 2008). Based on these findings, we propose that 1) negative connectivity may represent functional segregation or differentiation between distinct functional systems and 2) the strength of negative connectivity between such systems is a potential index of optimally balanced competition, which may be one of the keys to successful behavior. These theoretical accounts are consistent with the findings of Weissman et al. (2006), who demonstrated that less deactivation in the default mode network during an attention task was significantly related to inefficient behavior (i.e., longer response times). However, such a dichotomous view has been challenged by Sadaghiani et al. (2009), who demonstrated beneficial effects of increased activity in the default mode network on perceptual performance.
Second, we also found negative RSFC in the dorsolateral prefrontal and superior parietal cortices, which are strongly activated by effortful control tasks (Cazalis et al. 2003; MacDonald 2008) and tasks placing high demand on working memory (Marklund et al. 2007; Wendelken et al. 2008). This result is consistent with a recent report that negative connectivity between 2 task-positive networks (i.e., between the visual cortex and the visuospatial attention network) was induced by intensive visual perceptual training (Lewis et al. 2009). In most adults with extensive reading experience, word reading is a relatively automatized process and may not require (or may even require suppression of) high-level effortful control. This is best exemplified by the Stroop task, where word reading occurs obligatorily, despite controlled efforts to ignore the word (MacLeod 1991; Carter et al. 1995; Milham et al. 2001). Thus, the observed negative connectivity between the 2 task-positive regions and our reading seeds may reflect segregation of these functional organizations as a result of maturation of reading fluency/automatization. This assumption needs to be tested in future studies, particularly by comparing adults and children (i.e., less experienced readers).
Negative relationships, however, should be interpreted with some caution. Recent analyses have shown that negative RSFC is, at least in part, a consequence of preprocessing strategies such as global correction (Chang and Glover 2009; Fox et al. 2009; Murphy et al. 2009). On the other hand, intrinsic negative relationships are clearly present in direct neurophysiological recordings in animals (Walters et al. 2007; Hayden et al. 2009; Popa et al. 2009). Additionally, resting-state fMRI studies in humans have linked behavioral measures to the magnitude of intrinsic negative RSFC (Kelly et al. 2008; Di Martino et al. 2009), further supporting the potential functional significance of this measure.
Loci of Functional Interaction
The conjunction analysis demonstrated that RSFC for 5 of the 6 ROIs overlapped in the post-LMTG and the post-LIFG. These results suggest that these cortical areas represent important loci of functional interaction among intrinsic reading networks. Notably, the post-LMTG region was not included in the original set of reading seed regions nor was it reported in the aforementioned meta-analyses of normal word reading.
One may question why the meta-analyses failed to report the post-LMTG as a region reliably activated by word reading. This may be due to differences in the nature of the contrasts and tasks employed in the meta-analyses. One of the meta-analyses (Turkeltaub et al. 2002) strictly employed loose contrasts (e.g., reading aloud vs. rest or fixation), which tend to induce broad networks of reading-related activity that are often oversimplified in reporting (i.e., large clusters comprising several regions are represented by a single peak coordinate). Consequently, post-LMTG activations may be missed in meta-analyses performed on the basis of such peak coordinates. The other meta-analysis (Bolger et al. 2005) used both loose and tight contrasts (e.g., words vs. pseudowords). Both words and pseudowords involve visual–phonological recoding, subserved by the post-LMTG (Fiebach et al. 2002), which may consequently be activated by both reading conditions. Thus, tighter contrasts may underestimate this region's activation associated with word reading. However, when individuals with dyslexia have been studied, both abnormal (i.e., reduced) activations (Richlan et al. 2009) and normalized (i.e., increased) activations in response to intervention training (Simos, Fletcher, Sarkari, Billingsley, et al. 2007; Simos, Fletcher, Sarkari, Billingsley-Marshall, et al. 2007) have been detected in the post-LMTG. Recently, significant increases in gray-matter volumes were identified in several regions, including the post-LMTG, in a sample of late-literates who acquired reading skills in adulthood, relative to illiterate adults (Carreiras et al. 2009). These results further support the use of RSFC for investigating functional brain organization, particularly in clinical populations.
