Abstract
Although patients with frontotemporal dementia (FTD) are known to exhibit a wide range of cognitive and personality difficulties, some evidence suggests that there may be a degree of selectivity in their reasoning impairments. Based on a recent review of the neuroimaging literature on reasoning, the authors hypothesized that the presence or absence of familiar content may have a selective impact on the reasoning abilities of patients with FTD. Specifically, the authors predicted that patients with frontalvariant FTD would be more impaired when reasoning about transitive arguments involving familiar spatial environments than when reasoning about identical logical arguments involving unfamiliar spatial environments. As predicted, patients with FTD were less accurate than normal controls only when the content of arguments involved familiar spatial environments. These results indicate a degree of selectivity in the cognitive deficits of this patient population and suggest that the frontal-temporal lobe system may play a necessary role in reasoning about familiar material.
Keywords: executive function, frontotemporal dementia, reasoning, semantic memory
Patients with frontotemporal dementia (FTD) are known to exhibit a wide range of deficits in memory, attention, and executive function tasks (Harciarek & Jodzio, 2005), as well as personality difficulties such as impulsivity (The Lund & Manchester Groups, 1994). These factors would lead one to believe that there would be general impairment in the ability of patients with FTD to reason logically. However, there is emerging evidence indicating that there may be a degree of selectivity in the reasoning deficits exhibited by patients with FTD. For example, Waltz et al. (1999) demonstrated that patients with frontal-variant FTD were impaired in a transitive inference task (A > B; B > C; ∴ A > C) only when the arguments required relational integration for correct solution. Otherwise, they performed as well as normal controls. Waltz et al.’s (1999) results suggest that in the realm of transitive reasoning, the deficits exhibited by patients with frontal-variant FTD may be a function of the features of the problem under consideration, rather than a universal impairment.
Neuroimaging studies of reasoning in healthy subjects suggest that another factor—the presence or absence of believable content—may have a selective impact on reasoning abilities. Specifically, neuroimaging studies of reasoning link activation in the frontal-temporal lobe system to reasoning about meaningful, familiar, situations, drawing on the beliefs the subject has about the propositions constituting the argument (e.g., all dogs are pets; all poodles are dogs; ∴ all poodles are pets). By contrast, a bilateral parietal system has been implicated in reasoning about formally identical arguments with nonspecific content (e.g., all P are B; all C are P; ∴ all C are B) (Goel, 2003, 2007; Goel, Buchel, Frith, & Dolan, 2000; Goel & Dolan, 2003; Grafman & Goel, 2002).
This suggests some form of multiple-mechanism account of logical reasoning (Evans, 2008; Evans & Over, 1996; Goel, 2008; Newell & Simon, 1972; Sloman, 1996; Stanovich & West, 2000). Such accounts postulate that when a problem contains familiar, meaningful content, a system based on the activation of situation-specific background knowledge is engaged. This heuristic system is underpinned by the frontal-temporal lobe system. If this system fails—as would be the case when the person encounters unfamiliar or nonspecific information about which they have no relevant beliefs or background knowledge—a formal reasoning system, underpinned by the bilateral parietal system is engaged (Goel, 2003, 2005). This formal system may rely on visuospatial representations for problem solution (Knauff, Fangmeier, Ruff, & Johnson-Laird, 2004), in line with the critical role that the parietal lobes are known to play in spatial cognition (Hubbard, Piazza, Pinel, & Dehaene, 2005; Marshall & Fink, 2001).
The dissociation between the frontal-temporal and bilateral parietal systems for reasoning about familiar and unfamiliar content extends to transitive spatial reasoning (Goel, Makale, & Grafman, 2004). Evaluating arguments involving spatial relations among familiar entities (e.g., Spain is west of Italy, Italy is west of Greece, ∴ Spain is west of Greece) activated the temporal lobes including bilateral posterior hippocampi in normal healthy subjects, consistent with this region’s involvement in the storage and processing of spatial information. By contrast, arguments involving spatial relations between unfamiliar spatial environments (e.g., the AI lab is east of the Roth Centre; Cedar Hall is west of the Roth Centre; ∴ Cedar Hall is west of the AI lab) activated a bilateral parietal system in the same healthy subjects.
To confirm the necessity of the frontal-temporal system in reasoning about familiar spatial environments, we measured the performance of patients with frontal-variant FTD on transitive relational arguments. FTD is a degenerative disorder resulting in gray matter loss largely confined to the frontal and temporal lobes. Based on the neuroimaging data cited above, we hypothesized that compared to normal controls, patients with frontal-variant FTD should be more impaired when reasoning about transitive arguments involving familiar spatial environments than when reasoning about identical logical arguments involving unfamiliar spatial environments. Ours is the first study to focus on the contribution of the frontal-temporal system to transitive inference as a function of the content of arguments within this population.
