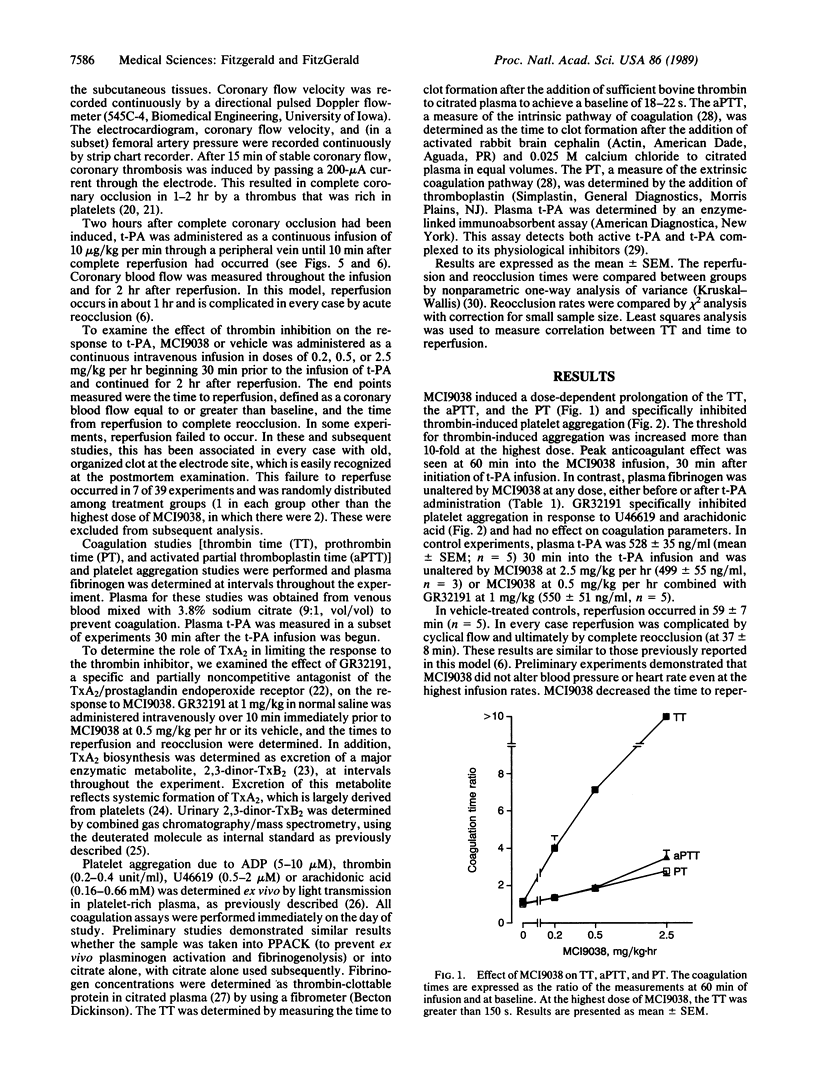
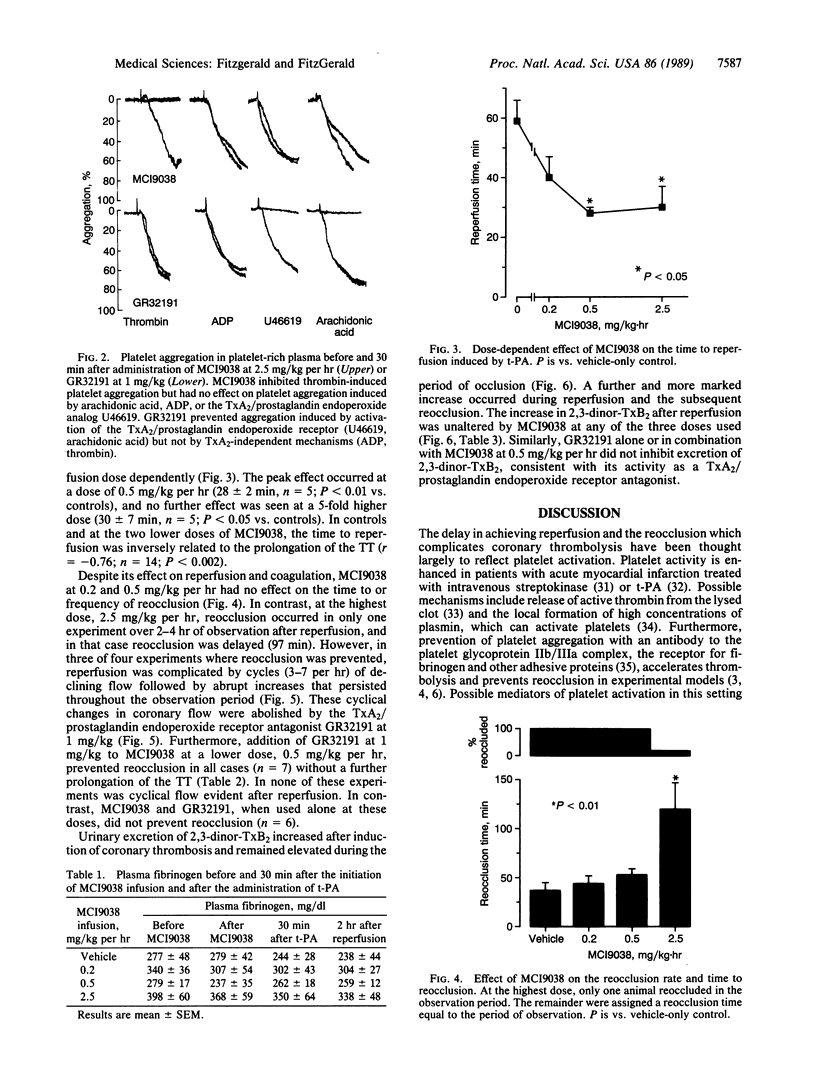
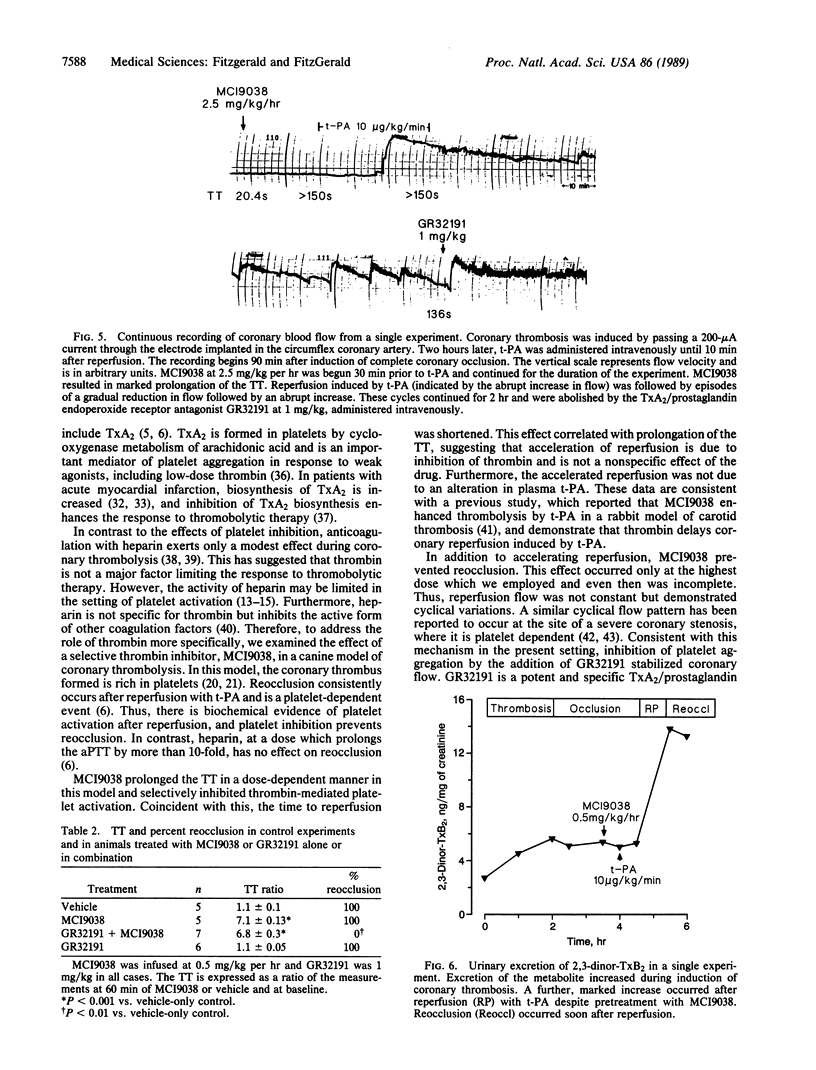
Abstract
Reocclusion of the coronary artery occurs after thrombolytic therapy of acute myocardial infarction despite routine use of the anticoagulant heparin. However, heparin is inhibited by platelet activation, which is greatly enhanced in this setting. Consequently, it is unclear whether thrombin induces acute reocclusion. To address this possibility, we examined the effect of argatroban [MCI9038, (2R,4R)-4-methyl-1-[N alpha-(3-methyl-1,2,3,4-tetrahydro-8- quinolinesulfonyl)-L-arginyl]-2-piperidinecarboxylic acid], a specific thrombin inhibitor, on the response to tissue-type plasminogen activator in a closed-chest canine model of coronary thrombosis. MCI9038 prolonged the thrombin time and shortened the time to reperfusion (28 +/- 2 min vs. 59 +/- 7 min in controls; mean +/- SEM, n = 5, P less than 0.01). At the highest dose, 2.5 mg/kg per hr, complete reocclusion was prevented in four of the five experimental animals, whereas reocclusion occurred in all five controls. However, reperfusion was complicated by cycles of decrease flow, which were abolished by the thromboxane A2 antagonist, GR32191. GR32191 at 1 mg/kg combined with MCI9038 at 0.5 mg/kg per hr prevented reocclusion, whereas, at these doses, either drug alone was without effect. In addition, thromboxane A2 biosynthesis, determined as excretion of its metabolite 2,3-dinor-thromboxane B2, was increased after reperfusion at all doses of MCI9038. These data demonstrate that thrombin impairs thrombolysis induced by tissue-type plasminogen activator in vivo and induces acute reocclusion. Furthermore, the response to thrombin inhibition may be impaired by continued formation of thromboxane A2.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton J. H., Schmitz J. M., Campbell W. B., Ogletree M. L., Raheja S., Taylor A. L., Fitzgerald C., Buja L. M., Willerson J. T. Inhibition of cyclic flow variations in stenosed canine coronary arteries by thromboxane A2/prostaglandin H2 receptor antagonists. Circ Res. 1986 Nov;59(5):568–578. doi: 10.1161/01.res.59.5.568. [DOI] [PubMed] [Google Scholar]