Considering that the post-LMTG is strongly activated both for reading real words (Fiebach et al. 2002) and for naming objects (Price et al. 2005), automatized phonological (and semantic) retrieval of visually presented items may be the cognitive process responsible for neural responses in the post-LMTG. Such automaticity for visually presented words is often impaired in dyslexic readers (Shaywitz and Shaywitz 2008). The post-LMTG region identified in our conjunction analysis is adjacent to the middle temporal area (MT), known to be the motion-sensitive cortex (Maunsell and Newsome 1987; Livingstone and Hubel 1988). Some, but not all, individuals with dyslexia are impaired in tasks that require sensitivity to visual motion (Stein and Walsh 1997; Talcott et al. 2000; Stein 2001) and show reduced activation in the MT during the perception of moving stimuli (Eden et al. 1996). Although the present study does not permit conclusive interpretation regarding the role of the post-LMTG in reading, it does suggest the potential merits of investigating RSFC patterns of the post-LMTG and their relationship with behavioral performance (e.g., reading and visual processing) in individuals with dyslexia.
Another locus of functional interaction, the post-IFG, is located immediately posterior to the pars opercularis of the IFG. This area has been a focus of literature investigating the functional organization of language (Bookheimer 2002; Davis et al. 2008; Keller et al. 2009) and reading (Paulesu et al. 1993; Cornelissen et al. 2009). Saur et al. (2008) combined diffusion tensor imaging with fMRI and reported that phonological processing (sound articulation) is subserved by structural connectivity between the posterior–frontal (including the post-LIFG) and superior–temporal regions. This is consistent with the classic Wernicke–Geschwind model of language (Geschwind 1965; Anderson et al. 1999) grounded in postmortem studies. From this finding, we can infer that the reported hyperactivation of the post-IFG in dyslexics (Shaywitz et al. 1998; Hoeft et al. 2007) reflects increased dependence on articulation mechanisms to compensate for impaired phonological processing. However, a recent subtraction meta-analysis of dyslexia arrived at the opposite result, that is, the post-IFG is hypoactivated in dyslexic readers (Richlan et al. 2009). Such an inconsistency may be ascribable to methodological differences such as the nature of tasks used or participant ages. Our results suggest the value of examining the functional connectivity of the IFG during rest, without task demands, in dyslexic participants.
Inferior Occipital RSFC
Notably, the IOG seed was not significantly correlated with either of the 2 identified loci of functional interaction. This suggests that the functional network associated with the IOG seed contributes to reading differently than the other 5 reading networks. Given that the lateral occipital cortex, including the IOG, is robustly activated during visual object recognition tasks (Grill-Spector et al. 2001; Kourtzi and Kanwisher 2000, 2001), the IOG network may be associated with visual perception of letters and words. This is consistent with patterns of deficits shown in alexic patients with damage to the IOG and adjacent posterior occipital areas; their deficits are not restricted to letter processing per se but are rather characterized by general impairments in visual perceptual processing including size identification (Behrmann et al. 1998; Sakurai et al. 2001). Moreover, the IOG activation pattern does not discriminate dyslexics from unimpaired readers, at least in alphabetic languages (Richlan et al. 2009). Although compelling, our examination of IOG connectivity only during rest leaves open the possibility that specific task demands (e.g., visual word recognition) could increase IOG coordination with reading-related areas. Effective connectivity approaches such as psychophysiological interaction (Friston et al. 1997, 2003; Hasson et al. 2009) are needed to determine whether task and visual demands impact IOG connectivity during reading.
Limitations of the Current Study
Our results suggest that reading-related networks are intrinsically represented in the human brain. Such representations likely reflect the result of learning and experience in adult readers. One way to test this hypothesis would be to map the patterns of RSFC for reading-related regions throughout development, as individuals systematically learn and gain reading experience (e.g., in children, adolescents, and adults). As reading is an acquired skill, RSFC patterns for reading in adults are expected to differ from those in children, who are less experienced with reading skills. Given robust developmental changes in RSFC patterns (Fair et al. 2007; Kelly, de Zubicaray, et al. 2009; Kelly, Di Martino, et al. 2009; Supekar et al. 2009), examining the developmental trajectory of RSFC associated with reading is a high priority in future studies.
