Abstract
An alcoholic extract of Artemisia dracunculus L (PMI 5011) has been shown to decrease glucose and improve insulin levels in animal models, suggesting an ability to enhance insulin sensitivity. We sought to assess the cellular mechanism by which this botanical affects carbohydrate metabolism in primary human skeletal muscle culture. We measured basal and insulin-stimulated glucose uptake, glycogen accumulation, phosphoinositide 3 (PI-3) kinase activity, and Akt phosphorylation in primary skeletal muscle culture from subjects with type 2 diabetes mellitus incubated with or without various concentrations of PMI 5011. We also analyzed the abundance of insulin receptor signaling proteins, for example, IRS-1, IRS-2, and PI-3 kinase. Glucose uptake was significantly increased in the presence of increasing concentrations of PMI 5011. In addition, glycogen accumulation, observed to be decreased with increasing free fatty acid levels, was partially restored with PMI 5011. PMI 5011 treatment did not appear to significantly affect protein abundance for IRS-1, IRS-2, PI-3 kinase, Akt, insulin receptor, or Glut-4. However, PMI 5011 significantly decreased levels of a specific protein tyrosine phosphatase, that is, PTP1B. Time course studies confirmed that protein abundance of PTP1B decreases in the presence of PMI 5011. The cellular mechanism of action to explain the effects by which an alcoholic extract of A dracunculus L improves carbohydrate metabolism on a clinical level may be secondary to enhancing insulin receptor signaling and modulating levels of a specific protein tyrosine phosphatase, that is, PTP1B.
1. Introduction
Insulin resistance is a key pathophysiologic feature of type 2 diabetes mellitus, obesity, and the “metabolic syndrome” and is strongly associated with coexisting cardiovascular risk factors and accelerated atherosclerosis [1–3]. As such, one of the most desirable goals of treatment for subjects with insulin resistance and type 2 diabetes mellitus is directed at increasing insulin sensitivity in vivo. Although it is well established that caloric restriction and exercise greatly improve insulin resistance, the success of lifestyle intervention in humans over a sustained period is poor [4]. Therefore, strategies to improve insulin resistance by pharmacologic approaches have remained as a major focus for drug development and is a primary and extremely viable clinical option in clinical medicine. Unfortunately, recent data have questioned the safety of the current pharmacologic approaches to improve insulin resistance [5]. In this regard, alternative strategies, for example, nutritional supplementation with over-the-counter botanical agents, are extensively practiced by a large number of patients with diabetes and are frequently undertaken without informing the medical provider. According to the Food and Drug Administration, there are more than 29,000 different nutritional supplements available to consumers; and Americans spend more than 12 billion dollars per year on these supplements [6,7].
As a source for alternative therapy, plants have traditionally been a rich source of medicinal compounds for many indications, including diabetes. In fact, metformin is one of the most prescribed glucose-lowering medicines currently used and is derived from a chemical isolated from a plant [8]. However, there is considerable controversy regarding the use of botanical supplements for human health in general and on carbohydrate metabolism in particular. In part, the controversy stems from the paucity of data in humans regarding the clinical efficacy and safety of most botanical preparations. In addition, the cellular mechanism of action of botanicals that are marketed for improving human metabolism would be required before firm recommendations can be made by clinicians regarding routine use as an adjunctive therapy. There are a number of reports, however, about the use of plants from the genus Artemisia as a traditional treatment for diabetes [9]. Artemisia dracunculus L or Russian tarragon is a perennial herb with a long history of medicinal and culinary use. Recently, the ethanolic extract of A dracunculus L was shown to significantly decrease blood glucose levels in both genetic and chemically induced murine models of diabetes and demonstrated an effect to improve insulin action [10]. The cellular mechanism by which A dracunculus L modulates insulin action, however, is not precisely known. The objective of our current studies is to determine the precise cellular mechanism by which an extract of A dracunculus L improves insulin action by assessing functional assays of glucose metabolism, that is, glucose uptake and glycogen accumulation, and insulin signaling through the insulin receptor in primary human skeletal muscle culture (HSMC).
2. Research design and methods
2.1. Preparation of extract
The A dracunculus L extract (PMI 5011) was originally identified at the Biotech Center of Rutgers from a screening of extracts for hypoglycemic activity in diabetic mice as the most promising candidate for the development of a nutritional supplement for diabetes [10]. Preparation and analysis for PMI 5011 have been previously described in detail [10]. The extract was produced only from plants grown hydroponically in greenhouses maintained under uniform and strictly controlled conditions, thereby standardizing the plants for their phytochemical content. The preparation was produced from the fresh herb.
2.2. Extraction
Four kilograms of the shoot material was heated to 80°C with 80% ethanol for 2 hours. The extraction was continued for an additional 10 hours at 20°C. The extract was then filtered through cheese cloth and evaporated with a rotary evaporator, and the final volume was reduced to 1 L. The aqueous extract was freeze-dried for 48 hours, and the dried extract was homogenized with a mortar and pestle.
2.3. Primary human skeletal muscle culture
Human skeletal muscle tissues were obtained by needle muscle biopsy on the lateral portion of the quadriceps femoris (vastus lateralis) from 5 obese male subjects with type 2 diabetes mellitus (age, 54 ± 2 years; body mass index, 32.8 ± 0.9; A1c, 7.2% ± 0.5%) at the baseline of a hyperinsulinemic, euglycemic clamp using a 5-mm–diameter side-cutting needle as described by Henry et al [11]. At the time of biopsy, muscle tissue (about 100 mg) was placed in 10 mL Ham F-10 media at 4°C and carefully dissected, minced, and washed with Ham F-10 media. Muscle tissues were dissociated by 3 successive treatments of 20 minutes each in 25 mL 0.05% trypsin/EDTA at room temperature. Dissociated cells were centrifuged at 600g for 4 minutes at 37°C and placed in human skeletal growth media (SkGM Bullet Kit; Cambrex, East Rutherford, NJ) containing 10 µg/L human epidermal growth factor, 500 mg/L bovine serum albumin, 500 mg fetuin, and 195 µg/L dexamethasone, with 10% fetal bovine serum and no insulin. Cells were placed on 100-mm dishes and placed in an incubator at 37°C containing 95% air and 5% CO2. Medium was changed every 2 to 3 days. For an individual experiment, myoblasts were subcultured onto 6-well culture plates or in 100-mm dishes and grown to 80% to 90% confluence. The cells were then differentiated into fused myotubes for 7 days by switching to culture media with 2% horse serum. After starvation, cells were treated with concentrations of the extract ranging from 0.1 to 100 mg/L. After incubation, cultures were evaluated with glucose uptake and insulin signaling studies as outlined below. All the primary cultured cells used in this study were within 5 passages.
