Abstract
β-Arrestins, which were originally characterized as terminators of heterotrimeric guanine nucleotide–binding protein (G protein)–coupled receptor (GPCR) signaling, also act as important signal transducers. An emerging concept in GPCR signaling is β-arrestin–biased agonism, in which specific ligand-activated GPCR conformational states selectively signal through β-arrestins, rather than through G proteins. Here, we show that mechanical stretch induced β-arrestin–biased signaling downstream of angiotensin II type I receptors (AT1Rs) in the absence of ligand or G protein activation. Mechanical stretch triggered an AT1R-mediated conformational change in β-arrestin similar to that induced by a β-arrestin–biased ligand to selectively stimulate receptor signaling in the absence of detectable G protein activation. Hearts from mice lacking β-arrestin or AT1Rs failed to induce responses to mechanical stretch, as shown by blunted extra-cellular signal–regulated kinase and Akt activation, impaired transactivation of the epidermal growth factor receptor, and enhanced myocyte apoptosis. These data show that the heart responds to acute increases in mechanical stress by activating β-arrestin–mediated cell survival signals.
INTRODUCTION
Angiotensin II (AngII) type I receptors (AT1Rs) are members of the large superfamily of GPCRs [heterotrimeric guanine nucleotide–binding protein (G protein)–coupled receptors] and are important in the regulation of blood pressure and hypertrophic cardiac growth in response to increased mechanical load (1). The classic paradigm for activation of AT1R signaling involves AngII stimulation of AT1Rs resulting in dissociation of G proteins into Gαq and βγ subunits, leading to the formation of the second messengers inositol triphosphate and diacylglycerol (DAG) by the effector enzyme phospholipase C (PLC) (1). This G protein–dependent signaling pathway is rapidly terminated by enzymes known as GPCR kinases (GRKs), which phosphorylate AT1Rs on their cytoplasmic tail (2) and enable the binding of the multifunctional protein β-arrestin (2, 3). The binding of β-arrestins to phosphorylated receptors sterically impairs further receptor–G protein coupling in a process known as receptor desensitization (2). Although the function of β-arrestins in AT1R desensitization is now appreciated, increasing evidence supports a broader role for β-arrestins as signal transduction scaffolds that regulate a large network of signaling pathways (3). Moreover, it is also becoming apparent that some GPCR ligands may be able to selectively activate either the G protein pathway or the β-arrestin pathway, a concept known as ligand bias or biased agonism (4–6).
Biased agonism is the ability of a ligand to selectively stabilize receptor conformations that preferentially activate or inhibit a subset of downstream pathways (4–6). Inherent in the concept of ligand bias is that the receptor can exist in multiple distinct conformations in response to ligand binding (4–6). For example, in the absence of AngII, the AT1R would reside in an inactive conformation, whereas in the presence of AngII, an active conformation that couples to G proteins would predominate. In contrast, a β-arrestin–biased agonist would elicit a different conformation in the receptor, which promotes the recruitment and activation of β-arrestin without stimulation of G protein coupling (7, 8).
Recent work suggests that load-induced membrane stretch on cardiomyocytes activates AT1R signaling (9) without requiring the AT1R agonist AngII (10, 11). Mechanical transduction in cells can involve various mechanisms (12–16), including AT1R-mediated activation of transient receptor potential (TRP) channels that depends on G protein coupling (10, 17) and the recruitment of β-arrestin to AT1Rs (10). Here, we show that mechanical activation of AT1Rs can stimulate β-arrestin–biased signaling, and investigate its pathophysiological role in the heart.
RESULTS
Mechanical stretch activates β-arrestin–mediated signaling of AT1R
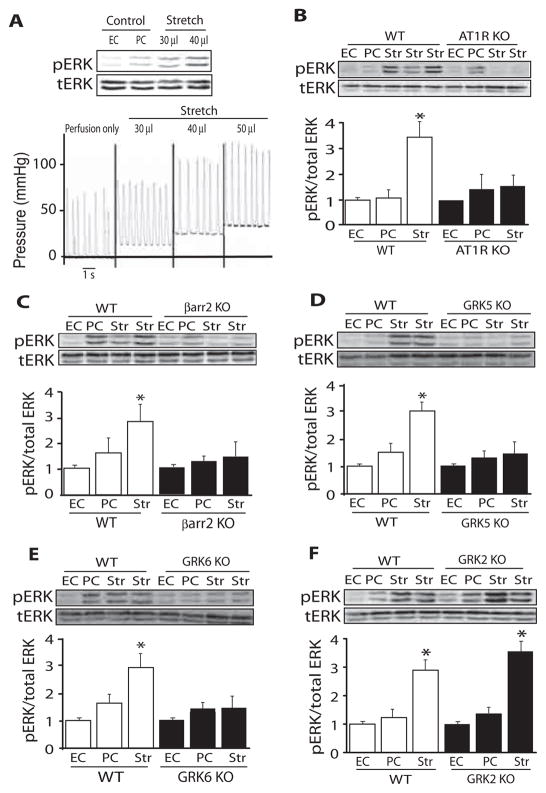
We measured the effect of tensile stretch of the left ventricle (LV) on the activation of extracellular signal–regulated kinase (ERK), a kinase that can be activated by AT1Rs in a β-arrestin–dependent manner (8). A handmade balloon was placed in the LV of a Langendorff-perfused mouse heart and inflated to 30 to 40 mmHg for 10 min (Fig. 1A and fig. S1A). Increasing mechanical stretch led to a graded increase in phosphorylated ERK in the heart (Fig. 1A). To determine whether ERK signaling that is activated by mechanical stretch in the heart requires AT1Rs and β-arrestin, we applied mechanical stretch to various knockout (KO) mouse hearts. Mechanical stretch induced an increase in the abundance of phosphorylated ERK in hearts from wild-type mice, but not in hearts from either AT1R KO or β-arrestin2 KO mice (Fig. 1, B and C). Stretch-induced ERK phosphorylation occurred in hearts from β-adrenergic receptor (βAR) double KO and β-arrestin1 KO mice (fig. S1, B and C). Because β-arrestin–mediated signaling depends on the initial receptor phosphorylation by a GRK (6), we tested whether specific GRKs were needed for ERK activation in the heart by mechanical stretch. Compared to that in wild-type hearts, mechanical stretch did not increase the abundance of phosphorylated ERK in GRK5 or GRK6 KO mouse hearts (Fig. 1, D and E). In contrast, hearts lacking GRK2, like wild-type hearts, showed increased amounts of phosphorylated ERK in response to mechanical stretch (Fig. 1F). Thus, ERK activation in response to mechanical stretch requires the presence of AT1Rs, β-arrestin2, GRK5, and GRK6.
Fig. 1.
Mechanical stretch activates ERK in an AT1R-, β-arrestin2–, and GRK5/6–dependent manner. Diastolic stress in Langendorff-perfused mouse hearts was acutely increased with a left ventricular balloon that was inflated for 10 min. (A) Representative immunoblot of five experiments showing ERK phosphorylation and pressure tracings from LVs after graded amounts of mechanical stretch. Acute increases in diastolic stretch resulted in increased left ventricular diastolic pressure and ERK phosphorylation. (B to E) Mechanical stretch induced ERK phosphorylation in hearts of wild-type (WT) mice, but not in hearts from AT1R KO, β-arrestin2 KO, GRK5 KO, or GRK6 KO mice. (F) Mechanical stretch induced similar amounts of phosphorylated ERK in WT mice and GRK2 KO mice. Summary data in (B) to (F) represent the mean ± SEM of eight to nine KO and WT hearts for stretch and four KO and WT littermate hearts for EC (excision control) and PC (perfusion control) conditions. Str (stretch), inflation of LV balloon for 10 min. *P < 0.0001, compared to EC or PC in the same group.