- Bergsdorf N., Nilsson T., Wallén P. An enzyme linked immunosorbent assay for determination of tissue plasminogen activator applied to patients with thromboembolic disease. Thromb Haemost. 1983 Oct 31;50(3):740–744. [PubMed] [Google Scholar]
- Bock P. E., Luscombe M., Marshall S. E., Pepper D. S., Holbrook J. J. The multiple complexes formed by the interaction of platelet factor 4 with heparin. Biochem J. 1980 Dec 1;191(3):769–776. doi: 10.1042/bj1910769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Catella F., FitzGerald G. A. Paired analysis of urinary thromboxane B2 metabolites in humans. Thromb Res. 1987 Sep 15;47(6):647–656. doi: 10.1016/0049-3848(87)90103-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg P. R., Sherman L. A., Jaffe A. S. Paradoxic elevation of fibrinopeptide A after streptokinase: evidence for continued thrombosis despite intense fibrinolysis. J Am Coll Cardiol. 1987 Sep;10(3):527–529. doi: 10.1016/s0735-1097(87)80194-8. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Bing D. H. Thrombin active-site regions. Semin Thromb Hemost. 1986 Jul;12(3):200–208. doi: 10.1055/s-2007-1003551. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Catella F., Roy L., FitzGerald G. A. Marked platelet activation in vivo after intravenous streptokinase in patients with acute myocardial infarction. Circulation. 1988 Jan;77(1):142–150. doi: 10.1161/01.cir.77.1.142. [DOI] [PubMed] [Google Scholar]
- Fitzgerald D. J., Doran J., Jackson E., FitzGerald G. A. Coronary vascular occlusion mediated via thromboxane A2-prostaglandin endoperoxide receptor activation in vivo. J Clin Invest. 1986 Feb;77(2):496–502. doi: 10.1172/JCI112329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Fragetta J., FitzGerald G. A. Prostaglandin endoperoxides modulate the response to thromboxane synthase inhibition during coronary thrombosis. J Clin Invest. 1988 Nov;82(5):1708–1713. doi: 10.1172/JCI113784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald D. J., Wright F., FitzGerald G. A. Increased thromboxane biosynthesis during coronary thrombolysis. Evidence that platelet activation and thromboxane A2 modulate the response to tissue-type plasminogen activator in vivo. Circ Res. 1989 Jul;65(1):83–94. doi: 10.1161/01.res.65.1.83. [DOI] [PubMed] [Google Scholar]