2.4. 2-Deoxy-glucose uptake
Primary human skeletal muscle culture (HSMC) was treated with various concentrations of extract overnight as described above, then insulin (100 nmol/L) was added during the last 15 minutes of incubation. Cells were washed twice, and glucose uptake was assayed in HEPES [2-(4-[2-hydroxyethyl]-1-piperazinyl)ethanesulfonic acid]-buffered saline solution (140 mmol/L NaCl, 20 mmol/L HEPES-Na, 2.5 mmol/L MgSO4, 1 mmol/L CaCl2, 5 mmol/L KCl [pH 7.4]) containing 100 µmol/L 2-deoxy-d-glucose (5 µCi/mL 2-deoxy-d-[3H]glucose). The measurements were carried out in triplicate. Nonspecific uptake was determined in the presence of 20 µmol/L cytochalasin B and was subtracted fromall values and normalized by protein concentration. Cells were dissolved in 0.2 N NaOH for scintillation counting.
2.5. Glycogen assay
Glycogen hydrolysis and glucose determination were performed according to Gomez-Lechon et al [12] with some modifications. Briefly, HSMC was grown in 24-well plates. At day 6 postdifferentiation, cells were starved from fetal bovine serum overnight (16 hours); and Krebs-Ringer-HEPES buffer was added containing 0.2% bovine serum albumin with or without 100 nmol/L insulin plus 30 mmol/L glucose for 2 hours. To assess whether PMI 5011 can enhance glycogen accumulation in the presence of free fatty acids, palmitic acid at concentrations of 150, 300, and 450 µmol/L was added to the media with or without 5 µg/mL of PMI 5011. After extensive washing with phosphate-buffered saline, 200 µL of 0.2 mol/L sodium acetate buffer (pH 4.8) was added to each well; and cells were sonicated. Forty microliters of lysate was taken for protein assay. Amyloglucosidase was added to each well at a final concentration of 500 mU/mL. Plates were incubated at 40°C for 2 hours with shaking to prevent the sediment of glycogen-protein aggregates. The products of enzyme digestion were collected in 1.5-mL microcentrifuge tubes and centrifuged at 3000 rpm for 10 minutes. For the glucose assay, 50 µL per well aliquots of the supernatants were transferred to a 96-well plate; and 150 µL of assay solution containing 20 U/L peroxidase, 10 U/L glucose oxidase, and 1 g/L 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) in 100 mmol/L phosphate buffer (pH 6) was added. The samples were incubated at room temperature in the dark for 30 minutes. The intensity of the color reaction was measured at 405 nm by a microplate reader. A blank of the reaction was performed by incubation of lysate without adding amyloglucosidase; this value represents the free glucose content and was subtracted from the total glucose obtained after enzymatic hydrolysis. A standard curve was constructed with known amounts of rabbit liver glycogen. Results were normalized by protein concentration measured by Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA), and glycogen content was presented as nanomoles glucose equivalent per well.
2.6. Insulin signaling studies
Protein content for phosphoinositide 3 (PI-3) kinase, IRS-1, IRS-2, insulin receptor, Akt, AS160, and Glut-4 was determined by Western blotting. For analysis, muscle culture, after overnight incubation, was first homogenized in 0.25 mL extraction buffer (1% Triton X-100, 100 mmol/L Tris [pH 7.4], 100 mmol/L sodium pyrophosphate, 100 mmol/L sodium fluoride, 10 mmol/L EDTA, 10 mmol/L sodium vanadate, 2 mmol/L phenylmethylsulfonyl fluoride, and 0.1 mg/mL aprotinin) at 4°C. The extracts were centrifuged at 100000g at 4°C for 15 minutes to remove insoluble material, and the supernatant was used for the assay. Extracts, corresponding to 50 µg of protein, were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis minigels using Western blotting techniques as described [13,14]. Specific antibodies used were anti–IRS-1 antibody, anti-Akt, anti–PI-3 kinase antibody, and anti-PTP1B (Upstate Biotechnology, Lake Placid, NY). In addition, we used anti–IRS-2, anti–insulin receptor β-subunit antibody, and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA).
2.7. Insulin-stimulated tyrosine phosphorylation and PI-3 kinase activity
To assess phosphorylation for the substrate of interest, muscle culture was assessed with or without insulin stimulation (100 nmol/L for 5 minutes). Homogenates made from muscle culture as outlined above were precipitated with an antiphosphotyrosine antibody (PY-20) (Transduction Laboratories, Lexington, KY). Immunoprecipitate collected with protein A–agarose was washed 5 times in ice-cold 50 mmol/L HEPES buffer containing 150 mmol/L NaCl, 0.1% Triton X-100, and 10 µmol/L Na3V04 (NHT buffer). The pellets were treated with Laemmli sample buffer containing 100 mmol/L dithiothreitol and heated in a boiling water bath for 5 minutes. The proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to nitrocellulose, and immunoblotted. For determination of PI-3 kinase activity, the immune complex was washed; and PI-3 kinase was measured as described [15,16].
2.8. Gene expression
Gene expression for glycogen synthase (GS) was assessed by reverse transcriptase polymerase chain reaction (PCR). Briefly, oligonucleotide primer pairs were synthesized (Integrated DNA Technologies, Coralville, IA); and total RNA was extracted from HSMC with RNazol (Cinna/MRC, Cincinnati, OH) and reverse-transcribed as described Macaulay et al [17]. For amplification of human GS-specific complementary DNAs (accession no. BC003182.1), a sense primer (5′-GTGCTGACGTCTTTCTGGAG-3′) and an antisense primer (5′-CCAGCATCTTGTTCATGTCG-3′), and for human β-actin (accession no. NM_001101), a sense primer (5′-GGACTTCGAGCAAGAGATGG-3′) and antisense primer (5′-AGCACTGTGTTGGCGTACAG-3′) were used in a reaction involving denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and elongation at 72°C for 1 minute. The number of cycles varied from 25 to 40. The PCR products were electrophoresed on a 1.5% agarose gel containing ethidium bromide.