Mechanical stretch induces G protein–independent AT1R signaling in the absence of ligand stimulation
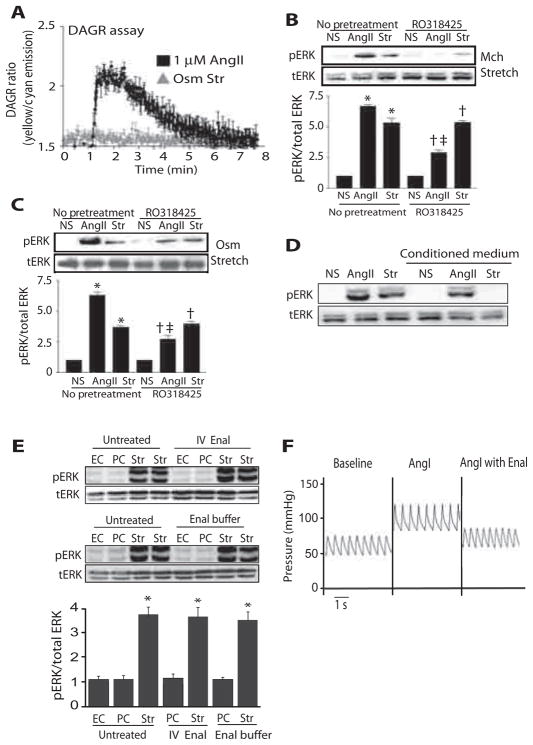
Ligand activation of AT1Rs induces a conformational change of the receptor that enables coupling to G proteins and leads to generation of the second messengers inositol triphosphate and DAG (18, 19). To determine whether mechanical stretch activates G protein signaling, we used DAG reporter (DAGR), a fluorescence resonance energy transfer (FRET)–based reporter for the second messenger DAG, to measure G protein activation in real time in live cells (20). DAGR translocates from cytosol to bind DAG in the plasma membrane, where it undergoes concentration-dependent inter-molecular FRET that is proportional to Gq-coupled AT1R activation (20). Human embryonic kidney (HEK) 293 cells stably expressing AT1Rs and DAGR were stimulated with AngII or hypo-osmotic stretch. AngII treatment induced a rapid increase in DAGR FRET, which peaked within 15 s and then slowly returned to baseline (Fig. 2A). In contrast, hypo-osmotic stretch did not induce a FRET signal, indicating absence of G protein activation (Fig. 2A). To further demonstrate the absence of G protein activation with mechanical stress, we measured the amount of phosphorylated ERK in the presence of the protein kinase C (PKC) inhibitor RO318425. Although RO318425 reduced ERK phosphorylation in response to AngII, it had no effect in cells stimulated with either mechanical or osmotic stretch (Fig. 2, B and C).
Fig. 2.
Mechanical stretch induces ligand- and G protein–independent signaling. (A) Real-time DAGR assay was performed on cells stably expressing AT1Rs, which showed increased DAGR fluorescence ratio with AngII treatment, whereas no DAGR accumulation occurred after hypo-osmotic stretch. (B and C) In HEK 293 cells stably expressing AT1Rs, pretreatment with the PKC inhibitor RO318425 (1 μM) significantly reduced ERK phosphorylation in response to 1 μM AngII, but not when cells were also exposed to mechanical (Mch) or hypo-osmotic stretch (Osm). *P < 0.001, No pretreatment NS compared to No pretreatment AngII or No pretreatment Str; †P < 0.001, RO318425 NS compared to RO318425 AngII or RO318425 Str; ‡P < 0.001, No pretreatment AngII compared to RO318425 AngII. n = 3 independent experiments for each condition with 2 × 106 to 3 × 106 cells per experiment. (D) Cells stably expressing AT1Rs were stimulated with AngII or cyclic stretch, and conditioned medium was used to treat a new set of cells. Cells treated with stretch-conditioned medium did not show ERK activation in response to AngII. n = 5 independent experiments for each condition with 2 × 106 to 3 × 106 cells per experiment. (E) Pretreatment of mice with enalaprilat (Enal) or perfusion of mice with buffer containing enalaprilat to block AngII production did not affect stretch-induced ERK phosphorylation in the heart. *P < 0.0001, compared to EC or PC in the same group. EC, n = 4 hearts; PC, n = 4 hearts; stretch, n = 8 hearts. IV, intravenous. (F) Enalaprilat blocked the AngI-mediated increase in blood pressure, confirming that enalaprilat inhibited angiotensin-converting enzyme and AngII production.
Previous in vitro studies show that activation of AT1Rs by membrane stretch occurs independently of released AngII ligand (10, 11). Therefore, we tested whether mechanical stretch of cells expressing AT1Rs promoted the release of AngII. Cells stably expressing AT1Rs were stimulated with AngII or cyclic stretch, and the conditioned medium was placed on unstimulated cells stably overexpressing AT1Rs. Conditioned medium from AngII-treated cells, but not from stretched cells, triggered an increase in ERK phosphorylation in unstimulated cells (Fig. 2D).
To demonstrate that ERK activation with mechanical stretch of the heart does not occur because of released AngII ligand, we infused enalaprilat intravenously into intact mice to inhibit conversion of AngI to AngII by angiotensin-converting enzyme (Fig. 2, E and F). Enalaprilat was effective because it prevented AngI-induced increases in blood pressure (Fig. 2F). In mouse hearts that received enalaprilat pretreatment or enalaprilat in the perfusion buffer, mechanical stretch induced ERK phosphorylation to the same degree as saline-treated controls (Fig. 2E). Together, these data indicate that the activation of ERK signaling by mechanical stress occurs in the absence of release of AngII and G protein–mediated second messenger generation.
Mechanical stretch induces AT1R trafficking and β-arrestin recruitment
We used mechanical and hypo-osmotic stretch in cells stably expressing AT1Rs to examine the molecular mechanisms of stretch-induced, β-arrestin–dependent signaling. We applied static mechanical stretch to cells plated on a rubber silicone membrane with a computer-regulated vacuum to achieve expansion of cell size by 20%. Cyclic stretch was achieved by applying cycles of 10% cell expansion at 1 Hz. Hypo-osmotic stretch was used to confirm reproducibility and to allow fluorescent imaging studies. In cells transfected with small interfering RNA (siRNA) directed against β-arrestin1 and β-arrestin2 (β-arrestin1/2), stimulation with cyclic mechanical stretch or hypo-osmotic stretch significantly attenuated ERK phosphorylation compared to that in cells transfected with scrambled control siRNA (Fig. 3, A and B). In contrast, knockdown of β-arrestin1/2 followed by AngII stimulation partially attenuated increases in ERK phosphorylation, consistent with the known G protein and β-arrestin pathways for ERK activation by AngII (8). In cells stimulated with 20% static mechanical stretch, ERK phosphorylation occurred at 5 min of stimulation in a β-arrestin–dependent manner, similar to that seen in cells stimulated with cyclic or hypo-osmotic stretch (fig. S2, A and B). Because stretch of AT1R-expressing cells led to β-arrestin–dependent ERK activation, we tested whether mechanical stretch could promote internalization and trafficking of AT1R, a process that typically occurs after ligand stimulation (21). HEK 293 cells stably expressing either hemagglutinin (HA)–tagged AT1Rs or Flag-tagged β1ARs were stimulated with 10% cyclic stretch for 10 min, and receptor internalization was visualized by confocal microscopy. In unstimulated cells, both AT1Rs and β1ARs showed a uniform distribution at the plasma membrane. Cyclic stretch induced the redistribution of AT1Rs into intracellular aggregates in a class B pattern (Fig. 3C), indicating a strong and long-lasting interaction with β-arrestin that is typically observed with AngII stimulation (22). Quantification of AT1R internalization was performed by measuring the percentage of cells showing intracellular aggregates in a given condition (table S1). AT1R internalization occurred in 77.0 ± 4.6% of cells after cyclic stretch and in 88.5 ± 2.7% of cells after AngII treatment compared to 2.5 ± 0.2% when unstimulated (P < 0.001) (table S1). In contrast, <2% of cells expressing β1ARs showed internalization after cyclic stretch (Fig. 3C and table S1).
