Since the earliest reports by White68 and Faber,19 it has been known that certain mechanical intestinal abnormalities could cause a hematologic syndrome which resembles pernicious anemia, and to date at least 100 examples have been reported. Location and etiology of the intestinal lesion varied, but the end result was stasis and retarded drainage from some portion of the gastrointestinal tract. Cases in which the stagnant loop was associated with certain specific absorption defects have been classified as examples of the blind-loop syndrome.6, 14, 26 It is probable that only a small number of patients with anatomic blind loops develop the blind-loop syndrome with its metabolically important changes.
Two of the most common operations performed today in which potential stagnant loops are created are Billroth II gastrectomy and gastroenterostomy. The duodenum is converted into a side arm which rejoins the main intestinal tract at the gastrojejunostomy. It is common knowledge that this afferent loop occasionally drains improperly.15, 30, 32, 37, 41, 42, 44, 46, 48, 53, 57, 58, 66, 67 However, prior to 1953, there were no well-documented reports of the blind-loop syndrome after either operation. Since that time, several examples of the blind-loop syndrome have been described after Billroth II resection or gastroenterostomy. The fact that only a handful of these cases has yet been reported may be due to the subtlety of the clinical manifestations, the difficulty of clearly establishing a diagnosis, and the general unawareness of this complication as a diagnostic possibility. In addition to its immediate clinical application, a consideration of the blind-loop syndrome may cast some light upon the nutritional superiority of the Billroth I over the Billroth II gastrectomy.
ANATOMIC CONFIGURATION OF BLIND LOOPS
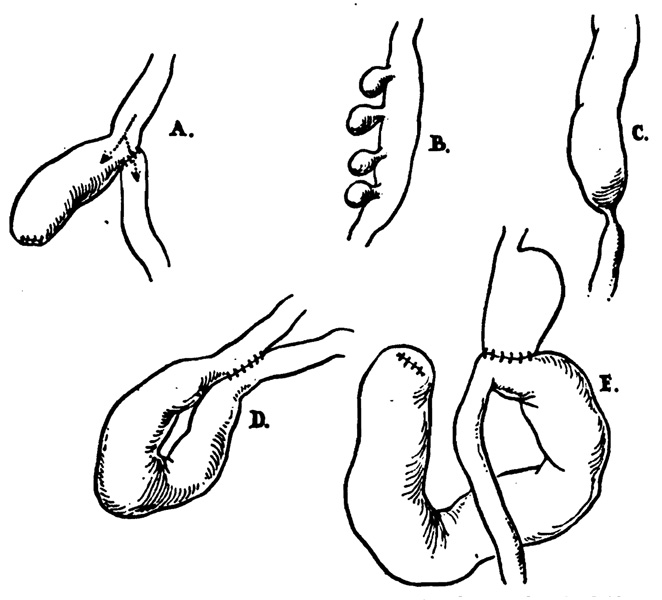
Various examples of gastrointestinal blind loops are shown in Fig. 1 for comparison with the blind loops observed after gastrectomy. These have occurred6, 26 after creation of dead-end segments by anastomoses (Fig. 1, A), with jejunal diverticulosis (Fig. 1, B), with intestinal strictures which have most commonly been tuberculous in origin (Fig. 1, C), and after enteroenterostomies and fistulas (Fig. 1, D).
Fig. 1.
Different types of gastrointestinal blind loops which have caused the blind-loop syndrome. A, Anastomosis with formation of a self-filled stagnant loop, B, with jejunal diverticulosis, C, with intestinal strictures, D, after enteroenterostomies or fistulas, and E, after gastric operation.
In experimental studies of the blind-loop syndrome by Tonnis and Brusis,61 Pearse,47 Watson and associates,64 Taylor,59 and Toon and Wangensteen,60 a side-arm loop has been employed as in Fig. 1, A, in which the loop is arranged so as to be self-filling. The side loop arrangement is the one which most resembles a blind loop which develops after gastrojejunostomy (Fig. 1, E).
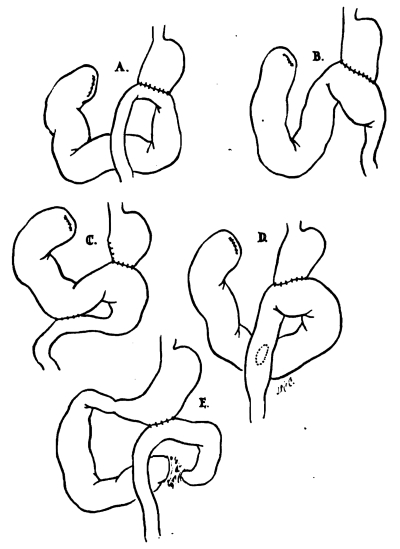
The structural conditions necessary for the blind-loop syndrome are not present after the Billroth I anastomosis. In all reported cases there has been either a Billroth II resection (8 patients) or a gastroenterostomy (1 patient). The anastomoses (Fig. 2, A and B) were both antiperistaltic, with the afferent loop to the greater curvature,25, 45 and isoperistaltic.13, 30, 45 In 2 cases (Fig. 2, C and D), an enterostomy had also been performed.1, 49 Commonly, the afferent loop was excessively long and dilated.25, 30, 45 The exact site of obstruction was sometimes difficult to define by roentgenogram or even at operation.13, 45, 49 Usually, the distended loop ended abruptly at the gastrojejunostomy, but in some cases it extended beyond this. Excessively long afferent loops, kinking at the site of anastomosis, and partially obstructing adhesions have all been described as the factor causing blind-loop stasis.1, 13, 25, 30, 45, 49
Fig. 2.