2.9. Statistical analysis
Data are presented as mean ± SEM. Statistical differences are determined by analysis of variance or by paired Student t test, as appropriate.
3. Results
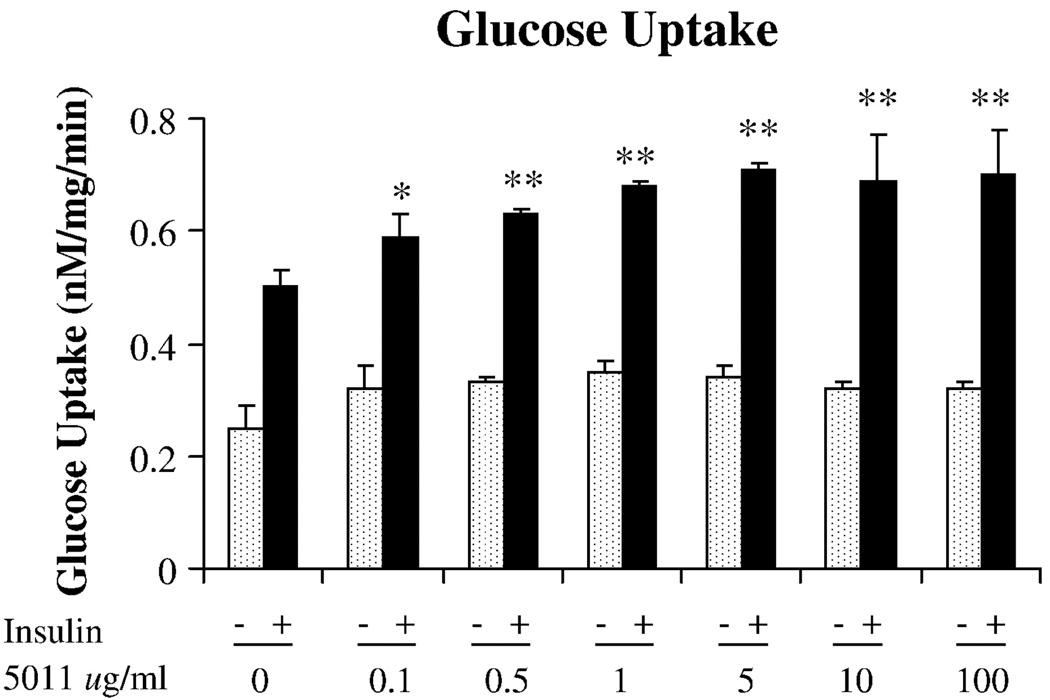
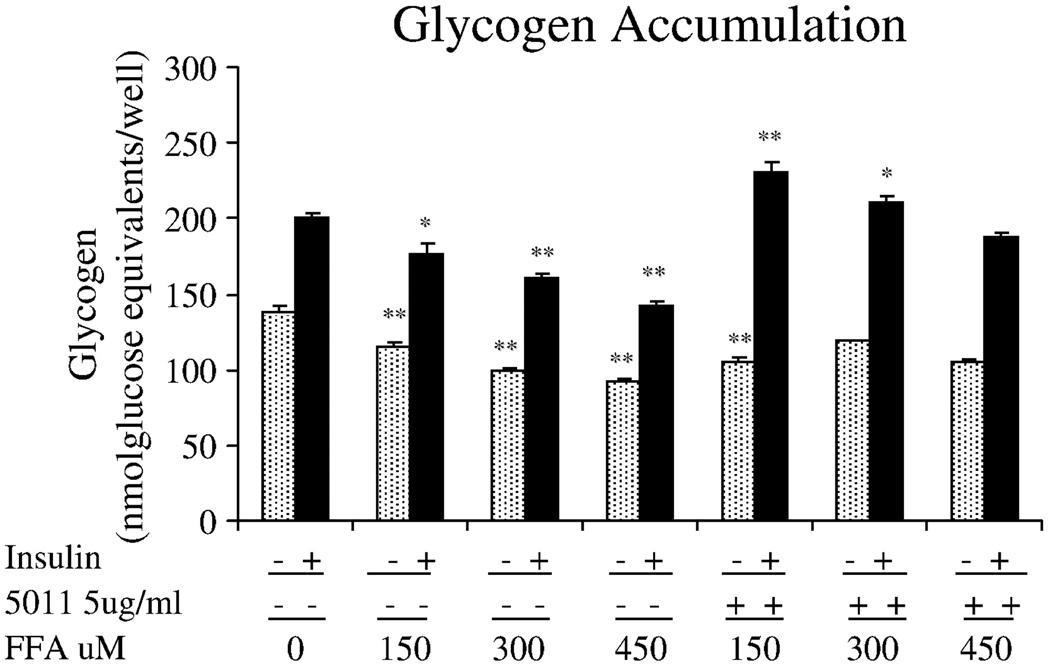
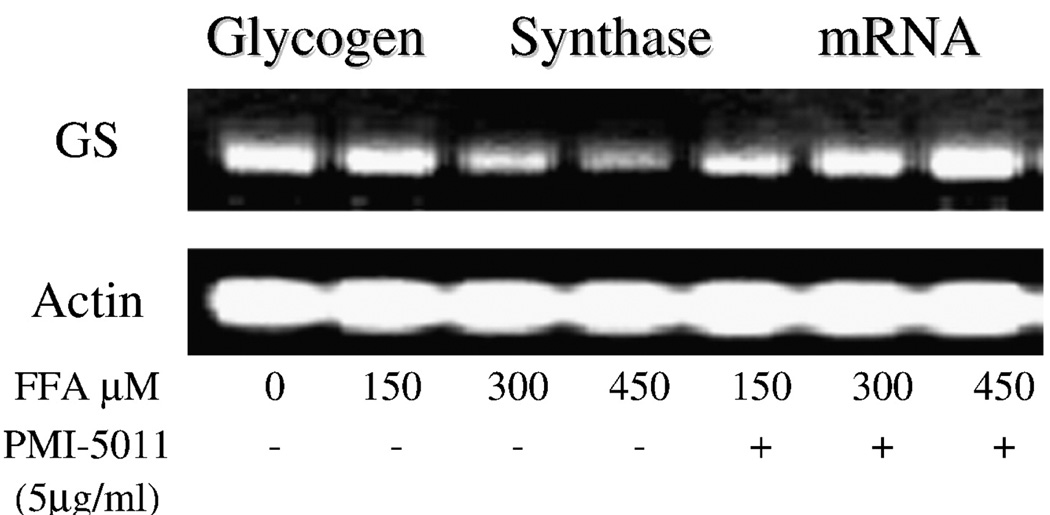
The alcoholic extract of A dracunculus L was shown to significantly increase glucose uptake in primary HSMC. As seen in Fig. 1, using concentrations of 0.1 to 100 µg/mL of PMI 5011, glucose uptake was shown to increase at the 0.1-µg/mL concentration and appeared to have maximal effect at the 5-µg/mL dose. Based on this response, additional experiments that evaluated glycogen and insulin signaling studies were assessed at the 5 µg/mL dose. Insulin-stimulated glycogen accumulation was assessed in the presence and absence of free fatty acids, that is, palmitic acid, as a means to induce attenuation in response. As shown in Fig. 2, increasing concentrations of free fatty acids in the media from 150 to 450 µmol/L was associated with reductions in insulin-stimulated glycogen accumulation. However, when 5 µg/mL of PMI 5011 was added to the media containing the free fatty acids, insulin-stimulated glycogen accumulation was partially restored, particularly at fatty acid concentrations of 150 and 300 