Abstract
This study sought to gain insights into the surface structural and mechanical changes leading to remineralization of dentin. Remineralization was compared between a continuous remineralization approach and a non-buffered static approach using solutions of the same initial composition. Artificial carious lesions were treated for 5 days and analyzed every twenty-four hours using nanoindentation in water, SEM, AFM. The continuous approach yielded a recovery of mechanical properties of up to 60% of normal dentin whereas the static approach lead to a recovery of only 10%. Image analysis revealed that the static approach yielded the formation of areas suggestive of an apatite precipitate on the surface of the dentin matrix. In contrast, surface precipitate was absent using the continuous approach suggesting that mineral formed within the lesion and re-associated with the collagenous matrix. This study provided evidence that mechanical recovery of dentin in near physiological conditions is attainable through the continuous delivery of calcium and phosphate ions.
Keywords: Dentin, remineralization, collagen, hydroxyapatite, mechanical properties, nanoindentation, AFM
Introduction
Dentin is the tissue underlying the enamel that forms the bulk of the tooth. The dentin matrix is formed by about 45–50 vol % mineral in the form of a carbonated hydroxyapatite; 30–35 vol % of organic matter, mostly as type I collagen with associated noncollagenous proteins; with the remainder being oral fluid (Marshall et al., 1997). In dental caries, oral bacteria metabolize carbohydrates, generating acids that diffuse into enamel and further into dentin, dissolving mineral, and eventually destroying the structural matrix. Apatite in dentin has a much smaller crystallite size, higher carbonate content and is more susceptible to acidic dissolution than enamel apatite. Hence, once the carious process enters dentin, the demineralization rate is accelerated. Moreover, the high organic content in dentin makes its remineralization a much more complex process than remineralization of enamel. Thus, a better understanding of the steps leading to remineralization of dentin is desirable.
It has been well documented that in dentin the apatite occurs in two specific regions, within the fibrils (intrafibrillar mineral) and between fibrils (extrafibrillar mineral) (Katz and Li, 1973; Katz et al., 1989; Landis, 1996). Primarily the intrafibrillar mineral (Kinney et al., 2003), has been suggested to be crucial for the normal mechanical properties of the tissue. Therefore, a critical aspect in treating carious dentin is not only to replace the lost mineral, but principally to provide the tight association of the re-grown mineral with the demineralized matrix (Bertassoni et al., 2009), thus enabling the recovery of the mechanical properties of the tissue.
Various in vitro studies have evaluated different saliva formulations (Hara et al., 2008), the effect of ozone and sodium hypochlorite (Zaura et al., 2007), CPP-ACP (Rahiotis and Vougiouklakis, 2007), nano-β-tricalcium phosphate (Shibata et al., 2008), bioactive glass (Vollenweider et al., 2007) and continuous mineral formation with the constant solution composition approach (Koutsoukos and Nancollas, 1981; Tomson et al., 1977); all reporting the reincorporation of mineral within the demineralized dentin matrix. However, to date, evidence of a successful remineralization has been based mostly upon evaluations of how much mineral the tissue has regained, without evaluation of mineral matrix binding and recovery of the mechanical properties of the tissue, particularly in near physiologic conditions of hydration.
Recent investigations suggest that recovery of mechanical properties of the hydrated dentin matrix provides insight into mineral matrix binding and occurs when there is an effective re-association of mineral with the organic matrix, a result termed functional remineralization (Bertassoni et al., 2009). On the other hand, little mechanical recovery occurs when mineral content is recovered, but the mineral is poorly attached to the organic matrix. Accordingly, this occurs because the unbound mineral, even in high concentration, floats in water while the collagen becomes highly deformable under load, thus yielding very low properties. However, if the mineral is bound and reinforces the collagen structure then, even in water, the properties are similar to normal dentin.
The objective of this investigation was to define the conditions that facilitate an improved association of mineral with the demineralized dentin matrix, thus allowing for an improved surface mechanical recovery. The hypothesis tested was that surface remineralization is facilitated by controlled and continuous mineral growth
Materials & methods
Dentin specimen preparation
Permanent fully formed human third molars were obtained from the UCSF dental hard tissue specimen core according to protocols approved by the UCSF Committee on Human Research. Immediately after extraction the teeth were sterilized with gamma irradiation and stored intact in de-ionized water and thymol at 4°C (White et al., 1994). Dentin blocks measuring 3.5 mm in length and width and 2 mm in thickness were cut from the mid-coronal region of the selected teeth perpendicular to the tubule direction. The specimens were ground with SiC abrasive papers from 600 to 1200 grit, and then polished with aqueous diamond suspensions (Buehler, Lake Bluff, IL) of 1.0 and 0.25 µm particle sizes. Each specimen surface was half covered with masking tape to provide a reference area of normal dentin, and the remaining surfaces were exposed to a demineralizing solution for 8 hours. The demineralizing solution contained 0.05 M acetate buffer, 2.2 mM calcium phosphate plus 0.1% thymol (McIntyre et al., 2000) and adjusted to pH 5.0 to create reproducible lesions approximately 30 µm deep as measured by polarized microscopy.