Finally, another interesting avenue for research is the investigation of the extent to which RSFC strength predicts individual differences in reading-related performance (Hampson et al. 2006) . Unfortunately, formal measures of reading performance were not available in the present data set. Future work will address these questions.
In summary, our resting-state fMRI study demonstrated large-scale resting-state functional connectivity networks associated with word reading. The patterns of positive RSFC replicated patterns of task-based activity and functional connectivity, previously demonstrated during word reading, despite the absence of reading tasks or word stimuli. Our approach also revealed potentially important loci of functional interaction within reading networks. The significance of one of these loci, the post-LMTG, has been underestimated in task-based approaches aiming to localize neural correlates of reading. Importantly, this cortical area has been previously implicated in dyslexia (Richlan et al. 2009) and has been shown to exhibit intervention-induced changes in cortical activity in dyslexic children (Simos, Fletcher, Sarkari, Billingsley, et al. 2007; Simos, Fletcher, Sarkari, Billingsley-Marshall, et al. 2007). In conclusion, the current study suggests that resting-state fMRI can inform our understanding of the functional organization of the brain underlying reading and reading disorders.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
NIMH grant (R01MH081218); Stavros Niarchos Foundation grant (to Dr Castellanos); Leon Levy Foundation grant (to Dr Milham); and gifts from Joseph Healy, Linda and Richard Schaps, and Jill and Bob Smith (Dr Castellanos).
Supplementary Material
Acknowledgments
The authors thank Dr Xinian Zuo for assistance with analyses and Mr Jonathan Adelstein for editorial assistance. Conflict of Interest: None declared.
References
- Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, Beversdorf DQ, Cibula J, Rogish M, Kortencamp S, et al. Conduction aphasia and the arcuate fasciculus: a reexamination of the Wernicke–Geschwind model. Brain Lang. 1999;70:1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- Behrmann M, Nelson J, Sekuler EB. Visual complexity in letter-by-letter reading: “pure” alexia is not pure. Neuropsychologia. 1998;36:1115–1132. doi: 10.1016/s0028-3932(98)00005-0. [DOI] [PubMed] [Google Scholar]
- Biswal B, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Res Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bitan T, Burman DD, Lu D, Cone NE, Gitelman DR, Mesulam MM, Booth JR. Weaker top-down modulation from the left inferior frontal gyrus in children. NeuroImage. 2006;33:991–998. doi: 10.1016/j.neuroimage.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger DJ, Perfetti CA, Schneider W. Cross-cultural effect on the brain revisited: universal structures plus writing system variation. Hum Brain Mapp. 2005;25:92–104. doi: 10.1002/hbm.20124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L. Specialization of phonological and semantic processing in Chinese word reading. Brain Res. 2006;1071:197–207. doi: 10.1016/j.brainres.2005.11.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Christoff K, Cusack R, Lancaster J. Using the Talairach atlas with the MNI template. Neuroimage. 2001;13:S85. [Google Scholar]
- Carreiras M, Seghier ML, Baquero S, Estevez A, Lozano A, Devlin JT, Price CJ. An anatomical signature for literacy. Nature. 2009;461:983–986. doi: 10.1038/nature08461. [DOI] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Cohen JD. Interference and facilitation effects during selective attention: an H215O PET study of Stroop task performance. NeuroImage. 1995;2:264–272. doi: 10.1006/nimg.1995.1034. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis F, Valabregue R, Pelegrini-Issac M, Asloun S, Robbins TW, Granon S. Individual differences in prefrontal cortical activation on the Tower of London planning task: implication for effortful processing. Eur J Neurosci. 2003;17:2219–2225. doi: 10.1046/j.1460-9568.2003.02633.x. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. NeuroImage. 2009;47:1448–1459. doi: 10.1016/j.neuroimage.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, Schlaggar BL. A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cereb Cortex. 2008;18:2054–2065. doi: 10.1093/cercor/bhm228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Kringelbach ML, Ellis AW, Whitney C, Holliday IE, Hansen PC. Activation of the left inferior frontal gyrus in the first 200 ms of reading: evidence from magnetoencephalography (MEG) PLoS One. 2009;4:e5359. doi: 10.1371/journal.pone.0005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C, Kleinman JT, Newhart M, Gingis L, Pawlak M, Hillis AE. Speech and language functions that require a functioning Broca's area. Brain Lang. 2008;105:50–58. doi: 10.1016/j.bandl.