µmol/L. To determine the possible mechanism for the effects on glycogen, reverse transcriptase PCR was assessed for the GS enzyme in human myotubes. As shown in Fig. 3, in the presence of free fatty acids, GS messenger RNA was decreased, but was partially restored in the presence of PMI 5011.
Fig. 1.
Effect of PMI 5011 on glucose uptake in primary HSMC. 2-Deoxy-d-glucose uptake was assessed in HSMC with or without various doses of PMI 5011. Data presented as mean ± SEM; *P < .05, **P < .01 vs control.
Fig. 2.
Effect of PMI 5011 treatment on glycogen accumulation in primary skeletal muscle culture. Glycogen accumulation was assessed in the presence of various concentrations of palmitic acid with or without PMI 5011. Data are mean ± SEM; *P < .05, **P < .01 vs control.
Fig. 3.
Determination of GS gene expression. Total RNA was isolated from human myotubes in the presence of free fatty acids and/or PMI 5011 and reverse-transcribed. As shown, GS gene expression was attenuated with free fatty acids, but was partially restored when PMI 5011 was added along with the free fatty acids.
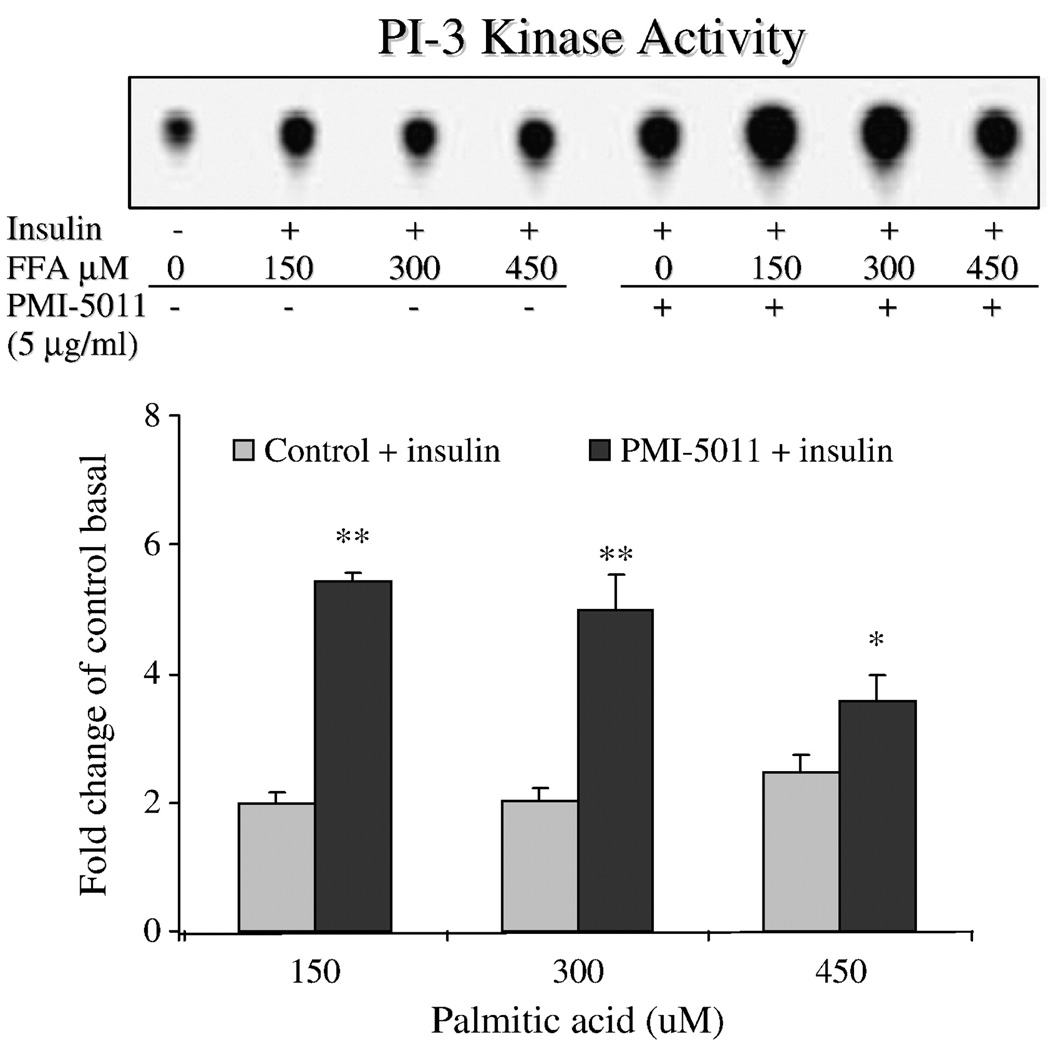
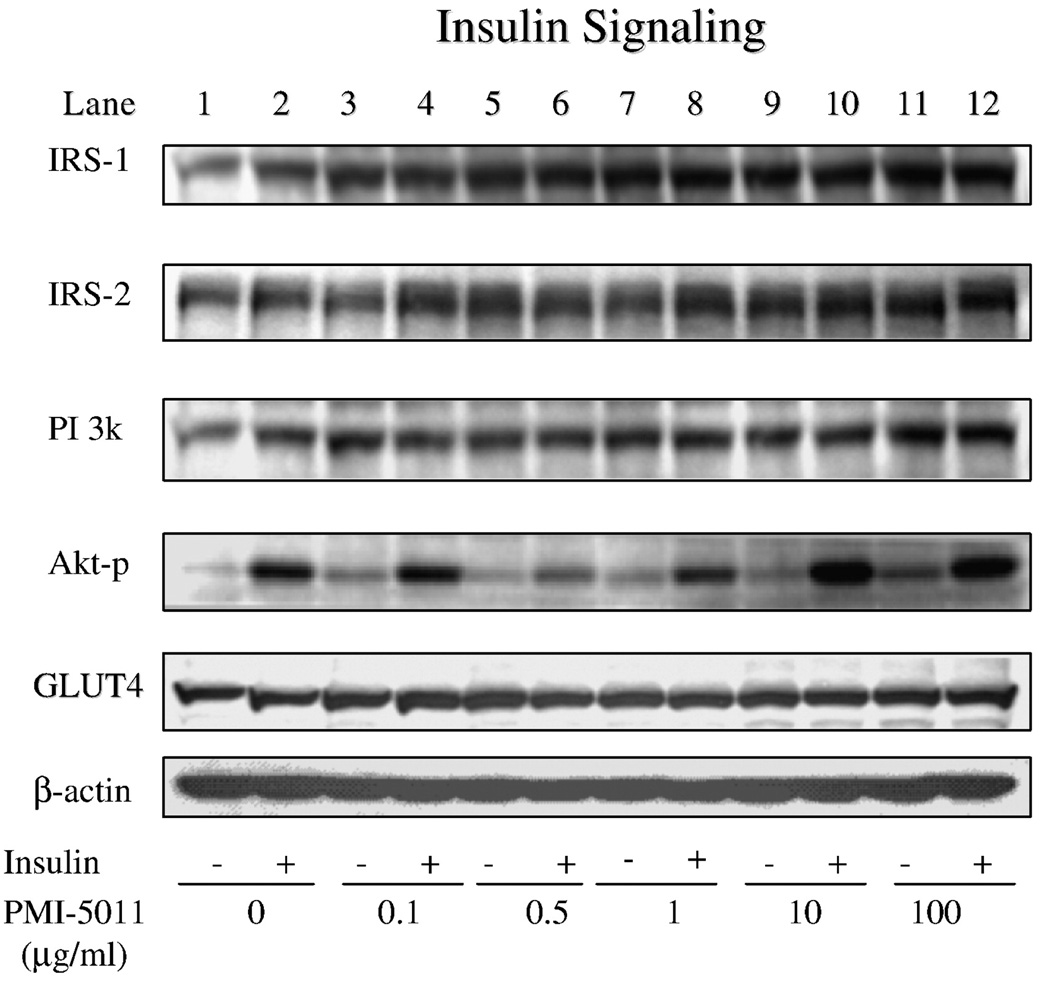
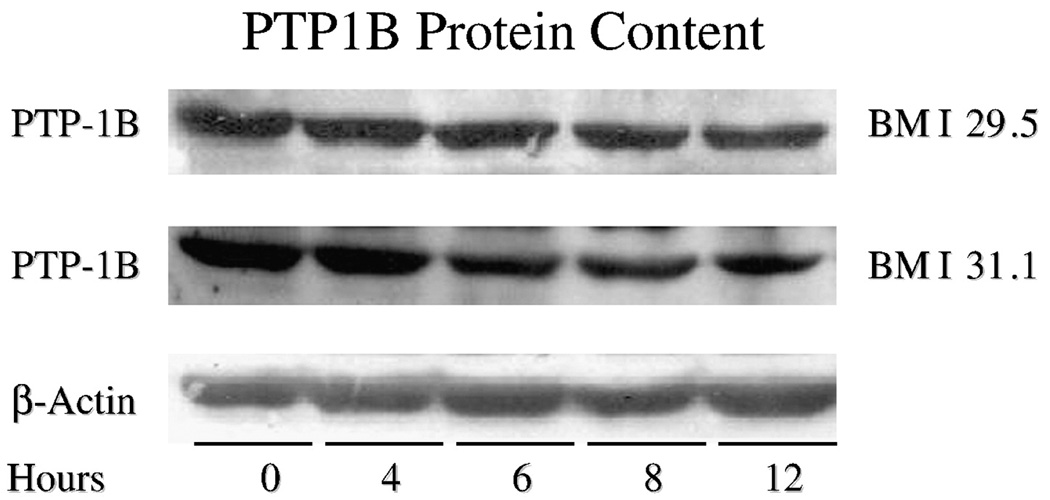