Remineralization experiments
Experiments used remineralization solutions with the same initial composition (1.5 mM CaCl2, 0.9 mM KH2PO4, 147 mM KCl and 1 mM KOH in a volume of 200 ml) and compared continuous and static approaches of mineral growth. The continuous remineralization was obtained using the constant composition method, first described by Thompson and Nancollas (Tomson and Nancollas, 1978), whereas the static approach was obtained using the starting remineralizing solutions.
In both approaches the solutions did not spontaneously precipitate and exhibited a degree of saturation (DS) of 10.62 with respect to stoichiometric hydroxyapatite. DS was calculated as equal to pKsp – pIP, where pKsp is the negative logarithm of the solubility activity product of hydroxyapatite and pIP is the negative logarithm of the ionic activity product. Software developed by M. J. Larsen was used for calculating DS (Larsen, 2001). All remineralization solutions had their temperature maintained at 37°C and had their initial pH carefully adjusted to 7.40. Each experiment lasted five days and started with five specimens. One specimen was removed every 24 hrs, mechanically tested and imaged at 1, 2, 3, 4 or 5 days. A minimum of three experiments was performed using both the continuous and static approaches (n=3 for each time period).
Continuous approach
The constant composition method allows continuous mineral growth with relatively sustained thermodynamic driving forces. The main remineralizing solution is controlled by a pH electrode. The addition of a specimen induces apatite precipitation, resulting in free H+ ions in solution, thus yielding a pH drop. The drop in pH bellow 7.40 triggers the simultaneous addition of two titrant solutions from two electrically coupled burettes with concentrations such that their addition compensates for the calcium and phosphate ions precipitated, according to calculations of mass balance described elsewhere (Wu and Nancollas, 1997). Titrant solutions of the continuous approach contained 8 mM CaCl2 and 284 mM KCl (titrant 1) and 4.8 mM KH2PO4 plus 9 mM KOH (titrant 2) for a volume of 200 ml.
Static approach
The static remineralization approach, on the other hand, relies on the use of non-replenished remineralizing solutions, and thus mineral formation occurs only until the solution reaches saturation equilibrium with respect to apatite.
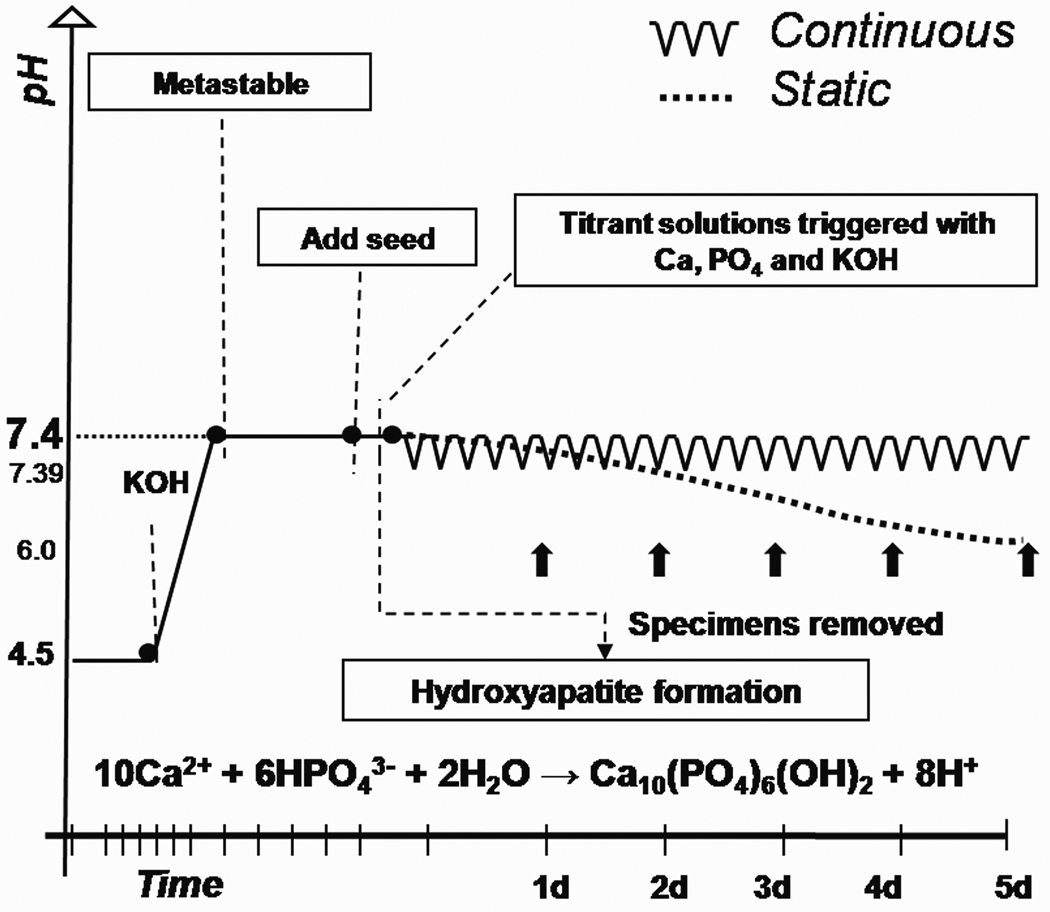
A graph illustrating typical experimental conditions for both methods is represented in figure 1.
Figure 1.
Schematic representation of a typical continuous and static experiment used for remineralization. Solutions containing 1.5 mmol/L CaCl2, 0.9 mmol/L KH2PO4 and 147 mmol/L KCl had their pH adjusted to 7.400 with the addition of potassium hydroxide. At this pH solutions did not precipitate and remained metastable until the addition of specimens (seeds). Once specimens were added an induction period was followed by mineral precipitation, thus yielding a drop in pH. In the continuous approach the pH drop triggered the addition two titrant solutions that compensated for the ions precipitated, whereas in the static approach the pH dropped freely (to pH 6.0±0.5) until the solution reached saturation equilibrium with respect to apatite.
AFM- nanoindentation