Fig. 3.
Mechanical stretch induces AT1R trafficking and β-arrestin recruitment. (A and B) HEK 293 cells stably expressing AT1R were transfected with scrambled siRNA (Ctrl siRNA) or β-arrestin1/2 siRNA and stimulated with 1 μM AngII, mechanical stretch (A), or hypo-osmotic stretch (B) for 10 min. n = 5 independent experiments with 2 × 106 to 3 × 106 cells per experiment. *P < 0.001, Ctrl siRNA NS compared to Ctrl siRNA AngII or Ctrl siRNA Str; †P < 0.05, Ctrl siRNA AngII or Ctrl siRNA Str compared to βarr1/2 siRNA AngII or βarr1/2 siRNA Str; ‡P < 0.01, βarr1/2 siRNA NS compared to βarr1/2 siRNA AngII. (C) HEK 293 cells stably expressing HA-tagged AT1R were stimulated with AngII (1 μM) or 10% cyclic mechanical stretch for 10 min. AT1R redistributed from the cell membrane to internalized vesicles. In cells expressing Flag-tagged β1ARs, isoproterenol stimulation (1 μM), but not mechanical stretch, resulted in receptor internalization. n = 4 independent experiments for each condition. Arrowheads show internalized AT1Rs. (D) Hypo-osmotic stretch induced redistribution of β-arrestin1–YFP and β-arrestin2–YFP into endocytic vesicles in a class B pattern similar to that induced by treatment with 1 μM AngII in HEK 293 cells stably expressing AT1R. n = 4 independent experiments for each treatment. Arrowheads, β-arrestin–YFP within endocytic vesicles. (E) Treatment with isoproterenol (Iso) (1 μM), but not cyclic (Cyc) or osmotic stretch (Osm Str), resulted in redistribution of β-arrestin1–YFP and β-arrestin2–YFP from the cytosol to the plasma membrane in a class A pattern, indicating a transient and weak interaction of β-arrestin with the receptor in HEK 293 cells stably expressing β1AR. Scale bar, 10 μm.
We next tested whether cyclic or osmotic stretch could induce AT1R-mediated β-arrestin recruitment. Cells stably expressing AT1Rs were transiently transfected with yellow fluorescent protein (YFP)–tagged β-arrestin1 or β-arrestin2 (in the presence of GRK5) and subjected to osmotic stretch (Fig. 3D). In the absence of stimulation, both β-arrestins showed a uniform distribution throughout the cell (Fig. 3D). In response to hypo-osmotic stretch, both β-arrestin1 and β-arrestin2 (in the presence of GRK5) localized to intracellular puncta, indicating the translocation of β-arrestin into endocytic vesicles (Fig. 3D). After AngII stimulation, 82.0 ± 2.9% of cells expressing β-arrestin1–YFP and 84.0 ± 2.4% of cells expressing β-arrestin2–YFP showed intracellular aggregates (P < 0.001 compared to unstimulated) (table S1). After hypo-osmotic stretch, intracellular aggregates were seen in 66.0 ± 2.2% of cells expressing β-arrestin1–YFP and in 66.5 ± 4.3% of cells expressing β-arrestin2–YFP (P < 0.001 compared to unstimulated cells) (table S1). In contrast, cells stably expressing β1ARs did not show trafficking of β-arrestin in response to either cyclic or hypo-osmotic stretch (Fig. 3E). These data demonstrate that mechanical stress promotes the intra-cellular trafficking of β-arrestin in a class B pattern that is typical for AT1Rs after stimulation by AngII, and suggest a stable interaction between β-arrestin and internalized AT1Rs.
We next tested whether mechanical stretch induces AT1R phosphorylation in a GRK5- and GRK6-dependent manner. Stimulation of HEK 293 cells transiently expressing AT1Rs with static mechanical stretch for 10 min led to robust phosphorylation of AT1Rs, to an extent comparable to that seen with AngII stimulation (fig. S2C). Moreover, cells transfected with siRNA targeting GRK5 and GRK6 showed decreased AT1R phosphorylation in response to stretch.
Mechanical stretch induces a conformational change in β-arrestin
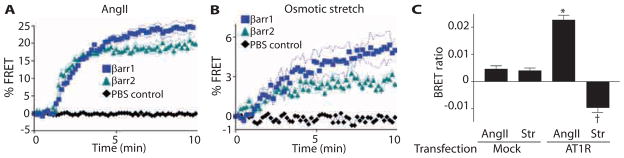
The absence of G protein activation with mechanical stress suggests that AT1Rs adopt a conformation that promotes β-arrestin recruitment and enables activation of downstream signaling (23, 24). To determine the time course of interaction between AT1R and β-arrestin, we performed FRET experiments in HEK 293 cells transiently transfected with AT1R–cyan fluorescent protein (CFP), GRK5, and β-arrestin1–YFP or β-arrestin2–YFP. We quantified the formation of the AT1R-mCFP−β-arrestin-mYFP complex by measuring the percentage of total whole-cell CFP-excited fluorescence. In response to AngII, the maximal FRET detected was ~25% for AT1R-CFP and β-arrestin1–YFP, and ~19% for AT1R-CFP and β-arrestin2–YFP (Fig. 4A). Similar to ligand stimulation, osmotic stretch induced β-arrestin recruitment to the AT1R, but to a lower extent: ~5% for the AT1R-CFP and β-arrestin1–YFP pair and 3% for the AT1R-CFP and β-arrestin2–YFP pair (Fig. 4B).
Fig. 4.
Specific β-arrestin conformation induced by mechanical stretch. (A and B) After agonist stimulation with AngII (1 μM) or hypo-osmotic stretch and GRK5 transfection, β-arrestin1 and β-arrestin2 (with GRK5 transfection)wererecruited to AT1Rs, as detected by increased FRET in HEK 293 cells transiently transfected with AT1R-CFP and β-arrestin1–YFP or β-arrestin2–YFP. n = 4 to 6 independent experiments with 1 × 106 to 2 × 106 cells per experiment. (C) Different conformations of β-arrestin2 were detected with the intramolecular BRET biosensor (Luc-βarr-YFP) when AT1R-expressing cells were stimulated with AngII (1 μM) or hypo-osmotic stretch. n = 5 independent experiments with 1 × 106 to 2 × 105 cells per experiment. *P < 0.0001, AngII AT1R compared to AngII Mock transfection; †P < 0.0001, Osm Str AT1R compared to AngII AT1R or Osm Str Mock transfection.
Biased signaling by β-arrestin presumes that the interaction of β-arrestin with the receptor causes β-arrestin to adopt a conformation that enables β-arrestin to trigger cellular signaling cascades (6, 25). To assess whether mechanical stress acts as a biased ligand by inducing conformational changes in β-arrestin, we used a β-arrestin–based biosensor and measured intra-molecular bioluminescence resonance energy transfer (BRET) (7, 26). The β-arrestin biosensor contains the bioluminescent Renilla luciferase (Rluc) and the YFP fused to the N and C termini of β-arrestin2, respectively (7, 26). Changes in β-arrestin conformation alter the distance or orientation of the two ends of the β-arrestin molecule (or both), leading to changes to the efficiency of BRET (7, 26). Cells cotransfected with AT1Rs and the β-arrestin reporter showed an increase in BRET ratio when stimulated with AngII (Fig. 4C). In contrast, when cells were stretched under hypo-osmotic conditions, the intramolecular BRET ratio for β-arrestin2 was decreased, indicating that β-arrestin adopted a different conformation with mechanical stretch compared to that with the ligand AngII (Fig. 4C). These data are similar to the changes in intramolecular BRET ratios observed when the β-arrestin–biased AT1R ligand SII (Sar1, Ile4, Ile8-AngII) is used to activate β-arrestin–mediated ERK signaling (8, 24). Together, these data demonstrate that membrane stretch stimulates AT1R to recruit β-arrestin and induces a conformation in β-arrestin that promotes receptor internalization and intracellular signaling.