Based on the effect of 5011 to enhance functional assays of glucose uptake and glycogen storage, insulin-stimulated PI-3 kinase activity was assessed. As shown in Fig. 4, insulin-stimulated PI-3 kinase activity, in the presence of 100 to 450 µmol/L of free fatty acids in the media, was blunted in skeletal muscle culture when compared with activities assessed at all concentrations of free fatty acids plus PMI 5011 at 5 µg/mL in the media. To evaluate the mechanism for the enhanced kinase activity, protein abundance of the insulin signaling proteins and insulin-stimulated phosphorylation of Akt were assessed at various concentrations of PMI 5011. There were no significant changes in protein abundance for insulin receptor (data not shown), PI-3 kinase, or Glut-4, whereas there was a trend for increases in protein abundance for IRS-1 and IRS-2 (Fig. 5). In addition, data suggested a reduction in PTP1B abundance (see additional studies, Fig. 6). However, despite no change in Akt levels (data not shown), significant increases for Akt phosphorylation were observed, particularly when assessed at doses of 10 and 100 µg/mL. To further evaluate this finding, additional experiments with cell cultures were assessed to evaluate the effect of PMI 5011 on PTP1B levels. As shown in Fig. 6, incubation of HSMC significantly decreased PTP1B content in primary cell culture in a time-dependent fashion.
