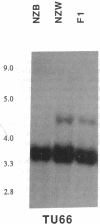
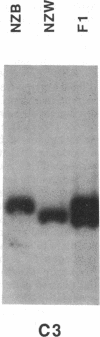
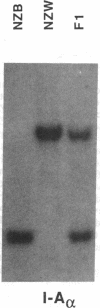
Abstract
Unlike parental New Zealand Black (NZB) or New Zealand White (NZW) mice, (NZB x NZW)F1 mice exhibit a lupus-like disease characterized by high serum levels of IgG anti-nuclear antibodies and a fatal immune complex glomerulonephritis. Previous results from studying [(NZB x NZW)F1 x NZB] backcross mice indicated that the NZW major histocompatibility complex (MHC) or gene(s) closely linked to this locus provides the major dominant NZW genetic contribution to the F1 disease. A surprising feature of the results was the 12% frequency of discordance between the autoimmune phenotype and the presence of the NZW H-2z haplotype. In the current study, we attempted to precisely define the position of the NZW gene(s) required for lupus-like renal disease by mapping genes in individual backcross mice that are both centromeric and telomeric to the MHC and then correlating genotypes for each locus with disease. The data indicate that an adjacent NZW locus does not provide a more accurate correlation with the autoimmune phenotype compared with MHC genes themselves. Thus, the imperfect association of MHC haplotype with disease in this murine model is not explained by genetic recombination with linked genes. These data may provide insight into the mechanisms by which MHC antigens increase the probability of developing autoimmune disease and may help explain the difficulty of defining MHC relationships in human systemic lupus erythematosus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman N. E., Watling D. L., McDevitt H. O. Treatment of (NZB x NZW)F1 disease with anti-I-A monoclonal antibodies. J Exp Med. 1983 Oct 1;158(4):1350–1355. doi: 10.1084/jem.158.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva F. P., Hoecker G. F., Day N. K., Vienne K., Rubinstein P. Murine complement component 3: genetic variation and linkage to H-2. Proc Natl Acad Sci U S A. 1978 Feb;75(2):963–965. doi: 10.1073/pnas.75.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S. K., Owen F. L., Womack J. E., Riblet R. J. Analysis of recombinant inbred lines derived from "autoimmune" (NZB) and "high leukemia" (C58) strains: independent multigenic systems control B cell hyperactivity, retrovirus expression, and autoimmunity. J Immunol. 1982 Oct;129(4):1539–1544. [PubMed] [Google Scholar]
- Fox H. S., Silver L. M., Martin G. R. An alpha globin pseudogene is located within the mouse t complex. Immunogenetics. 1984;19(2):125–130. doi: 10.1007/BF00387855. [DOI] [PubMed] [Google Scholar]
- Fronek Z., Timmerman L. A., Alper C. A., Hahn B. H., Kalunian K., Peterlin B. M., McDevitt H. O. Major histocompatibility complex associations with systemic lupus erythematosus. Am J Med. 1988 Dec 23;85(6A):42–44. doi: 10.1016/0002-9343(88)90382-8. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Ashwell J. D., Lechler R. I., Margulies D. H., Nickerson K. M., Suzuki G., Tou J. Y. "Exon-shuffling" maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide A beta. Proc Natl Acad Sci U S A. 1985 May;82(9):2940–2944. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Ueda G., Noguchi K., Okada T., Sekigawa I., Sato H., Shirai T. Requirement of H-2 heterozygosity for autoimmunity in (NZB X NZW)F1 hybrid mice. Eur J Immunol. 1986 Dec;16(12):1631–1633. doi: 10.1002/eji.1830161226. [DOI] [PubMed] [Google Scholar]
- Howie J. B., Helyer B. J. The immunology and pathology of NZB mice. Adv Immunol. 1968;9:215–266. doi: 10.1016/s0065-2776(08)60444-7. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Barr V. L., Palmer E. A large deletion within the T-cell receptor beta-chain gene complex in New Zealand white mice. Science. 1985 Jul 12;229(4709):167–171. doi: 10.1126/science.2990044. [DOI] [PubMed] [Google Scholar]
- Kotzin B. L., Palmer E. The contribution of NZW genes to lupus-like disease in (NZB x NZW)F1 mice. J Exp Med. 1987 May 1;165(5):1237–1251. doi: 10.1084/jem.165.5.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotzin B. L., Strober S. Reversal of nzb/nzw disease with total lymphoid irradiation. J Exp Med. 1979 Aug 1;150(2):371–378. doi: 10.1084/jem.150.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder A., Swan D., Ruddle F., D'Eustachio P., Leder P. Dispersion of alpha-like globin genes of the mouse to three different chromosomes. Nature. 1981 Sep 17;293(5829):196–200. doi: 10.1038/293196a0. [DOI] [PubMed] [Google Scholar]
- Maruyama N., Furukawa F., Nakai Y., Sasaki Y., Ohta K., Ozaki S., Hirose S., Shirai T. Genetic studies of autoimmunity in New Zealand mice. IV. Contribution of NZB and NZW genes to the spontaneous occurrence of retroviral gp70 immune complexes in (NZB X NZW)F1 hybrid and the correlation to renal disease. J Immunol. 1983 Feb;130(2):740–746. [PubMed] [Google Scholar]
- Miller M. L., Raveche E. S., Laskin C. A., Klinman D. M., Steinberg A. D. Genetic studies in NZB mice. VI. Association of autoimmune traits in recombinant inbred lines. J Immunol. 1984 Sep;133(3):1325–1331. [PubMed] [Google Scholar]
- Raveche E. S., Novotny E. A., Hansen C. T., Tjio J. H., Steinberg A. D. Genetic studies in NZB mice. V. Recombinant inbred lines demonstrate that separate genes control autoimmune phenotype. J Exp Med. 1981 May 1;153(5):1187–1197. doi: 10.1084/jem.153.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhme D., Fox H., Herrmann B., Frischauf A. M., Edström J. E., Mains P., Silver L. M., Lehrach H. Molecular clones of the mouse t complex derived from microdissected metaphase chromosomes. Cell. 1984 Mar;36(3):783–788. doi: 10.1016/0092-8674(84)90358-1. [DOI] [PubMed] [Google Scholar]
- Silver L. M. Mouse t haplotypes. Annu Rev Genet. 1985;19:179–208. doi: 10.1146/annurev.ge.19.120185.001143. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebauer K., Domdey H., Diggelmann H., Fey G. Isolation and analysis of genomic DNA clones encoding the third component of mouse complement. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7077–7081. doi: 10.1073/pnas.79.23.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kohno A., Ohta K., Hirose S., Maruyama N., Shirai T. Genetic studies of autoimmunity in New Zealand mice. III. Associations among anti-DNA antibodies, NTA, and renal disease in (NZB x NZW)F1 x NZW backcross mice. J Immunol. 1981 Aug;127(2):433–437. [PubMed] [Google Scholar]