Mechanical stretch stimulates AT1R-mediated EGFR transactivation and ERK signaling
β-Arrestins mediate the transactivation of epidermal growth factor receptors (EGFRs) in response to ligand stimulation of AT1R or β1ARs through the shedding of membrane-bound EGF to promote EGFR dimerization, autophosphorylation, and subsequent EGFR internalization (27, 28). We therefore tested whether mechanical stretch could induce transactivation of EGFR in a β-arrestin–dependent manner by monitoring EGFR–green fluorescent protein (GFP) internalization with confocal microscopy (28). In cells stably expressing AT1Rs transiently transfected with GFP-tagged EGFR, mechanical stretch induced EGFR internalization in 74.2 ± 2.4% of cells, indicating transactivation (Fig. 5A). Transactivation was blocked by the EGFR inhibitor AG1478 and the Src tyrosine kinase inhibitor PP2 (8.0 ± 3.5% and 11.0 ± 4.2% cells showed EGFR internalization, respectively) (Fig. 5A). Mechanical stretch induced EGFR internalization in 77.7 ± 5.5% of the AT1R-expressing cells transfected with control siRNA, and in 15.0 ± 0.5% in AT1R cells transfected with siRNA directed against β-arrestin1/2 (P < 0.001, control siRNA compared to β-arrestin1/2 siRNA) (Fig. 5B and table S1). AngII treatment induced internalization in 77.6 ± 3.5% of the control siRNA–transfected cells and in 12.0 ± 2.7% of the β-arrestin1/2 siRNA–transfected cells (P < 0.001 compared to control siRNA) (Fig. 5B and table S1). In contrast, mechanical stretch did not induce EGFR internalization in HEK 293 cells stably expressing β1ARs (fig. S1D). To measure EGFR phosphorylation induced by mechanical stretch, we transiently transfected HEK 293 cells stably expressing AT1Rs with EGFR-GFP, with or without siRNA targeting β-arrestin1/2, and stimulated with 1 μM AngII, EGF (10 ng/ml), osmotic stretch, or 10% cyclic stretch. Knockdown of β-arrestin1/2 significantly decreased the amount of EGFR phosphorylation in response to AngII or stretch (Fig. 5C). To determine whether mechanical stretch stimulates EGFR-mediated ERK activation in the heart, we examined whether pretreatment with the specific EGFR inhibitor erlotinib blocked mechanical stretch–induced ERK signaling. Stimulation of wild-type hearts with mechanical stretch or EGF ligand significantly increased the amount of phosphorylated ERK, an increase that was blocked by the EGFR inhibitor erlotinib (Fig. 5D), indicating that mechanical stress induces EGFR-dependent ERK signaling.
Fig. 5.
Mechanical stress induced AT1R-mediated EGFR transactivation. (A) EGFR-GFP was internalized in HEK 293 cells stably expressing AT1R that were stimulated with 1 μM AngII, EGF (10 ng/ml), or 10% cyclic stretch. This internalization was blocked by pretreatment with the Src inhibitor PP2 (5 μM) or the EGFR inhibitor AG1478 (1 μM). Arrowheads, internalized EGFRs. (B) Knockdown of β-arrestin1/2 by siRNA blocked AngII- and mechanical stretch–induced EGFR transactivation in HEK 293 cells stably expressing AT1Rs (n = 4 independent experiments with 50 to 80 cells per experiment). (C) Knockdown of β-arrestin1/2 in HEK 293 cells stably expressing AT1Rs prevented EGFR phosphorylation in response to 1 μM AngII, osmotic stretch, or 10% cyclic stretch, but not to EGF ligand (10 ng/ml). *P < 0.001, control siRNA no stimulation (NS) compared to control siRNA treated with AngII and Str; †P < 0.001, control siRNA Ang or Str compared to β-arrestin1/2 siRNA AngII or Str. n = 3 independent experiments with 2 × 106 to 3 × 106 cells per experiment. (D) Mechanical stretch in WT hearts stimulated activation of ERK, which was sensitive to the EGFR inhibitor erlotinib (Erl) (n = 4 hearts). *P < 0.0001, Str no treatment (open bar) compared to Str treated with Erl (solid bar); *P < 0.0001, EC EGF treated (solid bar) compared to EC no treatment (open bar). Scale bar, 10 μm.
Mechanical stress in the heart activates AT1R- and β-arrestin–dependent prosurvival signaling
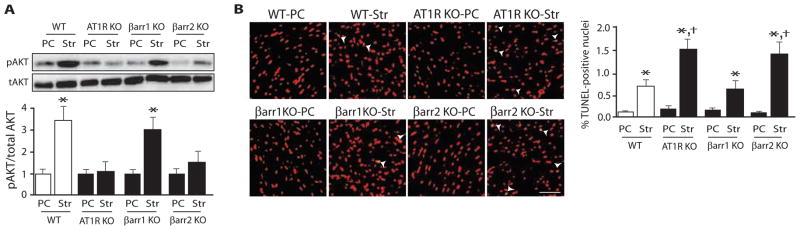
Because mechanical stretch can induce cardiomyocyte and smooth muscle cell apoptosis (12, 16), and β-arrestins have been implicated in pathways that promote cell survival (29), we tested whether mechanical stretch of the heart stimulates prosurvival pathways in a β-arrestin–dependent manner. Wild-type, AT1R KO, and β-arrestin KO mouse hearts were subjected to 30 min of balloon stretch to increase diastolic wall stress and induce apoptosis. Mechanical stretch of wild-type hearts led to Akt phosphorylation, which was absent in hearts from AT1R KO and β-arrestin2 KO mice (Fig. 6A). Moreover, the rate of apoptotic cell death as measured by TUNEL [terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate (dUTP) nick end labeling] staining was significantly increased in hearts from β-arrestin2 KO or AT1R KO mice compared to those from wild-type or β-arrestin1 KO mice (Fig. 6B).
Fig. 6.
Mechanical stress in the heart activates prosurvival antiapoptotic signaling in an AT1R- and β-arrestin2–dependent manner. (A) Diastolic stretch applied for 30 min stimulated Akt phosphorylation in WT hearts, but not in hearts from AT1R KO or β-arrestin2 KO mice. (B) Mechanical stretch–induced apoptosis was enhanced in hearts from AT1R KO and β-arrestin2 KO mice as measured by TUNEL staining compared to hearts from WT or β-arrestin1 KO mice. Str, balloon stretch for 30 min. n = 4 hearts for each group. *P < 0.0001, compared to PC in the same group; †P < 0.01, compared to stretch in WT or βarr1 KO mice. Scale bar, 500 μm.
The angiotensin receptor blocker losartan blocks stretch-induced AT1R signaling
To test whether stretch induces AT1Rs to adopt a signaling conformation, we measured ERK phosphorylation in the presence of an angiotensin receptor blocker (ARB), which stabilizes the receptor in an inactive conformation (30). Stimulation of HEK 293 cells stably expressing AT1R with AngII or stretch increased phosphorylated and activated ERK (Fig. 7A). In contrast, pretreatment with losartan abolished both the AngII- and the stretch-mediated activation of ERK. To block AT1Rs in vivo, we pretreated mice with losartan and losartan was added to the perfusion buffer during ex vivo stretch experiments. Pretreatment with losartan prevented the increase in blood pressure caused by AngII infusion (Fig. 7B, lower panel). Administration of losartan prevents mechanical stretch–induced ERK activation in hearts (Fig. 7B), consistent with the concept that an ARB locks the AT1R in an inactive conformation and prevents its activation by mechanical stretch. We further investigated whether treatment with losartan enhanced the rate of apoptosis in hearts subjected to ex vivo mechanical stress. We found that mechanical stretch of wild-type hearts treated with losartan significantly increased the rate of apoptotic cell death as measured by TUNEL staining compared to untreated wild-type hearts (Fig. 7C).
Fig. 7.