- Folts J. D., Gallagher K., Rowe G. G. Blood flow reductions in stenosed canine coronary arteries: vasospasm or platelet aggregation? Circulation. 1982 Feb;65(2):248–255. doi: 10.1161/01.cir.65.2.248. [DOI] [PubMed] [Google Scholar]
- Francis C. W., Markham R. E., Jr, Barlow G. H., Florack T. M., Dobrzynski D. M., Marder V. J. Thrombin activity of fibrin thrombi and soluble plasmic derivatives. J Lab Clin Med. 1983 Aug;102(2):220–230. [PubMed] [Google Scholar]
- Gold H. K., Coller B. S., Yasuda T., Saito T., Fallon J. T., Guerrero J. L., Leinbach R. C., Ziskind A. A., Collen D. Rapid and sustained coronary artery recanalization with combined bolus injection of recombinant tissue-type plasminogen activator and monoclonal antiplatelet GPIIb/IIIa antibody in a canine preparation. Circulation. 1988 Mar;77(3):670–677. doi: 10.1161/01.cir.77.3.670. [DOI] [PubMed] [Google Scholar]
- Golino P., Ashton J. H., Glas-Greenwalt P., McNatt J., Buja L. M., Willerson J. T. Mediation of reocclusion by thromboxane A2 and serotonin after thrombolysis with tissue-type plasminogen activator in a canine preparation of coronary thrombosis. Circulation. 1988 Mar;77(3):678–684. doi: 10.1161/01.cir.77.3.678. [DOI] [PubMed] [Google Scholar]
- Granström E., Diczfalusy U., Hamberg M., Hansson G., Malmsten C., Samuelsson B. Thromboxane a2: biosynthesis and effects on platelets. Adv Prostaglandin Thromboxane Leukot Res. 1982;10:15–58. [PubMed] [Google Scholar]
- Heldebrant C. M., Butkowski R. J., Bajaj S. P., Mann K. G. The activation of prothrombin. II. Partial reactions, physical and chemical characterization of the intermediates of activation. J Biol Chem. 1973 Oct 25;248(20):7149–7163. [PubMed] [Google Scholar]
- Kaplan K., Davison R., Parker M., Mayberry B., Feiereisel P., Salinger M. Role of heparin after intravenous thrombolytic therapy for acute myocardial infarction. Am J Cardiol. 1987 Feb 1;59(4):241–244. doi: 10.1016/0002-9149(87)90792-2. [DOI] [PubMed] [Google Scholar]