Anatomic conditions after gastric operation which have caused blind-loop syndrome. A, 3 cases, B, 3 cases, C, 1 case, D, 1 case, and E, 1 case.
ABNORMALITIES IN VITAMIN B12 METABOLISM IN BLOOD-LOOP SYNDROME
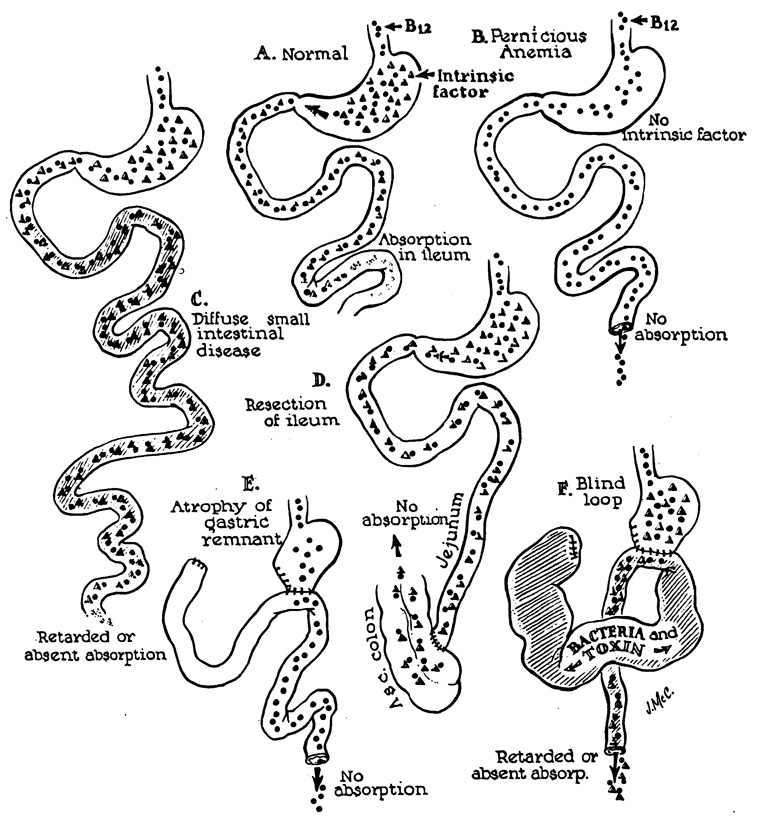
General information concerning the mechanism of the blind-loop syndrome has accumulated from observations on lesions at differing sites in the gastrointestinal tract. The principles involved apply, with variations, to blind loops at all levels. The best-known feature of the blind-loop syndrome is megaloblastic anemia, which is due to disruption of vitamin B12 absorption. Normally, dietary vitamin B12 (Castle’s extrinsic factor) is absorbed after an incompletely understood interaction with intrinsic factor (Fig. 3, A), a mucoprotein secreted by the gastric mucosa.22 In man, the principal site of B12 absorption is the ileum.7, 8
Fig. 3.
Mechanisms of B12 utilization in normal and diseased patients.
Vitamin B12 deficiency can develop by a number of alternative mechanisms. Rarely is there dietary deficiency of this nutritional factor. Commonly, as in pernicious anemia (Fig. 3, B) or after total gastrectomy,39 there is absent intrinsic factor due to gastric atrophy or the absence of the stomach, respectively. Malabsorption can also occur4, 23, 26, 27, 28, 35, 40, 43, 52, 54 with adequate intrinsic factor and dietary vitamin B12 in patients who have undergone ileal resection (Fig. 3, D) or who have diffuse small bowel disease (Fig. 3, C). Here, the malabsorption is due to damage or removal of the normal site of absorption. Vitamin B12 deficiency also develops in the blind-loop syndrome despite the presence of intrinsic factor and dietary B12 (Fig. 3, F). The malabsorption in this circumstance is thought to be due to bacterial overgrowth in a poorly emptying blind loop with consequent interference with B12 absorption in the remainder of the intestinal tract.19, 25, 26, 50, 55, 56, 60, 65, 69 As with pernicious anemia, patients with blind-loop syndrome can proceed to subacute combined degeneration of the spinal cord.1, 6, 26, 49
Virtually all authorities agree that a change in the bacterial flora of the torpid loop is responsible for the B12 malabsorption. Much of the evidence is based upon clinical impression, but there is solid experimental work to support this opinion. Seyderhelm,55 employing strictures to study the blind-loop effect in dogs, related the presence or absence of infection above the stenosis to the development or absence of anemia. Watson and Witts65 have demonstrated in rats that the bacterial flora of small intestinal blind loops resembles that normally found in the colon. They also showed that the bacterial growth in the small bowel distal to the blind loop was changed with a reduction in Lactobacilli and increases in Escherichia coli and alpha hemolytic streptococci. Perhaps the most conclusive evidence for the bacterial etiology of the malabsorption was provided by Toon and Wangensteen,60 and later confirmed by Watson and Witts.65 These authors showed that the anemia of the experimental blind loop syndrome could be prevented by oral administration of chlortetracycline. The therapeutic value of antibiotics in man has been confirmed by Siruala and Kaipainen56 and numerous other observers,1, 3, 17, 25, 26, 28, 30, 34, 43, 45, 49, 50, 54 who noted that certain antibiotics could not only prevent the development of, but also reverse, B12 deficiency by restoring normal absorption of this vitamin. The application of these disclosures to the diagnosis and treatment of the blind-loop syndrome will be discussed subsequently.