Blockade of stretch-induced signaling by an angiotensin receptor antagonist. (A) AngII and stretch increased phosphorylation of ERK. Pretreatment of cells with losartan abolished AngII- and stretch-mediated ERK phosphorylation (n = 4 independent experiments). (B) Mice pre-treated with losartan failed to activate stretch-induced ERK phosphorylation (left). Losartan blocked the AngII-induced increase in blood pressure, confirming that losartan inhibited AT1Rs (right) (n = 3 to 5 hearts for each condition). (C) Mechanical stretch–induced apoptosis was higher in losartan-treated WT mice as measured by TUNEL staining compared to untreated WT mice. Str, balloon stretch for 30 min. n = 4 hearts for each group. Arrowheads show TUNEL-positive nuclei. *P < 0.05, untreated PC compared to untreated Str hearts; *P < 0.001, losartan PC compared to losartan Str hearts; †P < 0.05, losartan Str hearts compared to untreated Str. Scale bar, 500 μm.
Because TUNEL staining cannot distinguish between apoptotic cells and cells undergoing DNA repair (31), additional assays for apoptosis were performed. In hearts subjected to mechanical stretch, losartan treatment increased DNA fragmentation, caspase 3/7 activity, and the ratio of Bax to Bcl2 protein compared to untreated stretched hearts (fig. S3).
DISCUSSION
An emerging concept in GPCR signaling is β-arrestin–biased agonism, the notion that distinct ligand-activated conformational states selectively stimulate GPCRs to signal through β-arrestin. Our studies provide evidence that membrane stretch activates AT1R-mediated β-arrestin signaling that is equivalent to a pure biased ligand. We show that (i) membrane stretch induces β-arrestin–biased signaling of AT1Rs in the absence of ligand or G protein activation; (ii) membrane stretch triggers an AT1R-mediated conformational change in β-arrestin that is identical to that found with a pure biased ligand; and (iii) in response to mechanical stretch, hearts lacking β-arrestin2 or AT1Rs fail to induce β-arrestin–biased AT1R signaling and show blunted ERK activation, impaired EGFR transactivation, and enhanced apoptosis.
Transduction of mechanical force on cells can involve various mechanisms, including GPCRs, cell cytoskeleton, integrins, and the TRP family of ion channels (12–16). Indeed, membrane stretch can lead to AT1R-mediated activation of TRP channels in a G protein– and PLC-dependent manner (10). Although mechanically stretched cultured cardiomyocytes show hypertrophic responses such as activation of EGFR (32) and ERK (9, 11), the precise molecular mechanism by which this occurs is not well understood. Here, we demonstrate that mechanical stretch can lead to β-arrestin–biased signaling through the AT1R that requires β-arrestin2, GRK5, and GRK6.
The concept of β-arrestin–biased signaling refers to the ability of a ligand to selectively stabilize a receptor conformation such that it promotes β-arrestin recruitment and β-arrestin–dependent signaling independently of G proteins (6). Stimulation of AT1R by AngII induces Gq-mediated activation of PLC and downstream second messenger signaling, which are important for the development of cardiac hypertrophy in response to pressure overload (33). Studies with the biased ligand SII show that β-arrestin activation of ERK signaling can occur in the absence of G protein coupling and second messenger generation (8, 24). It is becoming apparent that β-arrestin–biased signaling requires receptor phosphorylation by a subset of GRKs, specifically GRK5 or GRK6 (28, 34–36), which appears to induce a receptor–β-arrestin conformational state that activates signaling (7). Our data demonstrate that mechanical stress functions as a biased ligand by inducing a ligand-independent conformation of AT1R that is favorable for GRK5- and GRK6-mediated phosphorylation, β-arrestin translocation, and induction of cellular pathways that promote cell survival in the heart.
β-Arrestin1 and β-arrestin2 show a ubiquitous distribution and function in the desensitization of most seven-transmembrane receptors (29, 37). Although the amino acid sequences of the two β-arrestin isoforms (β-arrestin1 and β-arrestin2) are 78% identical, their activities do not appear to be redundant (38, 39). Although either β-arrestin isoform can mediate desensitization and signaling through AT1R, our results show that only β-arrestin2 appears to mediate stretch-induced biased signaling of AT1R in the heart, whereas our cellular studies show that stretch can mediate translocation of either β-arrestin1 or β-arrestin2 (in the presence of GRK5). In our cellular studies, we found that the abundance of endogenous GRK5 in HEK 293 cells was low, and therefore, β-arrestin2 recruitment with stretch could only be observed if exogenous GRK5 was transfected. Moreover, our ex vivo heart studies demonstrate selectivity for β-arrestin2, GRK5, and GRK6. These data are also consistent with data showing a role for only β-arrestin2 in mediating EGFR transactivation in vascular smooth muscle cells with endogenous amounts of AT1Rs (27).
Ligand activation of AT1Rs results in a conformational change of the receptor that enables coupling to G proteins and leads to generation of the second messengers inositol triphosphate and DAG (18, 19). Although mechanical stretch–activated signaling has been previously suggested to require G proteins (10, 11), we show that G protein activation is not required for activation of β-arrestin–dependent ERK signaling in cells expressing AT1Rs or in hearts. Our data support a model in which mechanical stretch under some conditions mediates the activation of β-arrestin signals in a manner that does not seem to require Gq or the generation of second messengers. Transactivation of EGFRs after ligand stimulation of AT1Rs and β1ARs requires β-arrestins (27, 28), as well as GRK5, GRK6, Src, matrix metalloproteinases, and shedding of heparin-binding EGF-like growth factor (27, 28). Consistent with our findings that mechanical stress activates β-arrestin–biased signaling, we show that stretch-induced AT1R-mediated EGFR transactivation requires the presence of β-arrestin and is sensitive to inhibitors of Src and EGFR.
A potential role for EGFR transactivation in the heart is to promote cell survival signaling (28). Indeed, ligand activation of both AT1Rs and βARs can activate antiapoptotic β-arrestin–dependent signaling in vascular smooth muscle cells and the heart (28, 40). It is possible that the high diastolic pressures we imposed on the hearts ex vivo may have induced other stresses, such as endocardial ischemia and hypoxic stress, which could have independently led to EGFR activation. Nonetheless, blood pressure in the mouse is similar to that in humans (41, 42), and previous studies have documented increased LV end-diastolic pressures in patients with coronary artery disease during exercise (43) or with severe aortic stenosis (44–46).
We therefore propose that mechanical stretch of the heart uses AT1Rs to stimulate β-arrestin–dependent prosurvival pathways under conditions of increased wall stress, such as that which occurs with hypertension and heart failure with preserved systolic function, conditions that are frequently treated with angiotensin-converting enzyme inhibitors and ARBs.
Controversy exists whether dual AT1R inhibition with a combination of an angiotensin-converting enzyme inhibitor and an ARB is favorable for patients with heart failure (47, 48). Indeed, the ARB candesartan can inhibit mechanical stress–induced ERK activation by AT1R (30). In our study, losartan blocked stretch-mediated β-arrestin–biased AT1R signaling and promoted apoptosis in hearts exposed to ex vivo diastolic stretch. We postulate that treatment of patients with an ARB may block stretch-mediated biased AT1R signaling, which potentially could inhibit beneficial β-arrestin signaling in the heart.
In summary, we demonstrate that mechanical stress in cells and in the heart activates AT1R-induced ERK signaling in a β-arrestin–dependent manner that does not require G proteins, second messenger generation, or AngII ligand release. Mechanical stress activates GRK5 and GRK6, which promotes AT1R internalization and β-arrestin recruitment, and subsequently triggers β-arrestin to adopt a signaling conformation that is functionally equivalent to that seen with a biased ligand. The formation of an AT1R–β-arrestin signalosome by mechanical stress induces EGFR transactivation and subsequent ERK signaling. The activation of β-arrestin–biased signaling in the heart in response to increases in diastolic mechanical stretch induces pro-survival pathways, which function to limit cardiomyocyte injury. These findings have important implications for patients who present to the hospital with acute diastolic heart failure, a common disease that is associated with high mortality (49).