Method
Participants
The participants were 14 patients with FTD (10 males, 4 females) diagnosed using consensus diagnostic criteria (McKhann et al., 2001), and 21 normal controls (16 males, 5 females). All patients with FTD were diagnosed with the frontal variant of FTD. The demographic characteristics of the patients are listed in Table 1. There was no significant difference in years of education between patients with FTD (M = 15.8, SD = 2.5) and normal controls (M = 14.9, SD = 2.2), t(32) =1.11, NS. There was also no significant difference in age between patients with FTD (M = 54.3, SD = 9.1) and normal controls (M = 49, SD = 7.2), t(32) = 1.87, NS.
Table 1.
The Demographic Characteristics of the 14 Patients With FTD
| Patient | Age (visit |
Age (onset) |
Years symptom onset |
Gender | Education (years) |
Handedness | First symptom type |
|---|---|---|---|---|---|---|---|
| 1 | 63 | 62 | 1 | M | 12 | L | Memory |
| 2 | 65 | 63 | 2 | M | 12 | R | Behavioral change |
| 3 | 58 | 54 | 4 | M | 17 | R | Memory |
| 4 | 53 | 52 | 1 | M | 16 | R | Depression |
| 5 | 51 | 44 | 7 | M | 16 | R | Poor judgment |
| 6 | 47 | 43 | 4 | M | 16 | R | Behavioral change |
| 7 | 39 | 38 | 1 | M | 16 | R | Lack of energy |
| 8 | 63 | 60 | 3 | F | 16 | R | Fatigue |
| 9 | 44 | 43 | 0.7 | F | 12 | R | Memory |
| 10 | 60 | 55 | 5 | F | 16 | R | Memory |
| 11 | 65 | 63 | 2 | F | 16 | R | Behavioral change |
| 12 | 48 | 43 | 5 | M | 16 | R | Memory |
| 13 | 64 | 60 | 4 | M | 20 | R | Memory |
| 14 | 54 | 49 | 5 | M | 20 | R | Memory |
Neuropsychological Characterization
Patients were administered an extensive battery of neuropsychological tests including the Dementia Rating Scale (DRS) (Mattis, 1988), Wechsler Adult Intelligence Scale—Revised (WAIS–R) (Wechsler, 1981), Wechsler Memory Scale—Revised (WMS-R) (Wechsler, 1987), verbal fluency (FAS) (Spreen & Strauss, 1991), the Wisconsin Card Sorting Test (Heaton et al., 1993), the California Card Sorting Test (CCST) (Delis, Squire, Bihrle, & Massman, 1992), and the Boston Naming Task (BNT) (Kaplan et al., 1983). The average scores for the patients on these tests are listed in Table 2.
Table 2.
Cognitive Baseline Scores of Patients With FTD
| Measures | Subscale | Score |
|---|---|---|
| BNT | 44.4 (17.7) | |
| CCST | Sort attempts overall | 12.14 (3.15) |
| Sort perseveration overall | 2.5 (2.24) | |
| Explanation perseveration overall | 2.35 (2.56) | |
| Explanation total score overall | 9.85 (8.70) | |
| DRS (raw scores) | Attention | 33.92 (2.58) |
| Initiation/perseveration | 26.92 (7.49) | |
| Construction | 5.85 (0.53) | |
| Conceptualization | 33.21 (4.11) | |
| Memory | 20.57 (3.77) | |
| DRS total | 120.07 (13.35) | |
| FAS | 25.8 (19.1) | |
| WAIS-R | Verbal | 87.76 (16.74) |
| Performance | 86.61 (16.49) | |
| Full scale | 86.38 (15.68) | |
| WCST Number | Number of cards | 125.21 (10.42) |
| Number correct | 49.07 (16.75) | |
| Number of errors | 76.14 (23.34) | |
| Perseverative errors | 49.96 (30.43) | |
| Nonperseverative errors | 26.42 (23.57) | |
| Category completion | 1.35 (1.78) | |
| WMS-R (Index scores) | General memory | 78.23 (15.65) |
| Working memory | 82.53 (13.86) |
Note. Standard deviations appear in parentheses. BNT = Boston Naming Task; CCST = California Card Sorting Test; DRS = Dementia Rating Scale; FAS = verbal fluency; WAIS-R = Wechsler Adult Intelligence Scale-Revised; WCST = Wisconsin Card Sorting Test; WMS-R = Wechsler Memory Scale-Revised.
Anatomical Analysis and Characterization
To determine the brain areas affected in the patients, we used a magnetic resonance imaging (MRI) analysis technique, called Voxel-Based Morphometry (VBM), to compare the scans of the patients to those of a group of 14 age-matched healthy control subjects. These control subjects were different subjects from those who received the reasoning task described above. VBM is an automated method of MRI analysis that allows a statistical summation of data from many patients and has the advantage of not requiring judgment by raters that can be biased by preexisting hypotheses. VBM has been used successfully and reliably to measure the association between symptoms and regional atrophy in patients with FTD (Whitwell et al., 2005) and other types of dementia (Grossman et al., 2004; Rosen et al., 2005).