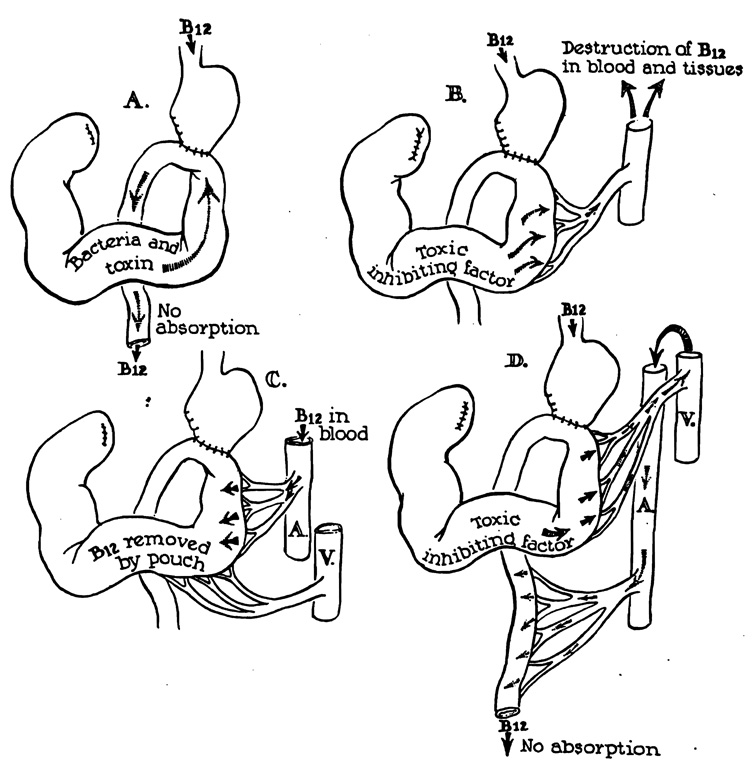
Despite the generous support accorded the bacterial theory, the precise mechanism of vitamin B12 malabsorption is not known. One widely accepted theory is that propounded by Witts69 in which B12 metabolism is supposedly affected by direct alimentary contamination of bacteria or their toxic byproducts which spill out of the stagnant loop (Fig. 4, A). Since certain strains of Escherichia coli and Streptococcus fecalis metabolize folic acid or vitamin B12, 11, 51 it has been suggested that the anomalously located microorganisms use up the available oral supply. Contrary to this reasoning is the fact that Neomycin and sulphonamides, which are nonabsorbable and which sterilize the nonstagnant intestine are of no therapeutic value17, 25, 26 despite the fact that they would be expected to come into contact with efflux from the loop. In contrast, oral antibiotics such as chlortetracycline, oxytetracycline, and tetracycline can restore normal absorption of B12.
Fig. 4.
Various hypotheses to explain malabsorption of vitamin B12.
However, if the offending agent were a toxin, its production in a sequestered loop would be suspended only with a systemic antibiotic. Drexler18 has shown on the basis of in vitro experiments that indole compounds are able to inhibit normal utilization of vitamin B12, and he has suggested that indole might be one of the blind-loop “toxins.” Recently, Hoffman and Spiro30 have failed to support either the bacterial or toxin theory of direct intestinal contamination. They instilled into a normal patient’s stomach the contents of a resected blind loop with Co60-tagged vitamin B12. B12 absorption was not depressed.
Other theories to explain the B12 deficiency involve blood-stream mechanisms. Ungley62 suggested that a toxin was absorbed from the blind loop which destroyed the vitamin in the blood and tissues (Fig. 4, B). He supported this opinion by the demonstration that plasma added from a resected blind loop suspended megaloblastic maturation in bone marrow culture. Card16 also proposed that B12 was actually absorbed, but that increments were returned by enteric recirculation (Fig. 4, C) to the blind loop and destroyed. Both theories were weakened by the results of studies of urinary and fecal excretion of radioactive vitamin B12 which show that the block in metabolism is primarily at, rather than after, the absorption phase.1, 3, 17, 25, 26, 28, 30, 34, 40, 43, 45, 49, 50, 54
An unexplored possibility is that bacterial toxins are picked up from the blind loop and circulated to the uninvolved portion of the small bowel where they alter the absorptive capacity (Fig. 4, D). Such a hypothesis is compatible with the evidence obtained from antibiotic therapy, and with present knowledge of the fundamental defect in B12 utilization.