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec' HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13:321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Sur un cas de cecite verbale avec agraphie, suivi d'autopsie. CR Soc Biol. 1891;43:197–201. [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;382:66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104:13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. J Cogn Neurosci. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci U S A. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122(Pt 2):199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hampson M, Tokoglu F, Sun Z, Schafer RJ, Skudlarski P, Gore JC, Constable RT. Connectivity-behavior analysis reveals that functional connectivity between left BA39 and Broca's area varies with reading ability. Neuroimage. 2006;31:513–519. doi: 10.1016/j.neuroimage.2005.12.040. [DOI] [PubMed] [Google Scholar]
- Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106:10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, et al. Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV. Plasticity in the developing brain: implications for rehabilitation. Dev Disabil Res Rev. 2009;15:94–101. doi: 10.1002/ddrr.64. [DOI] [PubMed] [Google Scholar]
- Keller SS, Crow T, Foundas A, Amunts K, Roberts N. Broca's area: nomenclature, anatomy, typology and asymmetry. Brain Lang. 2009;109:29–48. doi: 10.1016/j.bandl.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cereb Cortex. 2009;19:640–657. doi: 10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kelly AMC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, Castellanos FX, Milham MP, McMahon K. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS. Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex. 1998;8:372–384. doi: 10.1093/cercor/8.4.372. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Cortical regions involved in perceiving object shape. J Neurosci. 2000;20:3310–3318. doi: 10.1523/JNEUROSCI.20-09-03310.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Kanwisher N. Representation of perceived object shape by the human lateral occipital complex. Science. 2001;293:1506–1509. doi: 10.1126/science.1061133. [DOI] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lohmann G, Hoehl S, Brauer J, Danielmeier C, Bornkessel-Schlesewsky I, Bahlmann J, Turner R, Friederici A. Setting the frame: the human brain activates a basic low-frequency network for language processing. Cereb Cortex. 2009 doi: 10.1093/cercor/bhp190. doi:10.1093/cercor/bhq005, published ahead of print on 25 September 2009. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- MacDonald K. Effortful control, explicit processing, and the regulation of human evolved predispositions. Psychol Rev. 2008;115:1012–1031. doi: 10.1037/a0013327. [DOI] [PubMed] [Google Scholar]
- MacLeod CM. Half a century of research on the Stroop effect: an integrative review. Psychol Bull. 1991;109:163–203. doi: 10.1037/0033-2909.109.2.163. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-Based topographic parcellation of human cerebral white matter and nuclei II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. NeuroImage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Marklund P, Fransson P, Cabeza R, Larsson A, Ingvar M, Nyberg L. Unity and diversity of tonic and phasic executive control components in episodic and working memory. NeuroImage. 2007;36:1361–1373. doi: 10.1016/j.neuroimage.2007.03.058. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Newsome WT. Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Long S, Friston KJ, Lambon Ralph MA, Patterson K, McClelland JL, Price CJ. Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci. 2005;17:1753–1765. doi: 10.1162/089892905774589190. [DOI] [PubMed] [Google Scholar]
- Milham MP, Banich MT, Webb A, Barad V, Cohen NJ, Wszalek T, Kramer AF. The relative involvement of anterior cingulate and prefrontal cortex in attentional control depends on nature of conflict. Cogn Brain Res. 2001;12:467–473. doi: 10.1016/s0926-6410(01)00076-3. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Dehaene S, Jobert A, Le Bihan D, Kouider S. Task-specific change of unconscious neural priming in the cerebral language network. Proc Natl Acad Sci USA. 2007;104:19643–19648. doi: 10.1073/pnas.0704487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Owen WJ, Borowsky R, Sarty GE. FMRI of two measures of phonological processing in visual word recognition: ecological validity matters. Brain Lang. 2004;90:40–46. doi: 10.1016/S0093-934X(03)00418-8. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993;362:342–345. doi: 10.1038/362342a0. [DOI] [PubMed] [Google Scholar]
- Pinsk MA, Kastner S. Neuroscience: unconscious networking. Nature. 2007;447:46–47. doi: 10.1038/447046a. [DOI] [PubMed] [Google Scholar]
