Abstract
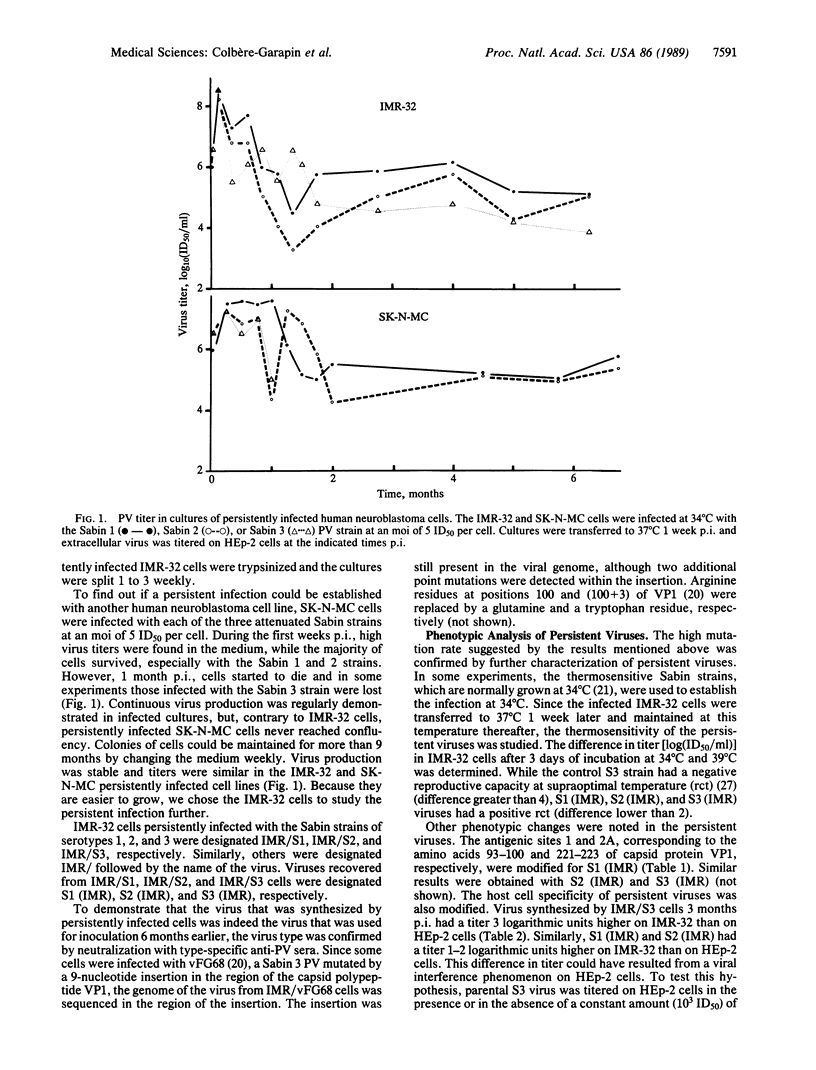
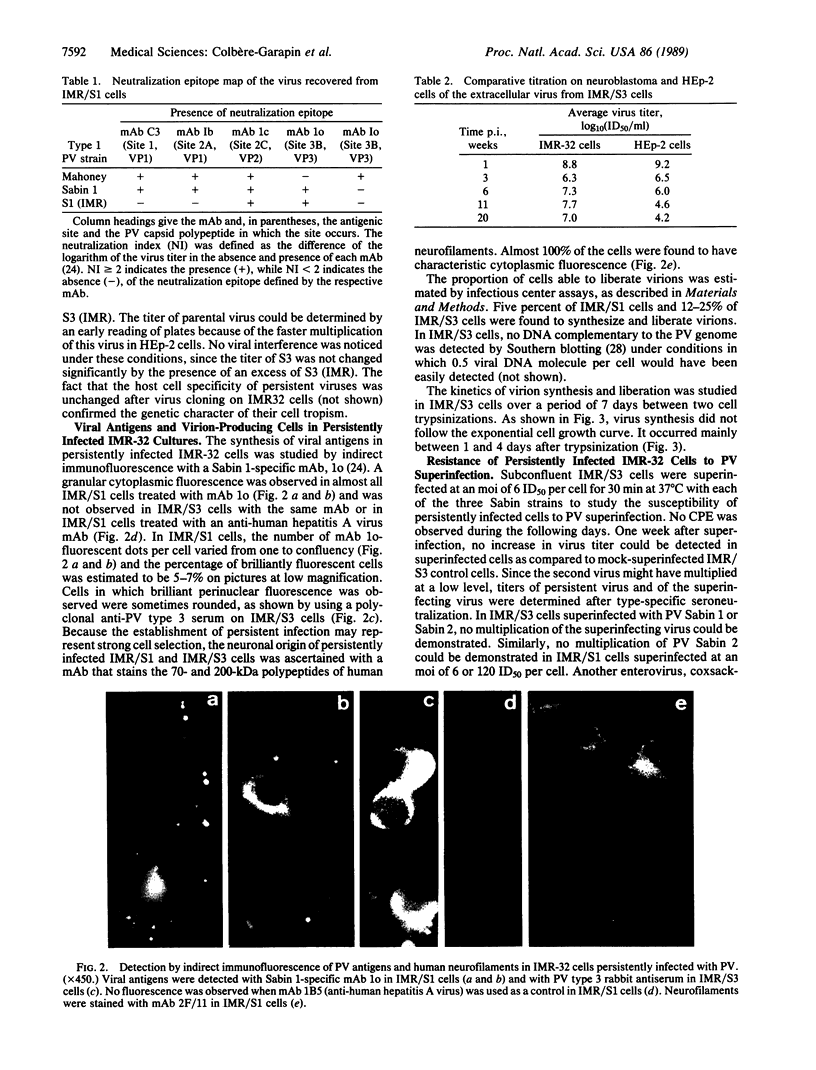
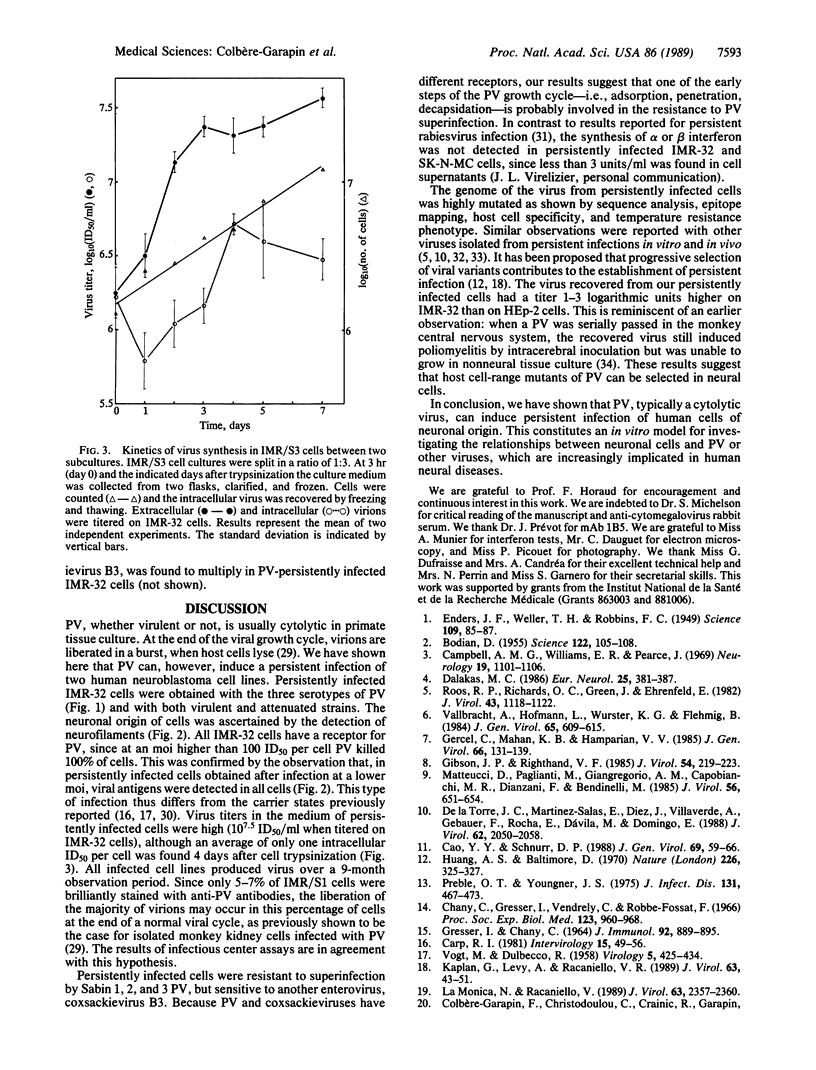
Two human neuroblastoma cell lines were persistently infected with poliovirus strains of all three serotypes. In persistently infected IMR-32 cells, which were studied in greatest detail, viral antigens were present in most cells, and over a 9-month period virions were found in the medium at high titers. Persistently infected cells were resistant to superinfection by Sabin 1, 2, and 3 poliovirus but sensitive to coxsackievirus B3. The viruses recovered from persistently infected cells were studied for conservation of epitopes, host cell specificity, and temperature resistance phenotype. The antigenic site 1 carried by the major capsid protein VP1 was modified on the persistent viruses of all three serotypes. This was confirmed for one virus by sequencing the corresponding genomic region in which two mutations were detected. The titers of persistent viruses were 1-3 log10 units higher on IMR-32 cells than on nonneuronal HEp-2 cells, while parental viruses had similar titers on both lines. When thermosensitive viruses were used to initiate the infection, the persistent viruses were found to be thermoresistant at 39 degrees C. Together the results indicate that the persistent infection correlated with the selection of highly mutated viral strains. Poliovirus-infected neuroblastoma cell lines thus constitute an in vitro model of chronic viral infections, which are increasingly implicated in human neural diseases.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., KURTZ H. Observations concerning a persisting infection of HeLa cells with poliomyelitis virus. J Exp Med. 1955 Nov 1;102(5):555–565. doi: 10.1084/jem.102.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODIAN D. Emerging concept of poliomyelitis infection. Science. 1955 Jul 15;122(3159):105–108. doi: 10.1126/science.122.3159.105. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Helson L., Spengler B. A. Morphology and growth, tumorigenicity, and cytogenetics of human neuroblastoma cells in continuous culture. Cancer Res. 1973 Nov;33(11):2643–2652. [PubMed] [Google Scholar]