OTHER NUTRITIONAL DEFICIENCIES IN BLIND-LOOP SYNDROME
Absorption of other nutritional substances is often impaired. Fat is probably the most commonly affected. Using experimental midintestinal loops, Aitken and colleagues2 found that virtually all rats exhibited steatorrhea whether or not the animals became anemic. The development of anemia alone, without steatorrhea, was uncommon.
As with vitamin B12 deficiency, it is thought that bacterial overgrowth in the blind loop is the causative factor in the steatorrhea. Specific evidence has been presented by Sammons51 and by Goldstein and his group25 that the bacterial growth adversely affects fat utilization. The latter authors have shown that the steatorrhea of the blind-loop syndrome is favorably influenced by antibiotics.
Why some blind loops do and others do not cause steatorrhea is not known. There is some evidence that the location of the blind loop is influential in this respect. Booth7 has pointed out that a blind loop of the ileum, where normal vitamin B12 absorption occurs, may lead to pure B12 deficiency. Blind loops of the jejunum, where fat is normally absorbed,7, 9, 25, 33 usually produce prominent steatorrhea.4, 54, 63 While such localization is admittedly crude, it may help to explain differences in the malabsorption defect in different cases. Of 9 patients who had blind-loop syndromes after gastric operations, 7 had steatorrhea.
Other nutritional deficiencies are also common. Numerous cases of protein deficiency have been noted with blind loops at various levels, including the blind loops after gastric operations.13, 25, 45 Usually this is reflected in low plasma protein levels, but 1 example of frank kwashiorkor has been reported in a patient with an intestinal blind loop.36 Pellagra and vitamin C and vitamin K deficiencies have been recorded. Badenach3 has warned against the dangerous complication of spontaneous retroperitoneal hemorrhage resulting from vitamin K deficiency.
INCIDENCE OF BLIND-LOOP SYNDROME AFTER GASTRIC OPERATIONS
The blind-loop syndrome has not been thoroughly evaluated as a cause of poor results after gastric operations. It is probable that this complication is more important than the 9 recorded cases1, 13, 25, 30, 45, 49 indicate. For example, Kinsella32 recently reported 7 cases which are probably examples of the blind-loop syndrome, but there is insufficient data to be certain of this. Several factors make the diagnosis an obscure and difficult one which is apt to be overlooked. First, the interval between operation and the onset of symptoms can be prolonged for many years, which tends to minimize the etiologic role of the remote operation in relation to the presenting complaints. In addition, the symptoms often are subtle and nonspecific and resemble psychoneurotic complaints.
Undoubtedly, an additional hindrance has been the preoccupation with macrocytic anemia as a prerequisite for this diagnosis. In point of fact, this feature may be absent as it was in 7 of the 9 cases collected in this review. Macrocytic anemia was frequently masked by prior vitamin B12 therapy or by the presence of other and equally important absorption defects of fat, iron, and other materials. Even in the untreated patient, the body stores of vitamin B12 are not dissipated for 3 to 4 years in the complete absence of B12 intake,20, 21, 23, 28 so the development of megaloblastic anemia is a late manifestation. However, techniques and knowledge acquired in the past few years allow the blind-loop syndrome to be defined in terms of specific and measurable parameters of malabsorption rather than in terms of their end results. These techniques, outlined in the section on diagnosis, should increase the frequency and accuracy of detection.
It should be emphasized not only that macrocytic anemia is not a prerequisite to the diagnosis of the blind-loop syndrome, but also that most cases of megaloblatic anemia after gastric operations are not due to this cause. McLean39 has summarized the results of investigations of patients with macrocytic anemia which developed after gastroenterostomy or partial gastrectomy. Almost invariably, these patients have developed atrophy of the gastric remnant with loss of the intrinsic factor (Fig. 3, E). Differentiating patients with the blind loop syndrome from those with gastric atrophy is a crucial step in correct diagnosis.
CLINICAL MANIFESTATIONS OF BLIND-LOOP SYNDROME AFTER GASTRIC OPERATIONS
The clinical features of the blind-loop syndrome are seldom overt. The diagnosis has usually been made only after months or years of disability. Commonly, a gastroenterostomy or Billroth II gastric resection performed as long as 26 years previously had initially been considered to have a good result. When anemia, weight loss, malaise, hypoproteinemia, steatorrhea, or neurologic complaints developed, the possible relation to the previous operation was often overlooked.
Anemia was present in all cases.1, 13, 25, 30, 45, 49 In only 2, however, was it macrocytic in type.1, 45 Steatorrhea was the next most common feature and was found in 7 patients.1, 13, 25, 45 Five of these had diarrhea. Some evidence of malnutrition was always present, and 5 patients had edema or hypoproteinemia.13, 25, 45 Glossitis, blepharitis, cheilosis, or other signs of vitamin deficiency were seen in 3 patients. Neurologic findings suggestive of subacute combined degeneration of the cord were present in 3 patients with calf pain, changes in deep tendon reflexes, and decreased sense of vibration and proprioception.1, 13, 49
Vomiting and abdominal pain were reported in l30 and 31, 13, 30 cases, respectively. The vomitus consisted of pure bile or foul brown material, and the emesis probably resulted from convulsive emptying of the blind loop. Sporadic bilious vomiting is known to be a characteristic symptom in patients with an obstructed or distended afferent loop,15, 30, 32, 37, 41, 42, 44, 46, 48, 53, 57, 58, 66, 67 and it is possible that this symptom will prove to be common as more information accumulates concerning the blind-loop syndrome.