- Popa D, Popescu AT, Pare D. Contrasting activity profile of two distributed cortical networks as a function of attentional demands. J Neurosci. 2009;29:1191–1201. doi: 10.1523/JNEUROSCI.4867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CA, Barker GJ, Noppeney U, Koepp MJ, Duncan JS. Hemispheric asymmetries in language-related pathways: a combined functional MRI and tractography study. NeuroImage. 2006;32:388–399. doi: 10.1016/j.neuroimage.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: effect of baseline. Hum Brain Mapp. 2005;25:70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Constable RT, Skudlarski P, Marchione KE, Jenner AR, et al. The angular gyrus in developmental dyslexia: task-specific differences in functional connectivity within posterior cortex. Psychol Sci. 2000;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: a quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;10:3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaghiani S, Hesselmann G, Kleinschmidt A. Distributed and antagonistic contributions of ongoing activity fluctuations to auditory stimulus detection. J Neurosci. 2009;29:13410–13417. doi: 10.1523/JNEUROSCI.2592-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai Y, Ichikawa Y, Mannen T. Pure alexia from a posterior occipital lesion. Neurology. 2001;56:778–781. doi: 10.1212/wnl.56.6.778. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Schwarzbauer C, Bullmore E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci. 2005;360:937–946. doi: 10.1098/rstb.2005.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kummerer D, Kellmeyer P, Vry MS, Umarova R, Musso M, Glauche V, Abel S, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci U S A. 2008;105:18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Wise RJS. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Paying attention to reading: the neurobiology of reading and dyslexia. Dev Psychopathol. 2008;20:1329–1349. doi: 10.1017/S0954579408000631. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, Shankweiler DP, Liberman AM, Skudlarski P, Fletcher JM, et al. Functional disruption in the organization of the brain for reading in dyslexia. Proc Natl Acad Sci U S A. 1998;95:2636–2641. doi: 10.1073/pnas.95.5.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley RL, Denton C, Papanicolaou AC. Altering the brain circuits for reading through intervention: a magnetic source imaging study. Neuropsychology. 2007;21:485–496. doi: 10.1037/0894-4105.21.4.485. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Sarkari S, Billingsley-Marshall R, Denton CA, Papanicolaou AC. Intensive instruction affects brain magnetic activity associated with oral word reading in children with persistent reading disabilities. J Learn Disabil. 2007;40:37–48. doi: 10.1177/00222194070400010301. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Spironelli C, Angrilli A. Language lateralization in phonological, semantic and orthographic tasks: a slow evoked potential study. Behav Brain Res. 2006;175:296–304. doi: 10.1016/j.bbr.2006.08.038. [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122(Pt 11):2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, Gee DG, Roy AK, Banich MT, Castellanos FX, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott JB, Witton C, McLean MF, Hansen PC, Rees A, Green GG, Stein JF. Dynamic sensory sensitivity and children's word decoding skills. Proc Natl Acad Sci U S A. 2000;97:2952–2957. doi: 10.1073/pnas.040546597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nat Neurosci. 2003;6:767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Margulies DS, Shehzad Z, Shaw D, Ghaffari M, Rotrosen J, Adler LA, Castellanos FX, et al. Network homogeneity reveals decreased integrity of default-mode network in ADHD. J Neurosci Methods. 2008;169:249–254. doi: 10.1016/j.jneumeth.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Walters JR, Hu D, Itoga CA, Parr-Brownlie LC, Bergstrom DA. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–776. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Bunge SA, Carter CS. Maintaining structured information: an investigation into functions of parietal and lateral prefrontal cortices. Neuropsychologia. 2008;46:665–678. doi: 10.1016/j.neuropsychologia.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Greene J, Buchel C, Scott SK. Brain regions involved in articulation. Lancet. 1999;353:1057–1061. doi: 10.1016/s0140-6736(98)07491-1. [DOI] [PubMed] [Google Scholar]
- Xiong J, Ma L, Wang B, Narayana S, Duff EP, Egan GF, Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2009;45:75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FG, Edens J, Simpson C, Krawczyk DC. Differences in task demands influence the hemispheric lateralization and neural correlates of metaphor. Brain Lang. 2009;111:114–124. doi: 10.1016/j.bandl.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.