Of the 14 FTD patients, 11 had MRI scans that could be used in the VBM analysis. The other three had not had an MRI scan (usually for behavioral reasons) or the scan showed excessive movement. The patients’ and normal controls’ structural images were preprocessed and analyzed using the optimized VBM protocol implemented in SPM5 (www.fil.ion.ucl.ac.uk/spm/) as described elsewhere (Adlam et al., 2006; Good et al., 2001). All images were segmented into gray matter, white matter, and cerebrospinal fluid. As part of the segmentation, the patient images were spatially normalized to the Montreal Neurological Institute brain template and then modulated by the Jacobian determinants from spatial normalization to correct for volume changes introduced during the transformations (Keller et al., 2004). The resulting gray matter images were then smoothed applying a 12 mm full width at half-maximum Gaussian kernel and then used in the analyses. An explicit mask encompassing the entire brain was used in the analyses to control for background signal outside the brain. This mask was downloaded from the SPM5 Anatomic Automatic Labeling toolbox (www.cyceron.fr/freeware). A 0.05 explicit absolute threshold for masking was used in the SPM second-level model interface (Ashburner & Friston, 2001). Total intracranial volume (TIV) was calculated in SPM5 from the unsmoothed, modulated gray matter, white matter, and CSF images from each patient, and used as a nuisance variable to account for the possible effect of varying brain volumes.
Differences in local gray matter volumes between the age-matched control group and the patient group were assessed with a two-group t test. The significance level for the t statistic was set at p < .05 at the voxel level, corrected for multiple comparisons using the family-wise error (FWE) threshold. Only those clusters composed of at least 50 voxels were considered significant.
Materials
Participants were administered 10 transitive arguments involving explicit spatial relations (see Table 3). The content of five arguments involved sentences that described spatial relations between familiar spatial environments (i.e., countries). The content of the remaining five arguments involved spatial relations that would have been unfamiliar to our subjects. Like their familiarcontent counterparts, unfamiliar-content arguments describe spatial relations between entities. However, the critical feature that distinguishes the two sets of arguments is that in the former case the sentences described relations between entities (i.e., countries) that subjects should be familiar with and should have stored semantic knowledge about—derived from maps, books, or personal experience of countries and cities accrued through travel (Goel et al., 2004), whereas in the latter case the entities are nonspecific so background knowledge related to their spatial location should be absent.
Table 3.
Complete List of Arguments Used in the Study
| Familiar spatial environments | Unfamiliar spatial environments |
|---|---|
| Cambodia is west of Vietnam | Buffalo are trailing deer |
| Vietnam is west of Japan | Deer are trailing caribou |
| Cambodia is east of Japan | Caribou are trailing buffalo |
| Estonia is east of Latvia | The plates are over the napkins |
| Latvia is east of Russia | The cups are over the plates |
| Estonia is east of Russia | The napkins are under the cups |
| Brazil is situated south of Peru | Tailors are standing to one side of lawyers |
| Argentina is situated north of Brazil | Lawyers are standing to one side of barbers |
| Peru is situated south of Argentina | Tailors are standing to the other side of barbers |
| Austria is east of Switzerland | The teacher is in front of the blackboard |
| Switzerland is east of France | The blackboard is in front of the desk |
| Austria is east of France | The teacher is in front of the desk |
| Spain is south of England | Children are seated to the right of adults |
| Scotland is north of Spain | Infants are seated to the right of adults |
| England is south of Scotland | Infants are seated to the left of children |
Note. Adjacent arguments are matched for validity and determinacy (see text). Familiar spatial arguments appearing in italics are congruent, else incongruent (see text).
Although the arguments involving familiar spatial environments involve descriptions of larger spaces (i.e., countries) compared to arguments involving unfamiliar spatial environments, a recent review of the literature found no evidence that spatial reasoning is influenced by spatial shcale (Gattis, 2005; see also Mandler, 1983). To verify that normal controls indeed possess sufficient geographic knowledge to have correct beliefs about the content of specific propositions, we instructed an independent group of 20 participants (7 males, 13 females, M = 40 years old, SD = 20) to determine whether each conclusion of our familiar arguments was true or false. Average accuracy was 86%. This is consistent with previous results from our lab demonstrating that normal participants possess adequate background geographical knowledge to have correct beliefs about the content of such propositions (80% accuracy, Goel et al., 2004).
The arguments for familiar and unfamiliar spatial environments were matched in terms of two critical factors: determinacy and validity. Determinate arguments are either valid or inconsistent. An example of a valid argument used in this study is “Estonia is east of Latvia; Latvia is east of Russia; ∴ Estonia is east of Russia.” This is a valid argument because the relationship between Estonia and Russia is absolutely determined by the information provided in the two premises (i.e., Estonia is east of Latvia and Latvia is east of Russia). Specifically, acceptance of the premises requires one to accept the conclusion. An argument is inconsistent if the conclusion contradicts the information provided in the premises. An example of an indeterminate argument used in this study is “Brazil is situated south of Peru; Argentina is situated north of Brazil; ∴ Peru is situated south of Argentina.” As is evident in this example, the two premises do not provide sufficient information to determine the relationship between Peru and Argentina. Like inconsistent arguments indeterminate arguments are also invalid, but not because of inconsistency. Rather, indeterminate arguments are invalid because the relationship between the entities stated in the conclusion is uncertain.