- Blondel B., Akacem O., Crainic R., Couillin P., Horodniceanu F. Detection by monoclonal antibodies of an antigenic determinant critical for poliovirus neutralization present on VP1 and on heat-inactivated virions. Virology. 1983 Apr 30;126(2):707–710. doi: 10.1016/s0042-6822(83)80027-0. [DOI] [PubMed] [Google Scholar]
- Campbell A. M., Williams E. R., Pearce J. Late motor neuron degeneration following poliomyelitis. Neurology. 1969 Nov;19(11):1101–1106. doi: 10.1212/wnl.19.11.1101. [DOI] [PubMed] [Google Scholar]
- Cao Y., Schnurr D. P. Persistent infection of YAC-1 cells by coxsackievirus B3. J Gen Virol. 1988 Jan;69(Pt 1):59–65. doi: 10.1099/0022-1317-69-1-59. [DOI] [PubMed] [Google Scholar]
- Carp R. I. Persistent infection of human lymphoid cells with poliovirus and development of temperature-sensitive mutants. Intervirology. 1981;15(1):49–56. doi: 10.1159/000149214. [DOI] [PubMed] [Google Scholar]
- Chany C., Gresser I., Vendrely C., Robbe-Fossat F. Persistent polioviral infection of the intact amniotic membrane. II. Existence of a mechanical barrier to viral infection. Proc Soc Exp Biol Med. 1966 Dec;123(3):960–968. doi: 10.3181/00379727-123-31648. [DOI] [PubMed] [Google Scholar]
- Crainic R., Couillin P., Blondel B., Cabau N., Boué A., Horodniceanu F. Natural variation of poliovirus neutralization epitopes. Infect Immun. 1983 Sep;41(3):1217–1225. doi: 10.1128/iai.41.3.1217-1225.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalakas M. C. New neuromuscular symptoms in patients with old poliomyelitis: a three-year follow-up study. Eur Neurol. 1986;25(5):381–387. doi: 10.1159/000116038. [DOI] [PubMed] [Google Scholar]