DIAGNOSIS
Radiologic studies may be helpful in establishing the presence of dilatation or stasis in the afferent loop. In some cases, barium entered a dilated, tortuous loop.25, 30, 45 In others, barium was held up, often for many hours, in an unidentifiable pouch (Fig. 5) near the gastroenterostomy.13, 49 In some, barium did not enter the afferent loop.49 In a recent study of afferent loop obstruction, Kinsella and Hennessy32 reported the various radiologic features of afferent loop obstruction to be (1) absence of barium passage into afferent loop, (2) passage of barium for 1 to 2 cm. into the afferent loop with an abrupt halt at an obstruction, and (3) pendulum effect with passage into the afferent loop and remittent emptying back into the stomach.
Fig. 5.
Gastrointestinal series in patient with blind loop syndrome after Billroth II gastrectomy. Note pouch at gastrojejunostomy site and dilated and elongated afferent loop (left). Barium remained in pouch for 6 hours.
During the last 10 years, laboratory techniques have been developed which allow the diagnosis of the blind-loop syndrome to be made with increasing precision. First among these are tests employing Co60-tagged vitamin B12 either for determination of fecal excretion,29 hepatic uptake,24 or urinary excretion.52 The urinary excretion method, the Schilling test,52 is the most widely used. The patient is given 0.5 to 1.0 µg of Co60-tagged B12 orally, and 2 hours later a flushing dose of 1,000 µg of nontagged B12 is given intramuscularly or subcutaneously. The amount of radioactivity in the ensuing 24-hour urine specimen provides an indirect measure of the amount of oral Co60-tagged B12 absorbed. Normally 8 to 40 per cent of the Co60-tagged B12 is excreted in the urine.
When urinary excretion of the Co60-tagged B12 is subnormal or absent, the test is repeated with concomitant oral administration of intrinsic factor. In cases of pernicious anemia and in postoperative patients in whom gastric atrophy has occurred, urinary excretion will be restored to normal, and the diagnosis of blind-loop syndrome is excluded.5, 10, 23, 38, 39
If the combination of Co60-tagged B12 and intrinsic factor does not increase urinary excretion, the blind-loop syndrome becomes a strong possibility. Co60-tagged B12 is again given, this time after several days of therapy with tetracycline, chlortetracycline, or oxytetracycline. In many cases, the antibiotics restore absorption and the urinary excretion will become normal. Should this occur, the diagnosis of blind-loop syndrome is virtually established.1, 3, 17, 25, 26, 28, 30, 34, 43, 45, 49, 50, 54 Failure of the antibiotics may be due to Whipple’s disease, sprue, or some other diffuse intestinal disease, or to surgical absence of the ileum. It does not preclude the possibility of the blind-loop syndrome, however, since the bacteria of the blind loop may not be sensitive to the antibiotics used.13, 17, 25, 43
Experimental and clinical data previously alluded to emphasize the importance of studying the absorption of other substances, particularly fat. Jackson and Linder31 and Butler and associates12 recognized the resemblance of gastroenterostomy and Billroth II gastrectomy to the better-known intestinal blind loops. They speculated that bacterial growth in the afferent limb might account for the higher incidence of steatorrhea after Billroth II than after Billroth I gastrectomy. Recently, Goldstein and his colleagues25 studied bacterial counts by afferent limb intubation in postoperative patients, and demonstrated a strong correlation between the degree of contamination in the afferent loop and the magnitude of steatorrhea. They also showed that antibiotic therapy reduced steatorrhea, presumably by the same mechanism as B12 absorption is improved in other blind loops. This type of testing may prove to be of great value in the study of postgastrectomy malabsorption syndromes and in the detection of afferent blind loops.
Other tests may be useful in individual cases. Gastric analysis for acid should always be carried out, since the presence of free acid excludes gastric atrophy as a cause of B12 malabsorption. Badenach5 has performed endoscopic gastric biopsy and studied urinary uropepsinogen in order to evaluate the secretory capacity of the stomach.
In the diagnosis of the blind-loop syndrome, reliance upon a single symptom, finding, or abnormality of absorption is not wise. The absorption defects are frequently multiple, and other deficiencies may be of greater importance than those which would lead to a specific hematologic picture. Unification of the different aspects presented by the blind-loop syndrome into a well-understood entity would undoubtedly lead to a higher rate of recognition of this complication of gastric operations.
TREATMENT OF THE BLIND-LOOP SYNDROME AFTER GASTRIC OPERATION
Medical therapy can temporarily improve the health of patients with the blind-loop syndrome. With tetracycline, chlortetracycline, oxytetracycline, chloramphenicol, and possibly other antibiotics, the absorption of vitamin B12, fat, and probably other nutrients may be restored toward normal. The improvement may outlast a course of antibiotic therapy by weeks or months,1, 25, 54 but relapse eventually is expected. Specific vitamin deficiencies, especially of B12, should be corrected. High protein, high calorie diet, correction of low blood volume, and restoration of electrolytes are other adjuncts.