Finally, arguments for familiar and unfamiliar spatial environments were also balanced in terms of inversion. Inversion indicates whether the direction in which the relationships in the argument are described is fixed or varies. For example, the argument “Austria is east of Switzerland; Switzerland is east of France; ∴ Austria is east of France” has no inverted relation because all three sentences use the term “is east of” to describe the relationships. In contrast, the argument “Cambodia is west of Vietnam; Vietnam is west of Japan; ∴ Cambodia is east of Japan” has an inverted relation because the terms used to describe the relationships vary between “is east of” and “is west of.”
Procedure
The items were presented using Macintosh computers and SuperLab software. The presentation of trials was randomized for each participant. Advancement through the items was self-paced to allow patients sufficient time to read the arguments and to make validity judgments. Each trial consisted of the appearance of the entire argument (i.e., two premises and the conclusion) on a single slide. Participants were instructed to enter their “Valid” and “Not Valid” responses using two keys on the keyboard, and the software recorded the response made by the participant. Before the start of the experiment, the concept of validity was explained to the participants using a tutorial consisting of six arguments. These examples were presented to the participants to ensure that the participants understood what was required of them in this task.
Results
Imaging Results
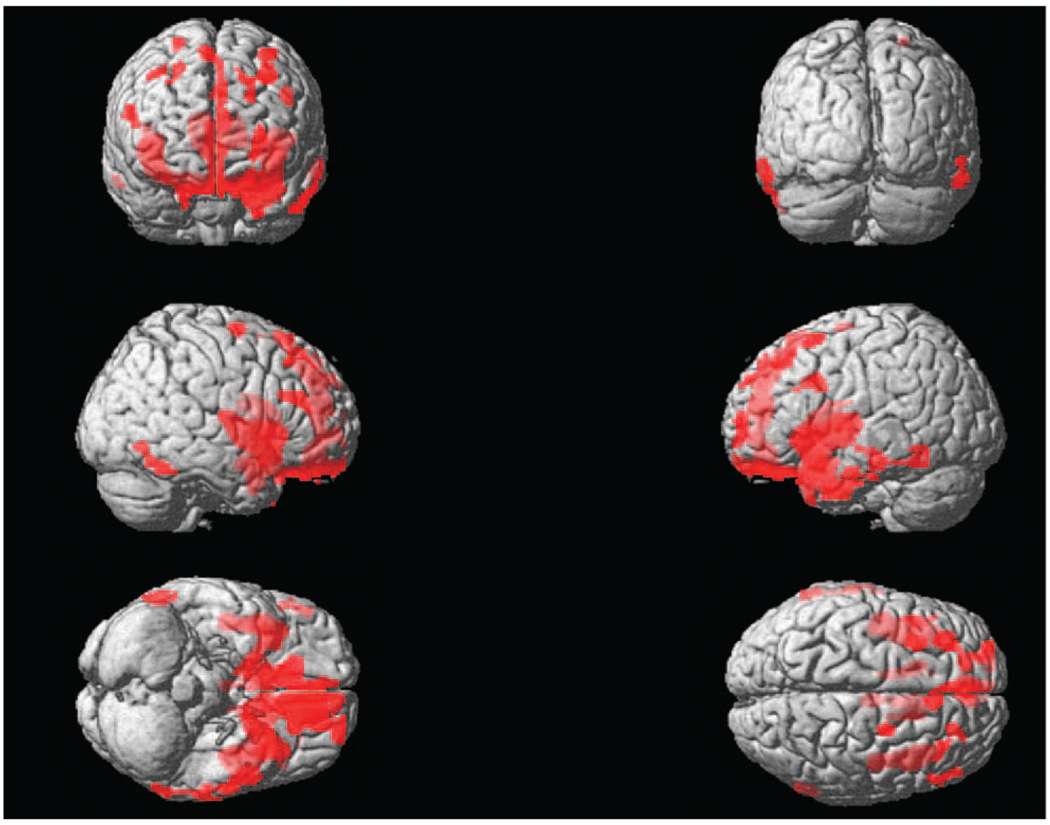
The FTD patients showed a predominance of frontal and anterior temporal lobe, and caudate, gray matter loss compared with the normal control subjects. Parietal gray matter loss was not observed (see Figure 1). These data are consistent with previous imaging findings on FTD (Cardenas et al., 2007).
Figure 1.
Colored areas indicate significantly higher gray matter loss in patients with FTD compared to normal controls. All areas listed are significant at the voxel level when corrected for multiple comparisons with the Family-Wise-Error correction at the p < .05 level.