MATERIALS AND METHODS
Experimental animals
Eight- to 12-week-old β-arrestin1 and β-arrestin2 KO [βarr1 KO (50) and βarr2 KO (51)], AT1R KO (52), double βAR KO (double β1AR and β2AR KO) (53), GRK2 KO, GRK5 KO, GRK6 KO (54), and control C57/B6 wild-type (WT) mice were used for this study.
Cell culture and transfections
HEK 293 cells were maintained as previously described (28). HEK 293 cells stably expressing AT1AR were cultured in zeocin (100 μg/ml; Invitrogen) (24). β-Arrestin1 and β-arrestin2 transfections were carried out with Fugene 6 (Roche) (55). Briefly, 2 μg of β-arrestin1 or β-arrestin2 plasmid was used for transfection. Cells were incubated for 24 to 48 hours and then replated in 35-mm confocal dishes or six-well plates containing a silicone membrane bottom. Cells were serum-starved for 12 hours before stimulation. Fugene 6 was also used to transfect EGFR into HEK 293 cells (24, 56).
Isolated perfused heart preparation
Mice were heparinized [500 U/kg intraperitoneally (ip)] and anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (2.5 mg/kg). Before dissection, the size of the LV was measured by echocardiography and the approximate LV volume was computed. Hearts were removed from animals on a heated operation board under a dissecting microscope (Nissho Optical T-240; Labtek) by median thoracotomy. Hearts were cannulated and retrogradely perfused at 37°C and 80 to 100 mmHg with Krebs-Henseleit buffer [118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 25 mM NaHCO3, 0.5 mM Na-EDTA, and 11 mM glucose, saturated with 95% O2–5% CO2 (pH 7.4)] through the aorta in a noncirculating Langendorff apparatus (Hugo Sachs Harvard Apparatus). Buffer was initially passed through a 5.0-μm filter on preparation.
Intraventricular balloon and myocardial stretch
The LV balloon was made by stretching a polypropylene membrane (57) into the shape of the LV cavity, inserted into the LV through the mitral valve, and inflated with water to yield an LV end-diastolic pressure of 30 to 50 mmHg. The water-filled balloon was secured to a polyethylene-50 tube and connected to a Statham P23Db pressure transducer (Gould Instruments). LV pressure was continuously recorded with a pressure-recording system (Mac Lab, Millar Instruments). Hearts of age- and sex-matched mice that were perfused without inflation of the balloon for identical periods of time served as the perfusion control (PC). Mice with neither perfusion nor inflation served as an excision control (EC). Balloon inflation pressure was held constant with minor adjustments of volume during the experiment. After 10 min of balloon inflation, experiments were terminated and hearts were snap-frozen for analysis. Hearts were excluded from analysis if they failed to beat spontaneously when perfused, or if balloon pressure was <25 or >50 mmHg for 10 min after inflation.
Application of mechanical strain and osmotic stress
Flexible silicone elastomer membrane bottom culture plates (BF-300-1U untreated BioFlex culture plate; Flexcell International) were coated with 0.002% collagen (Roche) for adherence. After cells were plated at a confluence of 70 to 80%, they were allowed to incubate for 24 hours and then serum-starved for 12 hours before mechanical stimulation. Mechanical stretch was applied with a computer-regulated vacuum strain apparatus (Flexcell Strain Unit FX-4000 Tension plus, Flexcell International). Alternate cycles of strain and relaxation were applied at 1 Hz (0.5 s of strain, 0.5 s of relaxation) with regulated vacuum pressure sufficient to generate 10% stretch of culture well diameter. Control samples were maintained at static conditions with no application of strain. Hypo-osmotic stretch was induced by adding double-distilled H2O at a ratio of 1:1. As a result, the osmolality of phosphate-buffered saline (PBS) changed from 285 to 147 mosm/kg, and the osmolality of imaging buffer (for FRET analysis) changed from 255 to 137 mosm/kg.
Knockdown of β-arrestin1/2 with siRNA
β-Arrestin–targeting siRNA is a 21-nucleotide siRNA that has been previously described (8, 58). Cells were plated at 30 to 40% confluence in 10-cm dishes. siRNA (3.5 μg) was used with GeneSilencer Transfection reagent (Gene Therapy Systems) (35). All experiments were performed 60 to 72 hours after siRNA transfection. Cells were serum-starved for 12 hours before stimulation (28).
Immunoblotting
LV tissue was homogenized in NP-40 lysis buffer containing 20 mM tris (pH 7.4), 137 mM NaCl, 1% NP-40, 20% glycerol, 10 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4, 10 mM NaF, aprotinin (2.5 μg/ml), and leupeptin (2.5 μg/ml). Protein concentrations were assayed with Bio-Rad protein assay reagent, and 100 μg of protein was denatured by heating at 95°C for 5 min before resolving by SDS–polyacrylamide gel electrophoresis. Immunoblotting for myocardial total ERK and phosphorylated ERK was performed as previously described (59). The following dilutions of primary antibody were used: total ERK (Upstate), 1:3000; ERK1/2 (Cell Signaling), 1:1200; total Akt (Cell Signaling), 1:1000; phosphorylated Akt (Cell Signaling), 1:1000; phosphorylated EGFR (Tyr845), 1:500; total EGFR (Cell Signaling), 1:500; Bax (Cell Signaling), 1:500; and Bcl2 (Cell Signaling), 1:500. Detection was carried out by ECL (Amersham Biosciences). Densitometric analysis was performed with Bio-Rad Fluoro-S Multi-Image software.
Confocal imaging
HEK 293 cells stably expressing HA-AT1Rs or Flag-β1ARs and transiently transfected with βarr1-YFP, βarr2-YFP, or EGFR-GFP were visualized by confocal imaging (56).
FRET assay
HEK 293 cells were transiently transfected with AT1R-CFP and βarr1-YFP or βarr2-YFP in 35-mm dishes. Imaging buffer [125 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 1.5 mM CaCl2, 10 mM glucose, 0.2% bovine serum albumin (BSA), 10 mM Hepes (pH 7.4)] was added to the dish on a 37°C heated stage. Images were acquired on a Zeiss Axiovert 200M microscope (Carl Zeiss Microimaging Inc.) (60). Calculation of percent FRET was performed as previously described (61).
DAGR assay
The DAGR assay was performed on cells stably expressing AT1R and DAGR (20). Cells were split and plated on a 35-mm dish coated with collagen. Twenty-four hours later, cells were washed with PBS and placed in imaging buffer [125 mM NaCl, 5 mM KCl, 1.5 mM MgCl2, 1.5 mM CaCl2, 10 mM glucose, 0.2% BSA, 10 mM Hepes (pH 7.4)]. After basal activity was recorded for 1 min, cells were stimulated with AngII or hypo-osmotic stretch and the DAGR ratio was recorded for 8 min.
BRET assay
BRET assays were performed as described (26). Briefly, HEK 293 cells were transiently transfected with Flag-AT1R and Rluc-βarr2-YFP with Fugene 6 reagent. After 48 hours of transfection, cells were plated into a 96-well microplate at ~50,000 per well. Luciferase substrate coelenterazine H at a final concentration of 5 μM was added 10 min before stimulation. Cells were then stimulated with AngII (1 μM) or H2O (1:1), and readings were collected at 10 min after stimulation. BRET signal was calculated as the ratio of light emitted by the acceptor (YFP) and light emitted by the donor (Rluc). The values for BRET signal were corrected by subtracting the background BRET signal detected when Rluc-βarr2 was expressed alone. The change in BRET signal was calculated as the difference between BRET signal after stimulation and BRET signal before stimulation.