Definitive therapy requires surgical correction of the blind afferent loop. In the 9 collected cases, 6 patients had been operated upon at the time of the report.13, 25, 30, 45, 49 In 4, the Billroth II anastomosis was taken down and converted to a Billroth I,13, 25, 45, 49 and in a fifth similar reconstruction was carried out with the interposition of a jejunal segment between the stomach and duodenum.45 A good result was obtained in all with complete or partial correction of the pre-existing absorption defects. In the sixth case, the dilated and elongated afferent loop was resected and a short-loop Billroth II anastomosis performed with a good result.30 From a mechanical point of view, other operations have been proposed for the treatment of afferent loop obstruction, such as enteroenterostomy, jejunoplasty, support of the afferent loop by suture, and defunctionalization of the afferent loop by Roux Y anastomosis. There are objections to each of these operations, especially in terms of late results, and they probably should not be used.
Acknowledgments
Aided by United States Public Health Service Grant No. A-3176.
REFERENCES
- 1.Adams JF. Postgastrectomy megaloblastic anemia and the loop syndrome. Gastroenterologia. 1958;89:326. doi: 10.1159/000201845. [DOI] [PubMed] [Google Scholar]
- 2.Aitken MA, Badenoch J, Spray GH. Fat excretion in rats with intestinal culs-de-sac. Brit. J. Exper. Path. 1950;31:355. [PMC free article] [PubMed] [Google Scholar]
- 3.Badenoch J. The blind loop syndrome. Proc. Roy. Soc. Med. 1960;53:657. [PMC free article] [PubMed] [Google Scholar]
- 4.Badenoch J, Bedford PD, Evans JR. Massive diverticulosis of the small intestine with steatorrhea and megaloblastic anemia. Quart. J. Med. 1955;24:321. [PubMed] [Google Scholar]
- 5.Badenoch J, Evans JR, Richards WCD, Witts LJ. Megaloblastic anemia following partial gastrectomy. Brit. J. Haemat. 1955;1:337. doi: 10.1111/j.1365-2141.1955.tb05518.x. [DOI] [PubMed] [Google Scholar]
- 6.Barker WH, Hummel LE. Macrocytic anemia in association with intestinal strictures and anastomoses. Bull. Johns Hopkins Hosp. 1939;64:215. [Google Scholar]
- 7.Booth CC, Mollin DL. The blind loop syndrome. Proc. Roy. Soc. Med. 1960;53:658. [PMC free article] [PubMed] [Google Scholar]
- 8.Booth CC, Mollin DL. The site of absorption of vitamin B12 in man. Lancet. 1959;1:18. doi: 10.1016/s0140-6736(59)90979-1. [DOI] [PubMed] [Google Scholar]
- 9.Borgstrom B, Dahlquist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J. Clin. Invest. 1957;36:1521. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodine C, Friedman BI, Saenger EL, Will JJ. The absorption of B12 following subtotal gastrectomy. J. Lab. & Clin. Med. 1959;53:220. [PubMed] [Google Scholar]
- 11.Burkholder PR. Microbiological studies on materials which potentiate oral vitamin B12 and intrinsic factor activity. Arch. Biochem. 1952;39:322. doi: 10.1016/0003-9861(52)90343-3. [DOI] [PubMed] [Google Scholar]
- 12.Butler TJ, Capper WM, Naish JM. Ileo-jejunal insufficiency following different types of gastrectomy. Gastroenterologia. 1954;81:104. [PubMed] [Google Scholar]
- 13.Butz GW, Jr, Hartman CF, Starzl TE. The blind loop syndrome following subtotal gastrectomy. A. M. A. Arch. Surg. In press. [PMC free article] [PubMed] [Google Scholar]
- 14.Cameron DG, Watson GM, Witts LJ. Clinical association of macrocytic anemia with intestinal stricture and anastomosis. Blood. 1949;4:793. [PubMed] [Google Scholar]
- 15.Capper WM, Welbourne RB. Postcibal symptoms following gastrectomy. Brit. J. Surg. 1955;43:24. doi: 10.1002/bjs.18004317704. [DOI] [PubMed] [Google Scholar]
- 16.Card WI. Blind loop syndrome. Proc. Roy. Soc. Med. 1959;52:28. [PMC free article] [PubMed] [Google Scholar]
- 17.Doscherholmen A, Hagen PS. Absorption of Co60 labeled vitamin B12 in intestinal blind loop megaloblastic anemia. J. Lab. & Clin. Med. 1954;44:790. [Google Scholar]
- 18.Drexler J. Effect of indole compounds on vitamin B12 utilization. Blood. 1958;13:239. [PubMed] [Google Scholar]
- 19.Faber K. Pernicious anemia following intestinal stricture. Klin. Wchnschr. 1897;34:643. [Google Scholar]
- 20.Girdwood RH. Megaloblastic anemia: their investigation and classification. Quart. J. Med. 1956;25:87. [PubMed] [Google Scholar]