- Davis L. E., Bodian D., Price D., Butler I. J., Vickers J. H. Chronic progressive poliomyelitis secondary to vaccination of an immunodeficient child. N Engl J Med. 1977 Aug 4;297(5):241–245. doi: 10.1056/NEJM197708042970503. [DOI] [PubMed] [Google Scholar]
- Enders J. F., Weller T. H., Robbins F. C. Cultivation of the Lansing Strain of Poliomyelitis Virus in Cultures of Various Human Embryonic Tissues. Science. 1949 Jan 28;109(2822):85–87. doi: 10.1126/science.109.2822.85. [DOI] [PubMed] [Google Scholar]
- GRESSER I., CHANY C. MULTIPLICATION OF POLIOVIRUS TYPE I IN PREPARATIONS OF HUMAN LEUKOCYTES AND ITS INHIBITION BY INTERFERON. J Immunol. 1964 Jun;92:889–895. [PubMed] [Google Scholar]
- Gercel C., Mahan K. B., Hamparian V. V. Preliminary characterization of a persistent infection of HeLa cells with human rhinovirus type 2. J Gen Virol. 1985 Jan;66(Pt 1):131–139. doi: 10.1099/0022-1317-66-1-131. [DOI] [PubMed] [Google Scholar]
- Gibson J. P., Righthand V. F. Persistence of echovirus 6 in cloned human cells. J Virol. 1985 Apr;54(1):219–223. doi: 10.1128/jvi.54.1.219-223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y., Kawai A., Matsumoto S. Persistent infection of rabies virus (HEP-Flury strain) in human neuroblastoma cells capable of producing interferon. J Gen Virol. 1985 May;66(Pt 5):957–967. doi: 10.1099/0022-1317-66-5-957. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D. Defective viral particles and viral disease processes. Nature. 1970 Apr 25;226(5243):325–327. doi: 10.1038/226325a0. [DOI] [PubMed] [Google Scholar]
- Kaplan G., Levy A., Racaniello V. R. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J Virol. 1989 Jan;63(1):43–51. doi: 10.1128/jvi.63.1.43-51.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LWOFF A., DULBECCO R., VOGT M., LWOFF M. Kinetics of the release of poliomyelitis virus from single cells. Virology. 1955 May;1(1):128–139. doi: 10.1016/0042-6822(55)90010-6. [DOI] [PubMed] [Google Scholar]