Treatment protocol for enalaprilat or losartan infusion in mice
Enalaprilat (1 μg/g, Sigma), the active metabolite of the angiotensin-converting enzyme inhibitor enalapril, was infused into WT mice for 10 min to block the formation of AngII. Angiotensin-converting enzyme inhibition was confirmed by monitoring the blood pressure in response to infusion of AngI (10 pmol/kg) for 3 min with and without pretreatment with enalaprilat. In similar experiments, the AT1R antagonist losartan (1 μg/g, Sigma) was administered to mice by intravenous infusion for 10 min. The efficacy of AT1R blockade was monitored by the infusion of AngII (10 pmol/kg) for 3 min in mice pretreated with losartan. In separate experiments, hearts from enalaprilat-and losartan-treated mice underwent balloon mechanical stretch as described above. Enalaprilat (1 μM) or losartan (100 μM) was also added to the Krebs-Henseleit buffer. After 10 min of ex vivo mechanical stretch, hearts were removed and assayed for phosphorylated ERK.
EGF or erlotinib treatment protocol for mice
WT mice were pretreated for 1 hour with erlotinib (20 mg/kg) or 10% dimethyl sulfoxide (DMSO) ip, followed by infusion of 10% DMSO or EGF (100 μg/kg). Five minutes after drug administration, hearts were excised and flash-frozen in liquid nitrogen for biochemical assays as described previously (56).
Histological analysis
Freshly harvested cardiac samples were placed in sucrose-PBS solution at 4°C for 2 to 4 hours, placed in cross section in optimum cutting temperature compound (Miles Pharmaceuticals), and snap-frozen in liquid nitrogen. DNA fragmentation was detected in situ by TUNEL (28). In brief, DNA fragments were labeled with fluorescein-conjugated dUTP using terminal deoxynucleotidyl transferase (Roche Diagnostics Corp.). The total number of nuclei was determined by manual counting of propidium iodide fluorescence–stained nuclei in six random fields per section (magnification, ×200). All TUNEL-positive nuclei were counted in each section.
Angiotensin receptor phosphorylation
Flag-tagged mutant AT1R in which the PKC phosphorylation sites have been substituted for alanine (34) were transfected into HEK 293 cells. Metabolic labeling was conducted for 1 hour in medium containing 200 μCi of [32P]Pi (inorganic phosphate)/ml (34, 56). After stimulation with 1 μM AngII or mechanical stretch for 10 min, Flag-AT1Rs were immunoprecipitated from each sample and their phosphorylation was analyzed by autoradiography with a PhosphorImager (Amersham Biosciences).
DNA laddering
DNA laddering was detected with the enhanced apoptotic DNA ladder detection kit (Biovision). Mouse hearts were subjected to the indicated treatments and 50 mg of heart tissue was cut into fine pieces, solubilized in tris-EDTA lysis buffer, and treated with ribonuclease-free deoxyribonuclease (Qiagen) for 1 hour at 37°C. Enzyme B was added to the sample and incubated at 50°C for 30 min. Samples were then treated with ammonium acetate and isopropanol and incubated at −20°C for 10 min. DNA was washed with 70% ethanol and loaded on a 1.8% agarose gel with ethidium bromide. DNA ladder was visualized by illuminating with ultraviolet light.
Caspase 3/7 activity assay
Caspase 3/7 activity in heart samples was measured with the Caspase-Glo 3/7 Assay kit (Promega) (62). Briefly, 10 μg of protein in a 50-μl total volume was mixed with 50 μl of caspase-Glo 3/7 reagent and incubated for 1 hour at room temperature. Luminescence was measured with a TD-20/20 luminometer (Turner Designs), with each heart sample run in triplicate. Results are expressed as relative units of luminescence.
Statistics
Data are expressed as mean ± SEM. Statistical significance was determined with a one-way analysis of variance (ANOVA) (with Bonferroni correction or Tukey’s test for multiple comparisons) with GraphPad Prism software. A P value of <0.05 was considered significant.
Supplementary Material
Table S1. Quantification of receptor internalization and β-arrestin translocation.
Fig. S1. βARs are not involved in stretch-mediated signaling.
Fig. S2. Time course of stretch-mediated ERK phosphorylation.
Fig. S3. Increased markers of apoptosis in mouse hearts treated with losartan.
Acknowledgments
Research carried out for this study with animals was handled according to approved protocols and animal welfare regulations of the authors’ institutional review boards. We thank R. J. Lefkowitz for his intellectual insight and L. Mao for technical assistance. The double brilliant BRET construct for β-arrestin2 was obtained from R. J. Lefkowitz with permission from M. Bouvier.
Funding: This work was supported by NIH grants HL56687 and HL75443 to H.A.R.
Footnotes
Author contributions: K.R., I.-M.K., and N.S. performed imaging experiments. B.Y. and K.-S.K. performed ex vivo heart experiments. K.R., B.Y., and I.-M.K. performed immunoblotting. B.Y. and K.-S.K. performed TUNEL staining. K.-S.K. and K.R. performed Bax, Bcl2, caspase, and DNA laddering assays. K.R. performed FRET, BRET, and DAGR experiments. H.A.R., K.R., B.Y., and I.-M.K. designed experiments, analyzed the data, and wrote the paper.
Competing interests: H.A.R. is a scientific cofounder of Trevena Inc., a company that is developing G protein coupled receptor–targeted drugs.
REFERENCES AND NOTES
- 1.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz RJ. G protein-coupled receptors. III. New roles for receptor kinases and β-arrestins in receptor signaling and desensitization. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 3.Shenoy SK, Lefkowitz RJ. Angiotensin II–stimulated signaling through G proteins and β-arrestin. Sci STKE 2005. 2005:cm14. doi: 10.1126/stke.3112005cm14. [DOI] [PubMed] [Google Scholar]
- 4.Kenakin T. Collateral efficacy in drug discovery: Taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Kenakin TP. Cellular assays as portals to seven-transmembrane receptor-based drug discovery. Nat Rev Drug Discov. 2009;8:617–626. doi: 10.1038/nrd2838. [DOI] [PubMed] [Google Scholar]
- 6.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 9.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 10.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. 2008;27:3092–3103. doi: 10.1038/emboj.2008.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 12.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- 13.Spassova MA, Hewavitharana T, Xu W, Soboloff J, Gill DL. A common mechanism underlies stretch activation and receptor activation of TRPC6 channels. Proc Natl Acad Sci USA. 2006;103:16586–16591. doi: 10.1073/pnas.0606894103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- 15.Christensen AP, Corey DP. TRP channels in mechanosensation: Direct or indirect activation? Nat Rev Neurosci. 2007;8:510–521. doi: 10.1038/nrn2149. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Xu Q. Mechanical stress-initiated signal transduction in vascular smooth muscle cells in vitro and in vivo. Cell Signal. 2007;19:881–891. doi: 10.1016/j.cellsig.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Seth M, Zhang ZS, Mao L, Graham V, Burch J, Stiber J, Tsiokas L, Winn M, Abramowitz J, Rockman HA, Birnbaumer L, Rosenberg P. TRPC1 channels are critical for hypertrophic signaling in the heart. Circ Res. 2009;105:1023–1030. doi: 10.1161/CIRCRESAHA.109.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghanouni P, Gryczynski Z, Steenhuis JJ, Lee TW, Farrens DL, Lakowicz JR, Kobilka BK. Functionally different agonists induce distinct conformations in the G protein coupling domain of the β2 adrenergic receptor. J Biol Chem. 2001;276:24433–24436. doi: 10.1074/jbc.C100162200. [DOI] [PubMed] [Google Scholar]
- 19.Granier S, Kim S, Shafer AM, Ratnala VR, Fung JJ, Zare RN, Kobilka B. Structure and conformational changes in the C-terminal domain of the β2-adrenoceptor: Insights from fluorescence resonance energy transfer studies. J Biol Chem. 2007;282:13895–13905. doi: 10.1074/jbc.M611904200. [DOI] [PubMed] [Google Scholar]
- 20.Violin JD, Dewire SM, Barnes WG, Lefkowitz RJ. G protein-coupled receptor kinase and β-arrestin-mediated desensitization of the angiotensin II type 1A receptor elucidated by diacylglycerol dynamics. J Biol Chem. 2006;281:36411–36419. doi: 10.1074/jbc.M607956200. [DOI] [PubMed] [Google Scholar]