In this study nanoindentation was used as a means of quantifying surface remineralization, particularly the mineral that was regained and attached to the demineralized matrix. Indentations were made with the specimens placed in a fluid cell filled with water and employed a calibrated Berkovich diamond indenter on an atomic force microscope (AFM - Nanoscope III Digital Instruments, Santa Barbara, CA, USA) with the standard head replaced by a Triboscope indenter system (Hysitron Inc., Minneapolis, MN, USA) to obtain values of reduced elastic modulus (E). The reduced elastic modulus is convoluted by the modulus of the indenter and the Poisson’s ratio of the sample and indenter. The nanoindenations were made to a fixed depth of 150 nm over and utilized a 3 second load, 3 second hold and 3 second unloading profile. This procedure was applied consistently but does not take into account the differences due to the state of the tissue. In a more mineralized state, higher loading is required to reach the designated depth, while in more demineralized areas or samples the loading rate will be lower. Thus possible load rate dependence limits the absolute values of the modulus reported here, and does not consider the viscoelastic response. Indeed the more demineralized the sample, the more important the viscoelastic properties of the tissue become, suggesting that more attention is needed in future studies. Each indentation yielded a load-deformation curve, from which the E was determined according to equation 1 (Doerner and Nix, 1986)
| (Eq. 1) |
where S represents the slope of the unloading curve based on the method of Oliver and Pharr (Oliver and Pharr, 1992) and a is the indentation contact area. In typical elastic–plastic analysis of indentation testing using the Oliver and Pharr method (Oliver and Pharr, 1992) two parameters are obtained, reduced elastic modulus (E) and the contact hardness (Hc). It has been reported that the contact hardness is directly related to the modulus for indentation tests on bone and tooth tissues (Angker et al., 2005; Chang et al., 2003; Mahoney et al., 2000; Zysset et al., 1999) and that this relationship is usually represented as a linear dependence of Hc on E. A more detailed explanation on this relationship can be found at (Oyen M., 2005). However, elastic modulus is a material property that is fundamentally related to atomic bonding in the material, whereas hardness is an engineering property more representative of the resistance to permanent deformation experienced at the maximum load. Thus, since our elastic modulus results were strictly correlated to hardness values, we opted to report only elastic modulus values. Specimens had both their normal (masked) and demineralized (unmasked) areas measured before remineralization and the results were used as controls. After remineralization a minimum of 15 indentations separated by at least 5 µm was performed on the unmasked area of each specimen. A minimum of three different areas was measured per specimen. The AFM-based nanoindentaion allows the positioning of the indenter with micrometer scale resolution, therefore, all measurements had the indenter carefully positioned in the intertubular region avoiding tubules, peritubular dentin and any mineral surface precipitates that were present. The values were averaged and statistically analyzed using a mixed effects regression model with fixed effects of treatment and day nested within treatment and a random tooth effect. The random tooth effect allows the proper analysis of the correlated observations within each tooth. These regression estimates were then used to produce estimated means for each of the four groups (normal, demineralized, continuous and static) and were followed by all the pair-wise comparisons which identified difference of least squares means of treatments and days within treatments.