Fig. 4.
IRS-1–associated PI-3 kinase activity in primary HSMC exposed overnight at day 7 of post differentiation to various concentrations of free fatty acids ranging from 150 to 450 µmol/L and with or without 5 µ/mL of PMI 5011. As shown, enhanced PI-3 kinase activity was observed in the presence of PMI 5011 in the incubation media. *P < .05, **P < .001 vs control (free fatty acids alone).
Fig. 5.
Insulin signaling studies demonstrating skeletal muscle protein content and phosphorylation as assessed by Western blots for intracellular proteins for HSMC exposed to various concentrations of PMI 5011. As shown, Akt phosphorylation was significantly enhanced at concentrations of 10 and 100 µg/mL.
Fig. 6.
Time course studies demonstrating reduction in PTP1B content in primary HSMC obtained from 2 obese subjects with type 2 diabetes mellitus (body mass index, 29.5 and 31.1) when exposed to 5 µg/mL of PMI 5011 over 12 hours. A reduction in PTP1B protein content of more than 50% was observed.
4. Discussion
Although one of the concerns with botanical supplements in the past has been the paucity of scientifically controlled clinical and animal studies that evaluated the effectiveness of any individual bioactive, additional concerns have been the demonstration of precise cellular mechanisms of action. In this regard, an ethanolic extract of A dracunculus L was recently shown to reduce blood glucose concentrations in streptozotocin-induced diabetic mice as well as in genetically diabetic KK-Aγ mice by 20% and 24%, respectively, after 7 days of treatment [10]. In addition, blood insulin concentrations were also significantly reduced in the genetically diabetic KK-Aγ mice with treatment with PMI 5011, suggesting a clinical effect to enhance insulin sensitivity. Confirmation of these findings in humans is the focus of ongoing research. However, with the series of experiments outlined in this article, we provide a mechanism by which the ethanolic extract of A dracunculus L enhances insulin sensitivity by demonstrating increases in insulin-stimulated phosphorylation of insulin receptor signaling proteins and enhanced downstream kinase activities. Thus, the clinical effect of PMI 5011 was supported by mechanistic studies in primary HSMC.
One of the unique aspects of our studies revolved around the use of primary HSMC. Clearly, it would be advantageous to test the extract in large-scale clinical studies and to obtain biopsies to assess specific mechanisms of interest. If the human studies are eventually shown to be positive, that approach can be suggested. However, it is well established that primary HSMC retains the intrinsic properties of skeletal muscle cells; and this serves as a major advantage. As such, an individual who is deemed insulin resistant on clinical grounds will yield muscle culture that will have attenuated insulin signaling and altered functional markers assessing carbohydrate metabolism. In support of this concept are studies showing that measurements of insulin sensitivity and fatty acid metabolism in culture closely parallel those observed in mature skeletal muscle cells [18–20]. Indeed, cultured skeletal muscle cells from nondiabetic and type 2 diabetes mellitus subjects respond to insulin stimulation in a manner consistent with in vivo changes in glucose utilization [18–20]. Moreover, the ability of skeletal muscle to respond to fasting conditions by switching from carbohydrate to lipid metabolism is retained in HSMCs [21], an important consideration in exploring the effects on metabolism. Thus, with this system, we demonstrated that the ethanolic extract of A dracunculus L clearly increased functional markers of carbohydrate metabolism in HSMC as assessed with glucose uptake and glycogen accumulation studies.
The cellular mechanism by which A dracunculus L increased carbohydrate metabolism was demonstrated to be secondary to enhanced signaling through the insulin receptor. The studies suggested an enhanced insulin-stimulated phosphorylation of downstream signals such as Akt and also demonstrated that the extract enhances insulin-stimulated intracellular kinase activities, that is, PI-3 kinase. It is important to note that the mechanisms studied apply only to the skeletal muscle cell and does not suggest that this might be the major mechanism to explain the clinical effect. Clearly, the extract contains many active compounds that may have beneficial effects on mechanisms operative in other tissues when given as a nutritional supplement. For example, an effect on hepatic glucose production was suggested from animal studies because the extract was also shown to decrease liver phosphoenolpyruvate carboxykinase messenger RNA expression by 28% in rats made diabetic by streptozotocin treatment [10]. Phosphoenolpyruvate carboxykinase is a key enzyme of hepatic gluconeogenesis, and its activity is closely correlated with hepatic glucose output [22].