- 21.Girdwood RH. The occurrence of growth factors for Lactobacillus leichmannii, Streptococcus fecalis, and Leuconostoc citrovorum in the tissues of pernicious anemia patients and controls. Biochem. J. 1952;52:58. doi: 10.1042/bj0520058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass GBJ. Hematopoietic activity of glandular mucoprotein of human gastric juice. Gastroenterology. 1953;23:219. [PubMed] [Google Scholar]
- 23.Glass GBJ. Intestinal absorption and hepatic uptake of vitamin B12 in diseases of the gastrointestinal tract. Gastroenterology. 1956;30:37. [PubMed] [Google Scholar]
- 24.Glass GBJ, Boyd LJ, Stephanson L. Intestinal absorption of vitamin B12 in humans as studied by isotope technique. Proc. Soc. Exper. Biol. & Med. 1954;86:522. doi: 10.3181/00379727-86-21153. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein F, Wirts CW, Krarner S. The relationship of afferent limb stasis and bacterial flora to the production of postgastrectomy steatorrhea. Gastroenterology. 1961;40:47. [PubMed] [Google Scholar]
- 26.Halsted JA, Lewis PM, Gasster M. Absorption of radioactive vitamin B12 in the syndrome of megaloblastic anemia associated with intestinal stricture of anastomosis. Am. J. Med. 1956;20:42. doi: 10.1016/0002-9343(56)90171-1. [DOI] [PubMed] [Google Scholar]
- 27.Halsted JA, Swendseid ME, Gasster M, Lewis PM. Absorption of radioactive B12 in disease of the small intestine: relation to macrocytic anemia. Tr. Am. Clin. & Climatol. A. 1954;66:18. [PMC free article] [PubMed] [Google Scholar]
- 28.Halsted JA, Swendseid ME, Lewis PM, Gasster M. Mechanisms involved in the development of vitamin B12 deficiency. Gastroenterology. 1956;30:21. [PubMed] [Google Scholar]
- 29.Heinle RW, Welch AD, Scharf V, Meacham GC, Prushoff WH. Studies of excretion (and absorption) of CO60 labeled vitamin B12 in pernicious anemia. Tr. A. Am. Physicians. 1952;65:214. [PubMed] [Google Scholar]
- 30.Hoffman WA, Spiro HM. Afferent loop problems. Gastroenterology. 1961;40:201. [PubMed] [Google Scholar]
- 31.Jackson WPU, Linder GC. Small gut insufficiency following intestinal surgery. South African J. Clin. Sc. 1951;2:205. [PubMed] [Google Scholar]
- 32.Kinsella VJ, Hennessy WB. Gastrectomy and the blind loop syndrome. Lancet. 1960;2:1205. doi: 10.1016/s0140-6736(60)92411-9. [DOI] [PubMed] [Google Scholar]
- 33.Kremen AJ, Linner JH, Nelson CH. An experimental evaluation of the nutritional importance of proximal and distal small intestine. Ann. Surg. 1954;140:439. doi: 10.1097/00000658-195409000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krevans JR, Conley C, Sachs M. Influence of certain diseases on the absorption of vitamin B12 from the gastrointestinal tract. J. Clin. Invest. 1954;33:949. [Google Scholar]
- 35.Krevans JR, Conley CL, Sachs MV. Radioactive tracer tests for the recognition and identification of vitamin B12 deficiency status. J. Chronic Dis. 1956;3:234. doi: 10.1016/0021-9681(56)90120-5. [DOI] [PubMed] [Google Scholar]
- 36.Krikler DM, Schrire V. “Kwashiorkor” in an adult due to an intestinal blind loop. Lancet. 1958;1:510. doi: 10.1016/s0140-6736(58)90815-8. [DOI] [PubMed] [Google Scholar]
- 37.Lake NC. The aftermath of gastrectomy. Brit. M. J. 1948;1:285. doi: 10.1136/bmj.1.4545.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowenstein F. Labeled vitamin B12 after subtotal gastrectomy. Blood. 1958;13:339. [PubMed] [Google Scholar]
- 39.MacLean LD. Megaloblastic anemia following total and subtotal gastrectomy. Surg. Gynec. & Obst. 1958;106:415. [PubMed] [Google Scholar]
- 40.McIntyre PA, Sachs MV, Conley CLJ. Pathogenesis and treatment of macrocytic anemia. A. M. A. Arch. Int. Med. 1956;98:541. doi: 10.1001/archinte.1956.00250290001001. [DOI] [PubMed] [Google Scholar]
- 41.McNealy RW. Problems with the duodenal stump in gastric resections. Surgery. 1942;12:207. [Google Scholar]
- 42.Mimpriss TW, Birt St JMC. Results of partial gastrectomy for peptic ulcer. Brit. M. J. 1948;2:1095. doi: 10.1136/bmj.2.4590.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mollin DL, Booth CC, Baker SJ. The absorption of vitamin B12 in control subjects, in Addisonian pernicious anemia and in malabsorption syndrome. Brit. J. Haemat. 1957;3:412. doi: 10.1111/j.1365-2141.1957.tb05540.x. [DOI] [PubMed] [Google Scholar]
- 44.Muir A. Postgastrectomy syndromes. Brit. J. Surg. 1949;37:165. doi: 10.1002/bjs.18003714606. [DOI] [PubMed] [Google Scholar]
- 45.Naish J, Capper WM. Intestinal cul-de-sac phenomena in man. Lancet. 1953;2:597. doi: 10.1016/s0140-6736(53)90328-6. [DOI] [PubMed] [Google Scholar]
