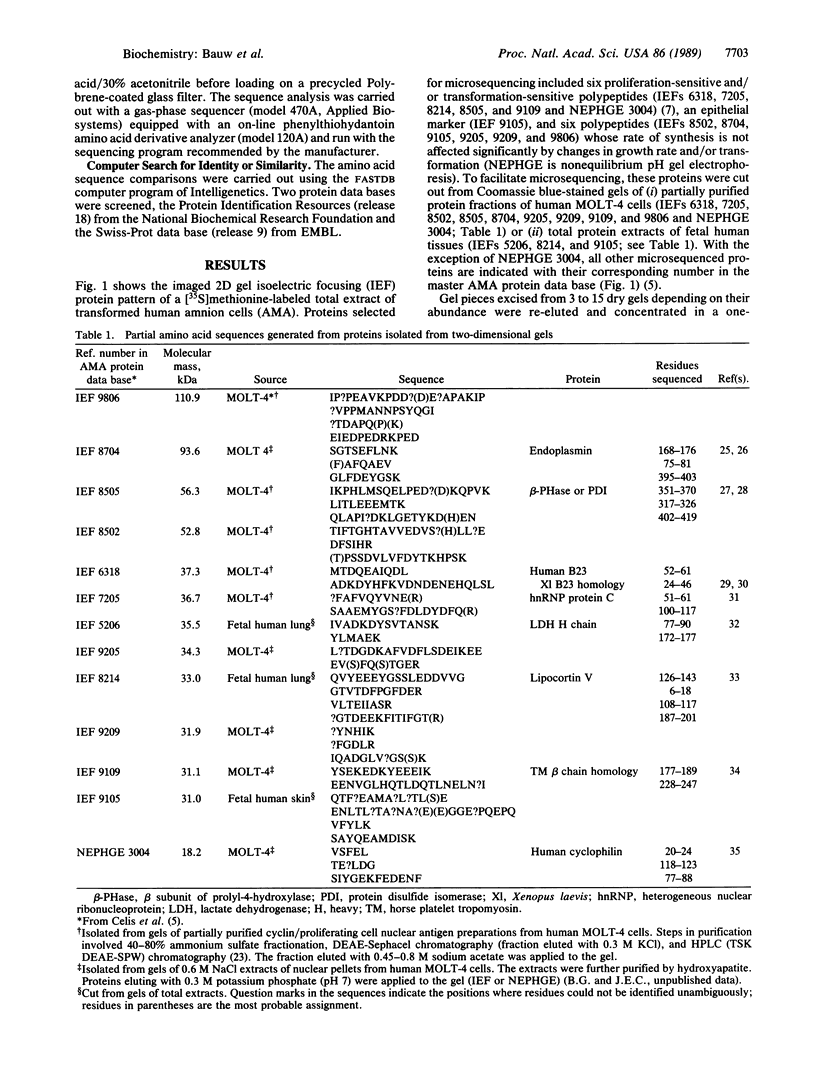
Abstract
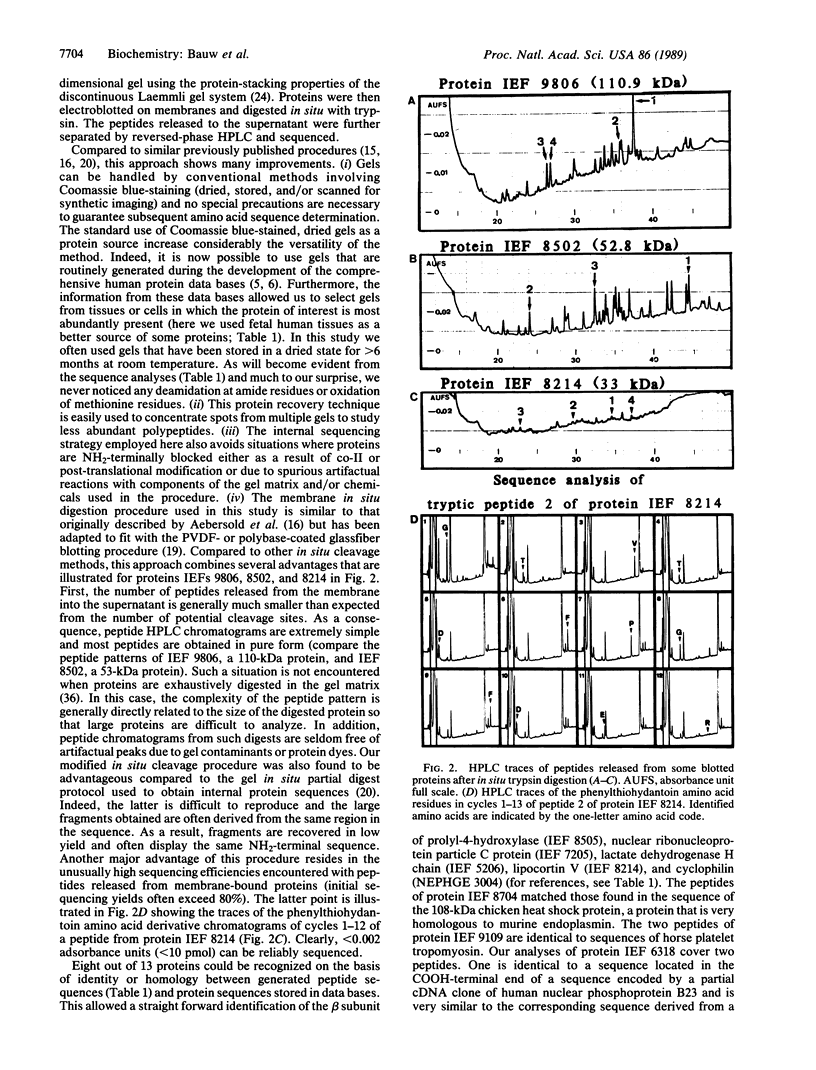
Coomassie blue-stained, heat-dried, and computer-imaged two-dimensional gels used to develop comprehensive human protein data bases served as the protein source to generate partial amino acid sequences. The protein spots were collected from multiple gels, rehydrated, concentrated by stacking into a new gel, electroblotted onto inert membranes, and in situ-digested with trypsin. Peptides eluting from the membranes were separated by HPLC and sequenced. Using this procedure, it was possible to generate partial sequences from 13 human proteins recorded in the amnion cell protein data base. Eight of these sequences matched those of proteins stored in data bases, demonstrating that a systematic analysis of proteins by computerized two-dimensional gel electrophoresis can be directly linked to protein microsequencing methods. The latter technique offers a unique opportunity to link information contained in protein data bases derived from the analysis of two-dimensional gels with forthcoming DNA sequence data on the human genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Human proteins sensitive to neoplastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982 Apr;28(4 Pt 2):949–954. [PubMed] [Google Scholar]
- Bravo R., Frank R., Blundell P. A., Macdonald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987 Apr 2;326(6112):515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Ratz G. P., Celis A., Madsen P., Gesser B., Kwee S., Madsen P. S., Nielsen H. V., Yde H., Lauridsen J. B. Towards establishing comprehensive databases of cellular proteins from transformed human epithelial amnion cells (AMA) and normal peripheral blood mononuclear cells. Leukemia. 1988 Sep;2(9):561–601. [PubMed] [Google Scholar]
- Celis J. E., Ratz G. P., Madsen P., Gesser B., Lauridsen J. B., Hansen K. P., Kwee S., Rasmussen H. H., Nielsen H. V., Crüger D. Computerized, comprehensive databases of cellular and secreted proteins from normal human embryonic lung MRC-5 fibroblasts: identification of transformation and/or proliferation sensitive proteins. Electrophoresis. 1989 Feb;10(2):76–115. doi: 10.1002/elps.1150100204. [DOI] [PubMed] [Google Scholar]