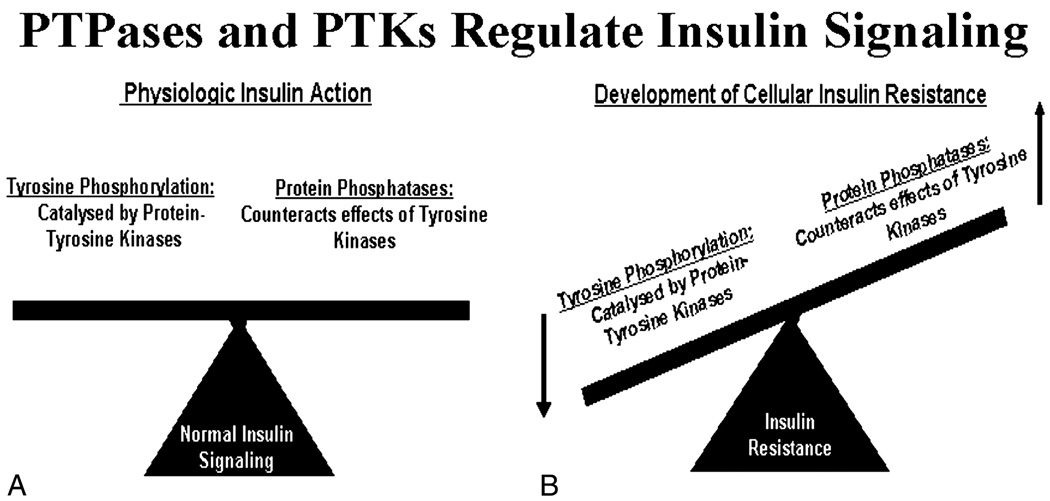
A very intriguing hypothesis based on our observations is that the extract may enhance insulin action by modulating levels of protein tyrosine phosphatases (PTPases). Phosphorylation of proteins at tyrosine residues serves as a switch in regulating the activity of biological processes and cellular signaling events [23,24]. Tyrosine phosphorylation is controlled by the coordinated actions of protein tyrosine kinases and PTPases. Protein tyrosine phosphatases comprise an extensive family of homologous enzymes that regulate various events in cellular signal transduction and have included receptors PTPα and PTPε, leukocyte antigen-related and PTP1B [23,25–27]. Both PTP1B and leukocyte antigen-related have been implicated as having important roles in the regulation of insulin action [26–29]. Specifically, PTP1B has been shown to be active against the autophosphorylated insulin receptor in in vitro studies, as PTP1B has unique structural features that promote its interaction with the receptor to promote dephosphorylation [23,24,26–34]. PTP1B, when compared with other PTPases, also has been shown to have enhanced specific activity toward the in vitro dephosphorylation of IRS-1 [35]. Thus, the 2 major families of enzymes, that is, protein tyrosine kinases and PTPases, provide opposing posttranslational regulations that maintain a dynamic balance of phosphotyrosine residues crucial for cellular homeostasis (Fig. 7A) [23]. As therefore proposed, hyperactivation of PTPases will have detrimental consequences on the level of tyrosine phosphorylation of cellular proteins and will inevitably lead to enhanced dephosphorylation of key insulin signaling proteins. Dephosphorylation of proteins will attenuate insulin signaling on a cellular level and development of insulin resistance on a whole-body level (Fig. 7B) [23,24]. Our data strongly support the hypothesis that the mechanism by which PMI 5011 enhances insulin action may be secondary to an effect to modulate PTPases, as we demonstrate that levels of PTP1B are significantly reduced in primary skeletal muscle culture exposed to PMI 5011.
Fig. 7.
Schematic demonstrating balance of tyrosine phosphorylation vs PTPases in mediating normal insulin signaling (A). With enhanced phosphatase activity (B), balanced is altered and phosphorylation is reduced, attenuating insulin signaling and resulting in clinical insulin resistance.
In summary, we provide evidence regarding the cellular mechanism by which the ethanolic extract of A dracunculus L alters carbohydrate metabolism. Our data suggest that the extract increases glucose uptake and enhances glycogen pathways and that these functional effects result from enhanced insulin receptor signaling. In addition, the data suggest that the effect of PMI 5011 to enhance downstream insulin receptor signaling may be secondary to modulation of PTPases. Studies to address this pathway and the resulting effect on insulin action are the focus of ongoing investigations.
Acknowledgments
Acknowledgment/Conflict of Interest
Supported by NIH Grant P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS), which funds the Botanical Research Center of Pennington Biomedical Research Center and The Biotech Center of Rutgers University.
References
- 1.DeFronzo RA. Insulin resistance, hyperinsulinemia, and coronary artery disease: a complex metabolic web. J Cardiovasc Pharmacol. 1992;20 Suppl 11:S1–S16. doi: 10.1097/00005344-199200111-00002. [DOI] [PubMed] [Google Scholar]
- 2.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 3.Haffner SM. The insulin resistance syndrome revisited. Diabetes Care. 1996;19:275–277. doi: 10.2337/diacare.19.3.275. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Nutrition recommendations and interventions for diabetes. Diabetes Care. 2007;30:S48–S65. doi: 10.2337/dc07-S048. [DOI] [PubMed] [Google Scholar]
- 5.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 6.Gibson JE, Taylor DA. Can claims, misleading information and manufacturing issues regulating dietary supplements be improved in the United States of America? J Pharmacol Exp Ther. 2005;314:939–944. doi: 10.1124/jpet.105.085712. [DOI] [PubMed] [Google Scholar]
- 7.Neuhouser ML. Dietary supplement use by American women: challenges in assessing patterns of use, motives and costs. J Nutr. 2003;133:1992S–1996S. doi: 10.1093/jn/133.6.1992S. [DOI] [PubMed] [Google Scholar]
- 8.Witters LA. The blooming of the French lilac. J Clin Invest. 2001;108:1105–1107. doi: 10.1172/JCI14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanston-Flatt SK, Flatt PR, Day C, Bailey CJ. Traditional dietary adjuncts for the treatment of diabetes mellitus. Proc Nutr Soc. 1991;50:641–651. doi: 10.1079/pns19910077. [DOI] [PubMed] [Google Scholar]
- 10.Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of TARRALIN, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13:550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Henry RR, Ciaraldi TP, Mudaliar S, Abrams L, Nikoulina SE. Acquired defects of glycogen synthase activity in cultured human skeletal muscle cells: influence of high glucose and insulin levels. Diabetes. 1995;45:400–407. doi: 10.2337/diab.45.4.400. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Lechon MJ, Ponsoda X, Cadtell JV. A microassay for measuring glycogen in 96-well cultured cells. Analytical Biochemistry. 1996;236:296–301. doi: 10.1006/abio.1996.0170. [DOI] [PubMed] [Google Scholar]