- 46.Noring O. The afferent-loop syndrome elucidated by three cases. Acta chir. scandinav. 1958;115:276. [PubMed] [Google Scholar]
- 47.Pearse AE. Experimental chronic intestinal obstruction from blind loops. Surg. Gynec. & Obst. 1934;59:726. [Google Scholar]
- 48.Quinn WF, Gifford JH. Syndrome of proximal jejunal loop obstruction following anterior gastric resection. California M. J. 1950;72:18. [PMC free article] [PubMed] [Google Scholar]
- 49.Ragins H, Oberhelman HA. Anemia associated with a stagnant (“blind”) jejunal loop. A. M. A. Arch. Surg. 1960;80:524. doi: 10.1001/archsurg.1960.01290200168030. [DOI] [PubMed] [Google Scholar]
- 50.Reilly RW, Kirsner JB. The blind loop syndrome. Gastroenterology. 1959;37:491. [PubMed] [Google Scholar]
- 51.Sammons HG, Vaughan DJ, Frazer AC. Synthesis of long-chain fats by bacteria isolated from human feces. Nature. 1956;177:237. doi: 10.1038/177237a0. [DOI] [PubMed] [Google Scholar]
- 52.Schilling RF. Intrinsic factor studies. II. The effect of gastric juice on the urinary excretion of radioactivity after the oral administration of radioactive vitamin B12. J. Lab. & Clin. Med. 1953;42:860. [PubMed] [Google Scholar]
- 53.Schofield JE, Anderson P St G. Postgastrectomy syndrome deviation of the afferent loop from the gastrointestinal anastomosis. Brit. M. J. 1953;2:598. doi: 10.1136/bmj.2.4836.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scudmore HH, Hagedorn AB, Wollaeger EE, Owen CA. Diverticulosis of the small intestine and macrocytic anemia with report of 2 cases and studies on absorption of radioactive vitamin B12. Gastroenterology. 1958;34:66. [PubMed] [Google Scholar]
- 55.Seyderhelm R, Lehman W, Wichels P. Experimental intestinal pernicious anemia in the dog. Klin. Wchnschr. 1924;3:1439. [Google Scholar]
- 56.Siurala M, Kaipainen WJ. Intestinal megaloblastic anemia treated with aureomycin and terramycin. Acta med. scandinav. 1953;147:197. doi: 10.1111/j.0954-6820.1954.tb12232.x. [DOI] [PubMed] [Google Scholar]
- 57.Stammers FAR. Complications associated with the use of a long afferent loop in the Polya type of partial gastrectomy. Brit. J. Surg. 1956;44:358. doi: 10.1002/bjs.18004418606. [DOI] [PubMed] [Google Scholar]
- 58.Stammers FAR. Remarks on 15 cases of small bowel obstruction following retrocolic partial gastrectomy. Brit. J. Surg. 1954;42:34. doi: 10.1002/bjs.18004217106. [DOI] [PubMed] [Google Scholar]
- 59.Taylor DW. Absorption from intestine of rats with blind self-filling pouch. Nature. 1952;170:80. doi: 10.1038/170081a0. [DOI] [PubMed] [Google Scholar]
- 60.Toon RW, Wangensteen OH. Anemia associated with blind intestinal segments and its prevention with aureomycin. Proc. Soc. Exper. Biol. & Med. 1950;75:762. doi: 10.3181/00379727-75-18335. [DOI] [PubMed] [Google Scholar]
- 61.Tonnis, Brusis A. Changes of morphological blood picture in acute and chronic intestinal obstruction. Deutsche Ztschr. Chir. 1931;233:133. [Google Scholar]
- 62.Ungley CC. Megaloblastic anemia following operations on intestine. Gastroenterologia. 1953;79:338. doi: 10.1159/000199805. [DOI] [PubMed] [Google Scholar]
- 63.Watkinson G, Feather DB, Marson FGW, Dossett JA. Massive jejunal diverticulosis with steatorrhea and mealoblastic anemia improved by excision of diverticula. Brit. M. J. 1959;2:58. doi: 10.1136/bmj.2.5141.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson GM, Cameron DG, Witts LJ. Experimental macrocytic anemia in the rat. Lancet. 1948;2:404. doi: 10.1016/s0140-6736(48)90984-2. [DOI] [PubMed] [Google Scholar]
- 65.Watson GM, Witts LJ. Intestinal macrocytic anemia. Brit. M. J. 1952;1:13. doi: 10.1136/bmj.1.4748.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watson PC. Acute distention of afferent loop after Polya gastrectomy. Brit. M. J. 1958;1:1334. doi: 10.1136/bmj.1.5083.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wells CA, Mac Phee IW. The afferent loop syndrome, bilious regurgitation after subtotal gastrectomy and its relief. Lancet. 1952;2:1189. doi: 10.1016/s0140-6736(52)90885-4. [DOI] [PubMed] [Google Scholar]
- 68.White WH. On the pathology and prognosis of pernicious anemia. Guy’s Hosp. Rep. 1890;47:149. [Google Scholar]
- 69.Witts LJ. Anaemia and the alimentary tract. Edinburgh: Royal College of Surgeons of Edinburgh; 1956. Publication No. 7. [Google Scholar]