Behavioral Results
Average accuracy across all trials for normal controls was 74.8% (SD = 17.8), comparable to results obtained using transitive relational problems in our lab in the past (77% in Goel & Dolan, 2001; 78.9% in Goel et al., 2004). To verify that the data could be analyzed using parametric analyses, we first ran separate Kolmogorov–Smirnov tests on distributions of scores for arguments with familiar and unfamiliar content. The results indicated that the distributions of scores did not deviate significantly from normality for arguments with familiar (z = 1.17, NS) and unfamiliar (z = 1.11, NS) content.
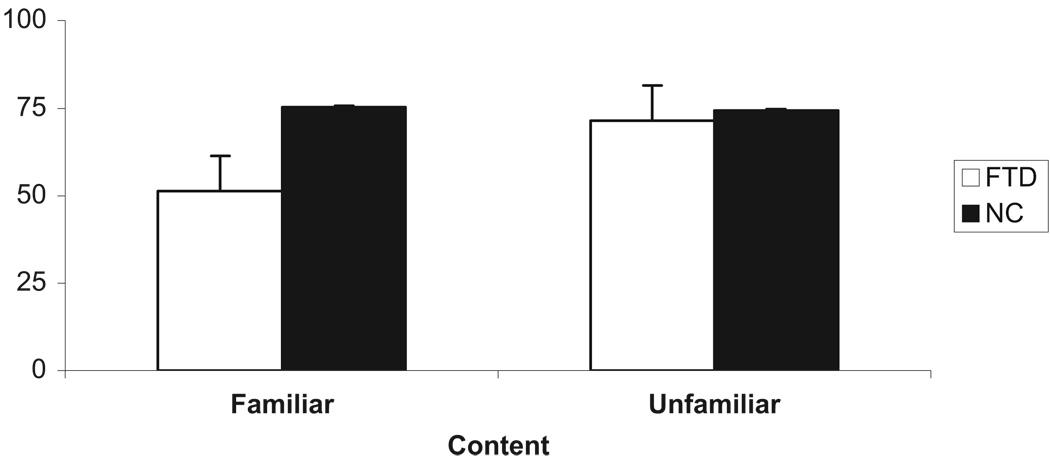
To test our key hypothesis, we carried out a mixed ANOVA to investigate the interaction between group (patients with FTD, normal controls), and content (familiar, unfamiliar). Specifically, group was entered as a between-subjects variable, and content was entered as within-subjects variable. To determine whether age or education should be included as covariates in this analysis we calculated the correlation between each variable and accuracy. The results demonstrated that age was not correlated significantly with overall accuracy (r[33] = −.07, NS) or accuracy on arguments with familiar (r[33] = −.19, NS) or unfamiliar (r[33] = .05, NS) content. Likewise, education was not correlated significantly with overall accuracy (r[33] = .04, NS) or accuracy on arguments with familiar (r[33] = .05, NS) or unfamiliar (r[33] = .03, NS) content. Therefore, age and education were not included as covariates in this analysis. There was a main effect for group such that overall, normal controls were more accurate than patients with FTD, F(1, 33) = 4.70, p < .05, partial η2 = .13. There was also a main effect for content such that accuracy was higher for unfamiliar (M = 73.1%, SD = 22.6) than familiar (M = 65.6%, SD = 22.4) spatial content, F(1, 33) = 5.58, p < .05, partial η2 = .13. However, critical for our hypothesis, there was a significant interaction between group and content such that the difference in accuracy between patients with FTD and normal controls was greater on arguments with familiar than unfamiliar spatial content, F(1, 33) = 6.77, p < .05, η2 = .16 (see Figure 2). Specifically, simple effects analyses indicated that in problems with familiar spatial content the difference in accuracy between patients with FTD and normal controls was significant, t(33) = −3.55, p < .001, r = .53 (Dunn, 2001). In fact, on problems with familiar content the performance of patients with FTD did not differ from chance, t(13) = .30, NS. In contrast, in problems with unfamiliar spatial content the difference between patients with FTD and normal controls failed to reach significance, t(33) = −.35, NS. Thus, as hypothesized, patients with FTD were more impaired when reasoning about transitive arguments involving familiar spatial environments than when reasoning about logically identical arguments involving unfamiliar spatial environments.
Figure 2.
The performance (accuracy) of patients with FTD and normal controls on transitive relational (spatial) arguments, broken down by content of arguments (familiar, unfamiliar). Note. FTD = Patients with Frontotemporal dementia; NC = Normal controls, bars indicate SEM.
One could argue that the observed difference between reasoning about arguments that contained familiar versus unfamiliar spatial content is that in the former case the subject must overcome general knowledge about the world to treat the problem as an abstract logical exercise, whereas in the latter case this is not necessary. In other words, while reasoning about arguments that contained familiar spatial content patients with FTD may have been unable to inhibit the more available, general-knowledge-based response to allow the less automatic, formal reasoning system to be invoked. This possibility is consistent with the results of a recent study involving frontal-variant FTD patients in which it was shown that intact prefrontal cortex may be necessary for controlling against perceptual and semantic interference during reasoning Krawszyk et al., 2008).