- 13.Cefalu WT, Wang ZQ, Zhang XH, Baldor LC, Russell JC. Oral chromium picolinate improves carbohydrate and lipid metabolism and enhances skeletal muscle Glut-4 translocation in obese, hyperinsulinemic (JCR-LA corpulent) rats. J Nutr. 2002;132:1107–1114. doi: 10.1093/jn/132.6.1107. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZQ, Bell-Farrow AD, Sonntag W, Cefalu WT. Effect of age and caloric restriction on insulin receptor binding and glucose transporter levels in aging rats. Exp Gerontol. 1997;32:671–684. doi: 10.1016/s0531-5565(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 15.Goodyear LJ, Giorgino F, Sherman LA, Carey J, Smith RJ, Dohm GL. Insulin receptor phosphorylation, insulin receptor substrate–1 phosphorylation, and phosphatidylinositol 3-kinase activity are decreased in intact skeletal muscle strips from obese subjects. J Clin Invest. 1995;95:2195–2204. doi: 10.1172/JCI117909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang ZQ, Zhang XH, Russell JC, Hulver M, Cefalu WT. Chromium picolinate enhances skeletal muscle cellular insulin signaling in vivo in obese, insulin-resistant JCR:LA-cp rats. J Nutr. 2006;136:415–420. doi: 10.1093/jn/136.2.415. [DOI] [PubMed] [Google Scholar]
- 17.Macaulay K, Blair AS, Hajduch E, Terashima T, Baba O, Sutherland C, et al. Constitutive activation of GSK3 down regulates glycogen synthase abundance and glycogen deposition in rat skeletal muscle cells. J Biol Chem. 2005;280:9509–9518. doi: 10.1074/jbc.M411648200. [DOI] [PubMed] [Google Scholar]
- 18.Cha BS, Ciaraldi TP, Park KS, Carter L, Mudaliar SR, Henry RR. Impaired fatty acid metabolism in type 2 diabetic skeletal muscle cells is reversed by PPARgamma agonists. Am J Physiol Endocrinol Metab. 2005;289:E151–E159. doi: 10.1152/ajpendo.00141.2004. [DOI] [PubMed] [Google Scholar]
- 19.Ciaraldi TP, Abrams L, Nikoulina S, Mudaliar S, Henry RR. Glucose transport in cultured human skeletal muscle cells. Regulation by insulin and glucose in nondiabetic and non–insulin-dependent diabetes mellitus subjects. J Clin Invest. 1995;96:2820–2827. doi: 10.1172/JCI118352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry RR, Abrams L, Nikoulina S, Ciaraldi TP. Insulin action and glucose metabolism in nondiabetic control and NIDDM subjects. Comparison using human skeletal muscle cell cultures. Diabetes. 1995;44:936–946. doi: 10.2337/diab.44.8.936. [DOI] [PubMed] [Google Scholar]
- 21.Ukropcova B, McNeil M, Sereda O, de Jonge L, Xie H, Bray GA, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. doi: 10.1172/JCI24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 23.Dube N, Tremblay ML. Involvement of the small protein tyrosine phosphatases TC-PTP and PTP1B in signal transduction and diseases: from diabetes, obesity to cell cycle, and cancer. Biochim Biophys Acta. 2005;1754:108–117. doi: 10.1016/j.bbapap.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein BJ. Protein-tyrosine phosphatase 1B (PTP1B): a novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr Drug Targets Immune Endocr Metabol Disord. 2001;1:265–275. doi: 10.2174/1568008013341163. [DOI] [PubMed] [Google Scholar]
- 25.Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. 2001;5:416–423. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad F, Azevedo JL, Cortright R, Dohm GL, Goldstein BJ. Alterations in skeletal muscle protein-tyrosine phosphatase activity and expression in insulin-resistant human obesity and diabetes. J Clin Invest. 1997;100:449–458. doi: 10.1172/JCI119552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstein BJ. Protein-tyrosine phosphatases: emerging targets for therapeutic intervention in type 2 diabetes and related states of insulin resistance. J Clin Endocrinol Metab. 2002;87:2474–2480. doi: 10.1210/jcem.87.6.8641. [DOI] [PubMed] [Google Scholar]
- 29.Santaniemi M, Ukkola O, Kesaniemi YA. Tyrosine phosphatase 1B and leptin receptor genes and their interaction in type 2 diabetes. J Intern Med. 2004;256:48–55. doi: 10.1111/j.1365-2796.2004.01339.x. [DOI] [PubMed] [Google Scholar]
- 30.Seely BL, Staubs PA, Reichart DR, Berhanu P, Milarski KL, Saltiel AR, et al. Protein tyrosine phosphatase 1B interacts with the activated insulin receptor. Diabetes. 1996;45:1379–1385. doi: 10.2337/diab.45.10.1379. [DOI] [PubMed] [Google Scholar]
- 31.Bandyopadhyay D, Kusari A, Kenner KA, Liu F, Chernoff J, Gustafson TA, et al. Protein-tyrosine phosphatase 1B complexes with the insulin receptor in vivo and is tyrosine-phosphorylated in the presence of insulin. J Biol Chem. 1997;272:1639–1645. doi: 10.1074/jbc.272.3.1639. [DOI] [PubMed] [Google Scholar]
- 32.Wang XY, Bergdahl K, Heijbel A, Liljebris C, Bleasdale JE. Analysis of in vitro interactions of protein tyrosine phosphatase 1B with insulin receptors. Mol Cell Endocrinol. 2001;173:109–120. doi: 10.1016/s0303-7207(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 33.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang ZY. Identification of a second aryl phosphate–binding site in protein-tyrosine phosphatase 1b—a paradigm for inhibitor design. Proc Natl Acad Sci. 1997;94:13420–13425. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salmeen A, Andersen JN, Myers MP, Tonks NK, Barford D. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B. Mol Cell. 2000;6:1401–1412. doi: 10.1016/s1097-2765(00)00137-4. [DOI] [PubMed] [Google Scholar]
- 35.Calera MR, Vallega G, Pilch PF. Dynamics of protein-tyrosine phosphatases in rat adipocytes. J Biol Chem. 2000;275:6308–6312. doi: 10.1074/jbc.275.9.6308. [DOI] [PubMed] [Google Scholar]