- Kikumoto R., Tamao Y., Tezuka T., Tonomura S., Hara H., Ninomiya K., Hijikata A., Okamoto S. Selective inhibition of thrombin by (2R,4R)-4-methyl-1-[N2-[(3-methyl-1,2,3,4-tetrahydro-8-quinolinyl++ +) sulfonyl]-l-arginyl)]-2-piperidinecarboxylic acid. Biochemistry. 1984 Jan 3;23(1):85–90. doi: 10.1021/bi00296a014. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Kitani M., Yamaguchi S., Suzuki T., Okada K., Tsunematsu T. Effects of an antithrombotic agent (MD-805) on progressing cerebral thrombosis. Thromb Res. 1989 Feb 1;53(3):305–317. doi: 10.1016/0049-3848(89)90105-9. [DOI] [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lee C. D., Mann K. G. Activation/inactivation of human factor V by plasmin. Blood. 1989 Jan;73(1):185–190. [PubMed] [Google Scholar]
- Loscalzo J., Braunwald E. Tissue plasminogen activator. N Engl J Med. 1988 Oct 6;319(14):925–931. doi: 10.1056/NEJM198810063191407. [DOI] [PubMed] [Google Scholar]
- Marder V. J., Sherry S. Thrombolytic therapy: current status (1). N Engl J Med. 1988 Jun 9;318(23):1512–1520. doi: 10.1056/NEJM198806093182306. [DOI] [PubMed] [Google Scholar]
- Miletich J. P., Jackson C. M., Majerus P. W. Interaction of coagulation factor Xa with human platelets. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4033–4036. doi: 10.1073/pnas.74.9.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak J., Murray J. J., Oates J. A., FitzGerald G. A. Biochemical evidence of a chronic abnormality in platelet and vascular function in healthy individuals who smoke cigarettes. Circulation. 1987 Jul;76(1):6–14. doi: 10.1161/01.cir.76.1.6. [DOI] [PubMed] [Google Scholar]
- Plow E. F., McEver R. P., Coller B. S., Woods V. L., Jr, Marguerie G. A., Ginsberg M. H. Related binding mechanisms for fibrinogen, fibronectin, von Willebrand factor, and thrombospondin on thrombin-stimulated human platelets. Blood. 1985 Sep;66(3):724–727. [PubMed] [Google Scholar]
- Roberts L. J., 2nd, Sweetman B. J., Oates J. A. Metabolism of thromboxane B2 in man. Identification of twenty urinary metabolites. J Biol Chem. 1981 Aug 25;256(16):8384–8393. [PubMed] [Google Scholar]