To test this possibility, we divided the five arguments that contained familiar spatial content into congruent (3) and incongruent (2) trials. Congruent trials consist of valid arguments with true conclusions (e.g., Austria is east of Switzerland, Switzerland is east of France, ∴ Austria is east of France), or invalid arguments with false conclusions (e.g., Cambodia is west of Vietnam, Vietnam is west of Japan, ∴ Cambodia is east of Japan). On congruent trials, subjects’ knowledge facilitated the logical task. In contrast, incongruent trials consist of valid arguments with false conclusions (e.g., Estonia is east of Latvia, Latvia is east of Russia, ∴ Estonia is east of Russia), or invalid arguments with true conclusions (e.g., Spain is south of England, Scotland is north of Spain, ∴ England is south of Scotland). On incongruent trials, subjects’ knowledge will inhibit the logical task. If the decreased accuracy of patients with FTD while reasoning about arguments with familiar spatial content were because of their inability to inhibit the more available, general-knowledge-based response to allow the formal reasoning system to be invoked, then one would expect them to be particularly impaired on incongruent trials. In fact, this was found not to be the case. Specifically, the interaction between group and congruency did not reach significance, F(1, 32) = 3.76, NS. Despite the limited number of items contributing to this analysis, the results would seem to suggest that inhibition may not be the likely mechanism impairing the performance of patients with FTD while reasoning about arguments with familiar spatial content.
Next, we conducted an analysis of reaction time (RT) data to rule out impulsivity as yet another possibility for the increased impairment of patients with FTD while reasoning about arguments that contained familiar spatial content. Previous evidence has shown that patients with FTD exhibit high impulsivity (The Lund & Manchester Groups, 1994). Impulsivity can in turn lead to impairments in reasoning because it can hamper a subject’s ability to maintain focus on the problem while reasoning about it. We reasoned that if impulsivity were a contributor to the increased impairment of patients with FTD while reasoning about arguments with familiar spatial content, then patients with FTD should exhibit shorter RT specifically on problems with specific familiar content. However, the interaction between group and familiarity did not reach significance, F(1, 33) < 1. This suggests that impulsivity may not be the likely mechanism impairing the performance of patients with FTD while reasoning about arguments with familiar spatial content.
Reasoning ability is highly correlated with general cognitive capacity. Thus, for the patient group we computed zero-order correlations between accuracy and measures of cognitive capacity including the BNT, CCST, DRS, FAS, WAIS–R, WCST, and WMS-R (see Table 2). The results demonstrated that there were significant correlations between accuracy on unfamiliar items and the Vocabulary (r[12] = .60, p < .05) and Comprehension (r[12] = .77, p < .05) subsets of the WAIS–R, and between accuracy on familiar items and the Picture completion (r[12] = .54, p < .05) subset of the WAIS–R. No other correlation achieved significance. In general, scores on measures of cognitive capacity did not correlate with performance on the transitive reasoning task.
Discussion
This study was conducted to investigate the performance of patients with frontal-variant FTD and normal controls on reasoning about transitive relations with content involving familiar and unfamiliar spatial environments. Based upon a recent review of the neuroimaging data on reasoning (Goel, 2007) we predicted that patients with FTD should be more impaired on those arguments that contain sentences describing familiar spatial environments than arguments containing sentences describing unfamiliar spatial environments. Consistent with our a priori prediction we observed an interaction such that patients with FTD were impaired more on problems with familiar spatial content, but performed as well as normal controls on problems with unfamiliar content (see Figure 2). Our results indicate that despite deficits in executive function (see Table 2), there may be some degree of selectivity in the reasoning deficits exhibited by patients with FTD. Specifically, our results suggest that impairment in reasoning in frontal-variant FTD may vary as a function of the features of the problem under consideration (see also Waltz et al., 1999).
The dissociation observed in this study is broadly consistent with cognitive theories that suggest multiple pathways for evaluating logical arguments (Evans, 2008; Evans & Over, 1996; Goel, 2008; Newell & Simon, 1972; Sloman, 1996; Stanovich & West, 2000). Although these theories differ in important ways, they all postulate the engagement of a heuristic system based on the activation of situation-specific background knowledge when a problem contains familiar, meaningful content. However, should this heuristic system fail—as would be the case when the person encounters nonspecific information about which they have no relevant beliefs or background knowledge, or a belief-logic conflict is detected—a formal reasoning system is engaged.