- Chan P. K., Chan W. Y., Yung B. Y., Cook R. G., Aldrich M. B., Ku D., Goldknopf I. L., Busch H. Amino acid sequence of a specific antigenic peptide of protein B23. J Biol Chem. 1986 Oct 25;261(30):14335–14341. [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Feuerstein N., Spiegel S., Mond J. J. The nuclear matrix protein, numatrin (B23), is associated with growth factor-induced mitogenesis in Swiss 3T3 fibroblasts and with T lymphocyte proliferation stimulated by lectins and anti-T cell antigen receptor antibody. J Cell Biol. 1988 Nov;107(5):1629–1642. doi: 10.1083/jcb.107.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr The REF52 protein database. Methods of database construction and analysis using the QUEST system and characterizations of protein patterns from proliferating and quiescent REF52 cells. J Biol Chem. 1989 Mar 25;264(9):5283–5298. [PubMed] [Google Scholar]
- Garrels J. I., Franza B. R., Jr Transformation-sensitive and growth-related changes of protein synthesis in REF52 cells. A two-dimensional gel analysis of SV40-, adenovirus-, and Kirsten murine sarcoma virus-transformed rat cells using the REF52 protein database. J Biol Chem. 1989 Mar 25;264(9):5299–5312. [PubMed] [Google Scholar]
- Garrels J. I. The QUEST system for quantitative analysis of two-dimensional gels. J Biol Chem. 1989 Mar 25;264(9):5269–5282. [PubMed] [Google Scholar]
- Garrels J. I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979 Aug 25;254(16):7961–7977. [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kennedy T. E., Gawinowicz M. A., Barzilai A., Kandel E. R., Sweatt J. D. Sequencing of proteins from two-dimensional gels by using in situ digestion and transfer of peptides to polyvinylidene difluoride membranes: application to proteins associated with sensitization in Aplysia. Proc Natl Acad Sci U S A. 1988 Sep;85(18):7008–7012. doi: 10.1073/pnas.85.18.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G. L., Booth C., Wooding F. B. Dissociation and re-assembly of the endoplasmic reticulum in live cells. J Cell Sci. 1988 Dec;91(Pt 4):511–522. doi: 10.1242/jcs.91.4.511. [DOI] [PubMed] [Google Scholar]
- Kulomaa M. S., Weigel N. L., Kleinsek D. A., Beattie W. G., Conneely O. M., March C., Zarucki-Schulz T., Schrader W. T., O'Malley B. W. Amino acid sequence of a chicken heat shock protein derived from the complementary DNA nucleotide sequence. Biochemistry. 1986 Oct 7;25(20):6244–6251. doi: 10.1021/bi00368a061. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewis W. G., Cote G. P., Mak A. S., Smillie L. B. Amino acid sequence of equine platelet tropomyosin. Correlation with interaction properties. FEBS Lett. 1983 Jun 13;156(2):269–273. doi: 10.1016/0014-5793(83)80511-0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Ogata Y., Nakamura R. M., Tan E. M. Purification and N-terminal amino acid sequence of proliferating cell nuclear antigen (PCNA)/cyclin and development of ELISA for anti-PCNA antibodies. J Immunol. 1985 Oct;135(4):2623–2627. [PubMed] [Google Scholar]
- Pepinsky R. B., Tizard R., Mattaliano R. J., Sinclair L. K., Miller G. T., Browning J. L., Chow E. P., Burne C., Huang K. S., Pratt D. Five distinct calcium and phospholipid binding proteins share homology with lipocortin I. J Biol Chem. 1988 Aug 5;263(22):10799–10811. [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai I., Sharief F. S., Pan Y. C., Li S. S. The cDNA and protein sequences of human lactate dehydrogenase B. Biochem J. 1987 Dec 15;248(3):933–936. doi: 10.1042/bj2480933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Zachmann M. S., Hügle-Dörr B., Franke W. W. A constitutive nucleolar protein identified as a member of the nucleoplasmin family. EMBO J. 1987 Jul;6(7):1881–1890. doi: 10.1002/j.1460-2075.1987.tb02447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Koch G. L. Isolation and identification of partial cDNA clones for endoplasmin, the major glycoprotein of mammalian endoplasmic reticulum. J Mol Biol. 1987 Mar 20;194(2):345–347. doi: 10.1016/0022-2836(87)90381-0. [DOI] [PubMed] [Google Scholar]
- Swanson M. S., Nakagawa T. Y., LeVan K., Dreyfuss G. Primary structure of human nuclear ribonucleoprotein particle C proteins: conservation of sequence and domain structures in heterogeneous nuclear RNA, mRNA, and pre-rRNA-binding proteins. Mol Cell Biol. 1987 May;7(5):1731–1739. doi: 10.1128/mcb.7.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Bauw G., Puype M., Van Damme J., Van Montagu M. Protein-blotting on Polybrene-coated glass-fiber sheets. A basis for acid hydrolysis and gas-phase sequencing of picomole quantities of protein previously separated on sodium dodecyl sulfate/polyacrylamide gel. Eur J Biochem. 1985 Oct 1;152(1):9–19. doi: 10.1111/j.1432-1033.1985.tb09157.x. [DOI] [PubMed] [Google Scholar]
- Walsh M. J., McDougall J., Wittmann-Liebold B. Extended N-terminal sequencing of proteins of archaebacterial ribosomes blotted from two-dimensional gels onto glass fiber and poly(vinylidene difluoride) membrane. Biochemistry. 1988 Sep 6;27(18):6867–6876. doi: 10.1021/bi00418a032. [DOI] [PubMed] [Google Scholar]