- Romson J. L., Haack D. W., Lucchesi B. R. Electrical induction of coronary artery thrombosis in the ambulatory canine: a model for in vivo evaluation of anti-thrombotic agents. Thromb Res. 1980 Mar 15;17(6):841–853. doi: 10.1016/0049-3848(80)90249-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Rosing J., van Rijn J. L., Bevers E. M., van Dieijen G., Comfurius P., Zwaal R. F. The role of activated human platelets in prothrombin and factor X activation. Blood. 1985 Feb;65(2):319–332. [PubMed] [Google Scholar]
- Schafer A. I., Maas A. K., Ware J. A., Johnson P. C., Rittenhouse S. E., Salzman E. W. Platelet protein phosphorylation, elevation of cytosolic calcium, and inositol phospholipid breakdown in platelet activation induced by plasmin. J Clin Invest. 1986 Jul;78(1):73–79. doi: 10.1172/JCI112576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamao Y., Yamamoto T., Kikumoto R., Hara H., Itoh J., Hirata T., Mineo K., Okamoto S. Effect of a selective thrombin inhibitor MCI-9038 on fibrinolysis in vitro and in vivo. Thromb Haemost. 1986 Aug 20;56(1):28–34. [PubMed] [Google Scholar]
- Teitel J. M., Rosenberg R. D. Protection of factor Xa from neutralization by the heparin-antithrombin complex. J Clin Invest. 1983 May;71(5):1383–1391. doi: 10.1172/JCI110891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol E. J., George B. S., Kereiakes D. J., Stump D. C., Candela R. J., Abbottsmith C. W., Aronson L., Pickel A., Boswick J. M., Lee K. L. A randomized controlled trial of intravenous tissue plasminogen activator and early intravenous heparin in acute myocardial infarction. Circulation. 1989 Feb;79(2):281–286. doi: 10.1161/01.cir.79.2.281. [DOI] [PubMed] [Google Scholar]
- Yasuda T., Gold H. K., Fallon J. T., Leinbach R. C., Guerrero J. L., Scudder L. E., Kanke M., Shealy D., Ross M. J., Collen D. Monoclonal antibody against the platelet glycoprotein (GP) IIb/IIIa receptor prevents coronary artery reocclusion after reperfusion with recombinant tissue-type plasminogen activator in dogs. J Clin Invest. 1988 Apr;81(4):1284–1291. doi: 10.1172/JCI113446. [DOI] [PMC free article] [PubMed] [Google Scholar]