- La Monica N., Racaniello V. R. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J Virol. 1989 May;63(5):2357–2360. doi: 10.1128/jvi.63.5.2357-2360.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteucci D., Paglianti M., Giangregorio A. M., Capobianchi M. R., Dianzani F., Bendinelli M. Group B coxsackieviruses readily establish persistent infections in human lymphoid cell lines. J Virol. 1985 Nov;56(2):651–654. doi: 10.1128/jvi.56.2.651-654.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Roos R. P., Richards O. C., Green J., Ehrenfeld E. Characterization of a cell culture persistently infected with the DA strain of Theiler's murine encephalomyelitis virus. J Virol. 1982 Sep;43(3):1118–1122. doi: 10.1128/jvi.43.3.1118-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhon E. J., Wilson A. K., Jubelt B. Characterization of genetic changes occurring in attenuated poliovirus 2 during persistent infection in mouse central nervous systems. J Virol. 1984 Apr;50(1):137–144. doi: 10.1128/jvi.50.1.137-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- VOGT M., DULBECCO R. Properties of a HeLa cell culture with increased resistance to poliomyelitis virus. Virology. 1958 Jun;5(3):425–434. doi: 10.1016/0042-6822(58)90037-0. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Hofmann L., Wurster K. G., Flehmig B. Persistent infection of human fibroblasts by hepatitis A virus. J Gen Virol. 1984 Mar;65(Pt 3):609–615. doi: 10.1099/0022-1317-65-3-609. [DOI] [PubMed] [Google Scholar]
- de la Torre J. C., Martínez-Salas E., Diez J., Villaverde A., Gebauer F., Rocha E., Dávila M., Domingo E. Coevolution of cells and viruses in a persistent infection of foot-and-mouth disease virus in cell culture. J Virol. 1988 Jun;62(6):2050–2058. doi: 10.1128/jvi.62.6.2050-2058.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