- 21.Hunyady L, Baukal AJ, Balla T, Catt KJ. Independence of type I angiotensin II receptor endocytosis from G protein coupling and signal transduction. J Biol Chem. 1994;269:24798–24804. [PubMed] [Google Scholar]
- 22.Shenoy SK, Lefkowitz RJ. Trafficking patterns of β-arrestin and G protein-coupled receptors determined by the kinetics of β-arrestin deubiquitination. J Biol Chem. 2003;278:14498–14506. doi: 10.1074/jbc.M209626200. [DOI] [PubMed] [Google Scholar]
- 23.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 24.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, Lefkowitz RJ. Independent β-arrestin 2 and G protein-mediated pathways for angiotensin II activation of extracellular signal-regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzi M, Charest PG, Angers S, Rousseau G, Kohout T, Bouvier M, Pineyro G. β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc Natl Acad Sci USA. 2003;100:11406–11411. doi: 10.1073/pnas.1936664100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of β-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J, Ahn S, Rajagopal K, Lefkowitz RJ. Independent β-arrestin2 and Gq/protein kinase Cζ pathways for ERK stimulated by angiotensin type 1A receptors in vascular smooth muscle cells converge on transactivation of the epidermal growth factor receptor. J Biol Chem. 2009;284:11953–11962. doi: 10.1074/jbc.M808176200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noma T, Lemaire A, Naga Prasad SV, Barki-Harrington L, Tilley DG, Chen J, Le Corvoisier P, Violin JD, Wei H, Lefkowitz RJ, Rockman HA. β-Arrestin-mediated β1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefkowitz RJ, Rajagopal K, Whalen EJ. New roles for β-arrestins in cell signaling: Not just for seven-transmembrane receptors. Mol Cell. 2006;24:643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Yasuda N, Miura S, Akazawa H, Tanaka T, Qin Y, Kiya Y, Imaizumi S, Fujino M, Ito K, Zou Y, Fukuhara S, Kunimoto S, Fukuzaki K, Sato T, Ge J, Mochizuki N, Nakaya H, Saku K, Komuro I. Conformational switch of angiotensin II type 1 receptor underlying mechanical stress-induced activation. EMBO Rep. 2008;9:179–186. doi: 10.1038/sj.embor.7401157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, Noda T, Fujiwara T, Fukuda K, Minatoguchi S, Fujiwara H. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: Not apoptosis but DNA repair. Circulation. 1999;99:2757–2764. doi: 10.1161/01.cir.99.21.2757. [DOI] [PubMed] [Google Scholar]
- 32.Duquesnes N, Vincent F, Morel E, Lezoualc’h F, Crozatier B. The EGF receptor activates ERK but not JNK Ras-dependently in basal conditions but ERK and JNK activation pathways are predominantly Ras-independent during cardiomyocyte stretch. Int J Biochem Cell Biol. 2009;41:1173–1181. doi: 10.1016/j.biocel.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 33.Akhter SA, Luttrell LM, Rockman HA, Iaccarino G, Lefkowitz RJ, Koch WJ. Targeting the receptor-Gq interface to inhibit in vivo pressure overload myocardial hypertrophy. Science. 1998;280:574–577. doi: 10.1126/science.280.5363.574. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc Natl Acad Sci USA. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 37.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 38.Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. β-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. β-Arrestins regulate protease-activated receptor-1 desensitization but not internalization or down-regulation. J Biol Chem. 2002;277:1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 40.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 mediates anti-apoptotic signaling through regulation of BAD phosphorylation. J Biol Chem. 2009;284:8855–8865. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deschepper CF, Olson JL, Otis M, Gallo-Payet N. Characterization of blood pressure and morphological traits in cardiovascular-related organs in 13 different inbred mouse strains. J Appl Physiol. 2004;97:369–376. doi: 10.1152/japplphysiol.00073.2004. [DOI] [PubMed] [Google Scholar]
- 42.Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics. 2002;79:679–685. doi: 10.1006/geno.2002.6754. [DOI] [PubMed] [Google Scholar]
- 43.Carroll JD, Hess OM, Hirzel HO, Krayenbuehl HP. Exercise-induced ischemia: The influence of altered relaxation on early diastolic pressures. Circulation. 1983;67:521–528. doi: 10.1161/01.cir.67.3.521. [DOI] [PubMed] [Google Scholar]
- 44.Bristow JD, Van Zee BE, Judkins MP. Systolic and diastolic abnormalities of the left ventricle in coronary artery disease. Studies in patients with little or no enlargement of ventricular volume. Circulation. 1970;42:219–228. doi: 10.1161/01.cir.42.2.219. [DOI] [PubMed] [Google Scholar]
- 45.Murakami T, Hess OM, Gage JE, Grimm J, Krayenbuehl HP. Diastolic filling dynamics in patients with aortic stenosis. Circulation. 1986;73:1162–1174. doi: 10.1161/01.cir.73.6.1162. [DOI] [PubMed] [Google Scholar]
- 46.Villari B, Vassalli G, Monrad ES, Chiariello M, Turina M, Hess OM. Normalization of diastolic dysfunction in aortic stenosis late after valve replacement. Circulation. 1995;91:2353–2358. doi: 10.1161/01.cir.91.9.2353. [DOI] [PubMed] [Google Scholar]
- 47.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 48.Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: A quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167:1930–1936. doi: 10.1001/archinte.167.18.1930. [DOI] [PubMed] [Google Scholar]
- 49.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 50.Conner DA, Mathier MA, Mortensen RM, Christe M, Vatner SF, Seidman CE, Seidman JG. β-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to β-adrenergic stimulation. Circ Res. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 51.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking β-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 52.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci USA. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaber M, Koch WJ, Rockman H, Smith B, Bond RA, Sulik KK, Ross J, Jr, Lefkowitz RJ, Caron MG, Giros B. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc Natl Acad Sci USA. 1996;93:12974–12979. doi: 10.1073/pnas.93.23.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates β-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 56.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. β-Blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc Natl Acad Sci USA. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Omens JH, Rockman HA, Covell JW. Passive ventricular mechanics in tight-skin mice. Am J Physiol. 1994;266:H1169–H1176. doi: 10.1152/ajpheart.1994.266.3.H1169. [DOI] [PubMed] [Google Scholar]
- 58.Ahn S, Wei H, Garrison TR, Lefkowitz RJ. Reciprocal regulation of angiotensin receptor-activated extracellular signal-regulated kinases by β-arrestins 1 and 2. J Biol Chem. 2004;279:7807–7811. doi: 10.1074/jbc.C300443200. [DOI] [PubMed] [Google Scholar]
- 59.Perrino C, Naga Prasad SV, Mao L, Noma T, Yan Z, Kim HS, Smithies O, Rockman HA. Intermittent pressure overload triggers hypertrophy-independent cardiac dysfunction and vascular rarefaction. J Clin Invest. 2006;116:1547–1560. doi: 10.1172/JCI25397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tilley DG, Kim IM, Patel PA, Violin JD, Rockman HA. β-Arrestin mediates β1-adrenergic receptor-epidermal growth factor receptor interaction and downstream signaling. J Biol Chem. 2009;284:20375–20386. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, Ward JE, Connors LH, Sawyer DB, Semigran MJ, Macgillivray TE, Seldin DC, Falk R, Liao R. Amyloido-genic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38α MAPK pathway. Proc Natl Acad Sci USA. 2010;107:4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Quantification of receptor internalization and β-arrestin translocation.
Fig. S1. βARs are not involved in stretch-mediated signaling.
Fig. S2. Time course of stretch-mediated ERK phosphorylation.
Fig. S3. Increased markers of apoptosis in mouse hearts treated with losartan.