Neuroimaging studies suggest that whereas the heuristic system is underpinned by a frontal-temporal lobe system, the formal system is underpinned by a bilateral parietal system (Goel, 2003, 2005, 2007). Although the heuristic system may rely on memory representations for problem solution, the formal system may rely on visuospatial representations for arriving at logical inferences. Given that our patients show predominantly frontal, anterior temporal, and caudate gray matter loss (see Figure 1), our findings support earlier neuroimaging studies linking the frontal-temporal lobe system to reasoning about familiar content that people can be expected to have background knowledge about (Goel et al., 2000, 2004; Goel & Dolan, 2003; Goel, Shuren, Sheesley, & Grafman, 2004). In addition, the patients’ good performance on the nonspecific items is consistent with previous evidence demonstrating that patients with FTD perform well on spatial tasks, an ability that has been attributed to the relative sparing of the parietal lobes in FTD (Hodges, 2001).
Based on this framework, we attribute the impairment of patients with FTD on arguments involving familiar spatial environments to a combination of two factors. When one encounters an argument, the heuristic system based on the activation of situation-specific background knowledge is engaged. However, because the heuristic system is underpinned by the frontal-temporal lobe system (Goel, 2003, 2007), and the frontal-temporal lobes are compromised by FTD (see Figure 1), this results in the engagement of a faulty system. In other words, patients with FTD exhibit impairment because of reliance on faulty memory representations for reasoning about content that appears familiar, but is in fact erroneous. This idea is supported by studies linking atrophy in the temporal lobes to the loss of geographic knowledge (Beatty & Bernstein, 1989; Beatty & Salmon, 1991; Yasuda, Watanabe, & Ono, 1997). However, when the heuristic system fails, switching to the formal system requires executive control including working memory resources (De Neys, 2006; Stanovich & West, 2000). In line with previous evidence, our frontal-variant FTD sample was also characterized by executive function deficits (see Table 2). Therefore, an additional factor contributing to the inability of patients to reason about problems with familiar content is that in the face of faulty memory representations they may have lacked the necessary executive resources for switching to the formal system, as would be expected to be the case here given the extensive gray matter loss in the FTD patients (see Figure 1). We argue that the engagement of a faulty heuristic system coupled with the inability to switch to the formal system may underlie reasoning deficits on arguments involving familiar spatial environments in patients with FTD.
Our results speak to the involvement of the frontal-temporal system in reasoning about familiar situations. Specifically, our results complement the results of neuroimaging studies by suggesting the necessity of the frontal-temporal system in this type of reasoning, presumably to access and bring world knowledge to bear on the task, or to facilitate switching to the formal reasoning system. In addition, our results add to a growing body of literature suggesting that impairment in reasoning in frontal-variant FTD may not be linked consistently to executive impairment alone (Giovagnoli, Erbeta, Riati, & Bugiani, 2008), but that it may vary as a function of the features of the problem under consideration (see Waltz et al., 1999). As such, the results encourage a more nuanced approach toward characterizing cognitive deficits associated with FTD.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research, National Science & Engineering Research Council of Canada, and a Premier’s Research Excellence Award to the second author.
Contributor Information
Oshin Vartanian, Department of Psychology, York University, Toronto, Ontario, Canada.
Vinod Goel, Department of Psychology, York University, Toronto, Ontario, Canada, and Department of Psychology, University of Hull, Hull, United Kingdom.
Michael Tierney, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD.
Edward D. Huey, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD
Jordan Grafman, Cognitive Neuroscience Section, National Institute of Neurological Disorders and Stroke, Bethesda, MD.
References
- Adlam AL, Patterson K, Rogers TT, Nestor PJ, Salmond CH, Acosta-Cabronero J, et al. Semantic dementia and fluent primary progressive aphasia: Two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. NeuroImage. 2001;14:1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Bernstein N. Geographical knowledge in patients with Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology. 1989;2:76–82. doi: 10.1177/089198878900200204. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Salmon DP. Remote memory for visuospatial information in patients with Alzheimer’s disease. Journal of Geriatric Psychiatry and Neurology. 1991;4:14–17. doi: 10.1177/089198879100400103. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Boxer AL, Chao LL, Gorno-Tempini ML, Miller BL, Weiner MW, et al. Deformation-based morphometry reveals brain atrophy in frontotemporal dementia. Archives of Neurology. 2007;64:873–877. doi: 10.1001/archneur.64.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Squire LR, Bihrle A, Massman P. Componential analysis of problem-solving ability: Performance of patients with frontal lobe damage and amnestic patients on a new sorting test. Neuropsychologia. 1992;30:683–697. doi: 10.1016/0028-3932(92)90039-o. [DOI] [PubMed] [Google Scholar]
- De Neys W. Dual processing in reasoning: Two systems but one reasoner. Psychological Science. 2006;17:428–433. doi: 10.1111/j.1467-9280.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- Dunn D. Statistics and data analysis for the behavioral sciences. 2nd ed. New York: McGraw-Hill; 2001. [Google Scholar]
- Evans J, St. B T. Dual-processing accounts of reasoning, judgment and social cognition. Annual Review of Psychology. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Evans J, St. B T, Over DE. Rationality and reasoning. Hove: Psychology Press; 1996. [Google Scholar]
- Gattis M. Inferencing from spatial information. Spatial Cognition and Computation. 2005;5:119–137. [Google Scholar]
- Giovagnoli AR, Erbetta A, Riati F, Bugiani O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia. 2008;46:1495–1504. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Goel V. Evidence for dual neural pathways for syllogistic reasoning. Psychologica. 2003;32:301–309. [Google Scholar]
- Goel V. Cognitive neuroscience of deductive reasoning. In: Holyoak K, Morrison R, editors. Cambridge handbook of thinking & reasoning. Cambridge: University Press; 2005. pp. 475–492. [Google Scholar]
- Goel V. Anatomy of reason. Trends in Cognitive Sciences. 2007;11:435–441. doi: 10.1016/j.tics.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Goel V. Fractionating the system of deductive reasoning. In: Kraft E, Guylas B, Poppel E, editors. Neural correlates of thinking. New York: Springer Press; 2008. [Google Scholar]
- Goel V, Buchel C, Frith C, Dolan RJ. Dissociation of mechanisms underlying syllogistic reasoning. NeuroImage. 2000;12:504–514. doi: 10.1006/nimg.2000.0636. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Functional neuroanatomy of three-term relational reasoning. Neuropsychologia. 2001;39:901–909. doi: 10.1016/s0028-3932(01)00024-0. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Explaining modulation of reasoning by belief. Cognition. 2003;87:B11–B22. doi: 10.1016/s0010-0277(02)00185-3. [DOI] [PubMed] [Google Scholar]
- Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. Journal of Cognitive Neuroscience. 2004;16:654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- Goel V, Shuren J, Sheesley L, Grafman J. Asymmetrical involvement of the frontal lobes in social reasoning. Brain. 2004;127:783–790. doi: 10.1093/brain/awh086. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grafman J, Goel V. Encyclopedia of Cognitive Science. London: Macmillan; 2002. Neural basis of reasoning; pp. 875–880. [Google Scholar]
- Grossman M, McMillan C, Moore P, Ding L, Glosser G, Work M, et al. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain. 2004;127:628–649. doi: 10.1093/brain/awh075. [DOI] [PubMed] [Google Scholar]
- Harciarek M, Jodzio K. Neuropsychological differences between frontotemporal dementia and Alzheimer’s disease: A review. Neuropsychology Review. 2005;15:131–145. doi: 10.1007/s11065-005-7093-4. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GC, Curtiss G. Wisconsin card sorting test. Manual. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Hodges JR. Frontotemporal dementia (Pick’s disease): Clinical features and assessment. Neurology. 2001;56:S6–S10. doi: 10.1212/wnl.56.suppl_4.s6. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions between number and space in parietal cortex. Nature Reviews Neuroscience. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Keller SS, Wilke M, Wieshmann UC, Sluming VA, Roberts N. Comparison of standard and optimized voxel-based morphometry for analysis of brain changes associated with temporal lobe epilepsy. NeuroImage. 2004;23:860–868. doi: 10.1016/j.neuroimage.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: Behavioral measures and cortical activity. Journal of Cognitive Neuroscience. 2004;15:559–573. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC, Morrison RG, Viskontas I, Holyoak KJ, Chow TW, Mendez MF, et al. Distraction during relational reasoning: The role of prefrontal cortex in interference control. Neuropsychologia. 2008;46:2020–2032. doi: 10.1016/j.neuropsychologia.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Mandler JM. Representation. In: Flavell JH, Markman EM, editors. Handbook of child psychology. New York: Wiley; 1983. pp. 420–494. [Google Scholar]
- Marshall JC, Fink GR. Spatial cognition: Where we were and where we are. NeuroImage. 2001;14:S2–S7. doi: 10.1006/nimg.2001.0834. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale professional manual. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: Report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Archives of Neurology. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- Newell A, Simon HA. Human problem solving. Englewood Cliffs, NJ: Prentice Hall; 1972. [Google Scholar]
- Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioral disorders in dementia. Brain. 2005;128:2612–2625. doi: 10.1093/brain/awh628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloman SA. The empirical case for two systems of reasoning. Psychological Bulletin. 1996;119:3–22. [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. New York: Oxford University Press; 1991. [Google Scholar]
- Stanovich KE, West RF. Individual differences in reasoning: Implications for the rationality debate. Behavioral and Brain Sciences. 2000;22:645–665. doi: 10.1017/s0140525x00003435. [DOI] [PubMed] [Google Scholar]
- The Lund and Manchester Groups. Clinical and neuropathological criteria for frontotemporal dementia. Journal of Neurology, Neurosurgery, and Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Mishkin FS, de Menezes, et al. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- Wechsler D. WAIS-R manual. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Archives of Neurology. 2005;62:1402–1408. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Watanabe O, Ono Y. Dissociation between semantic and autobiographical memory: A case study. Cortex. 1997;33:623–638. doi: 10.1016/s0010-9452(08)70721-4. [DOI] [PubMed] [Google Scholar]