Surface characterization
After nanoindentation scanning electron microscopy (SEM - ISI SX- 40A; Topcon Technologies Inc, Paramus, NJ) was used to evaluate surface morphology changes with particular interest in the presence of surface precipitates after remineralization treatments. Representative specimens of all groups were selected and coated with gold (60 nm) without further treatments. Additionally, selected specimens were imaged using an atomic force microscope (AFM - Nanoscope III, Digital Instruments, Santa Barbara, CA) in order to analyze surface morphology changes that were suggestive of mineral association with the collagen network. Imaging was performed in the contact mode in air using a silicon tip at a scan speed of 0.5–1 Hz.
Results
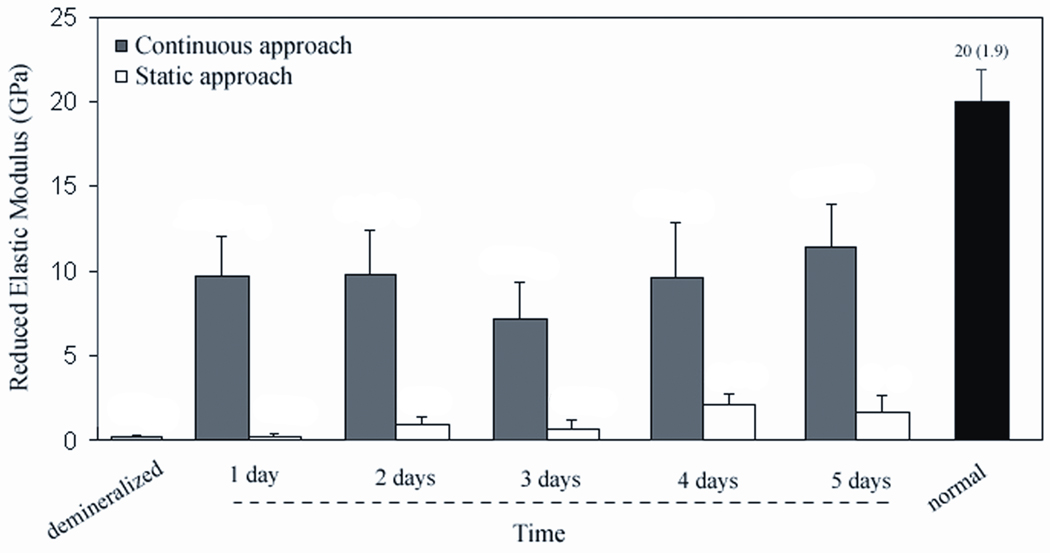
Values of reduced elastic modus (E) of the remineralized specimens were compared to the ones of normal and demineralized dentin and are graphically depicted in figure 2. Values obtained with the continuous approach were all significantly higher than the ones obtained with the static approach but were also still lower than normal dentin (p<.001). After one, two and four days using the continuous approach the E of demineralized dentin increased significantly by nearly a factor of 30 (p<.001). After five days, it had recovered approximately 60% of the value of normal dentin (p<.001). Interestingly only the specimens treated with the continuous approach exhibited a dramatic increase in mechanical properties from the first day of remineralization, whereas the static approach yielded a much more gradual and lower increase. Values obtained with the static approach were only significantly higher than demineralized dentin at the fourth day (p<.001), but were still significantly lower than normal dentin (p<.001).
Figure 2.
Reduced elastic modulus, E, of demineralized dentin followed by values obtained after remineralization treatments (one to five days), and normal dentin. Continuous group was significantly higher than the static and demineralized groups at all time points (p<.001). Both remineralization treatments had values significantly lower than normal dentin at all days (p<.001). Static group was only significantly higher than the demineralized dentin at the day four (p<.001).
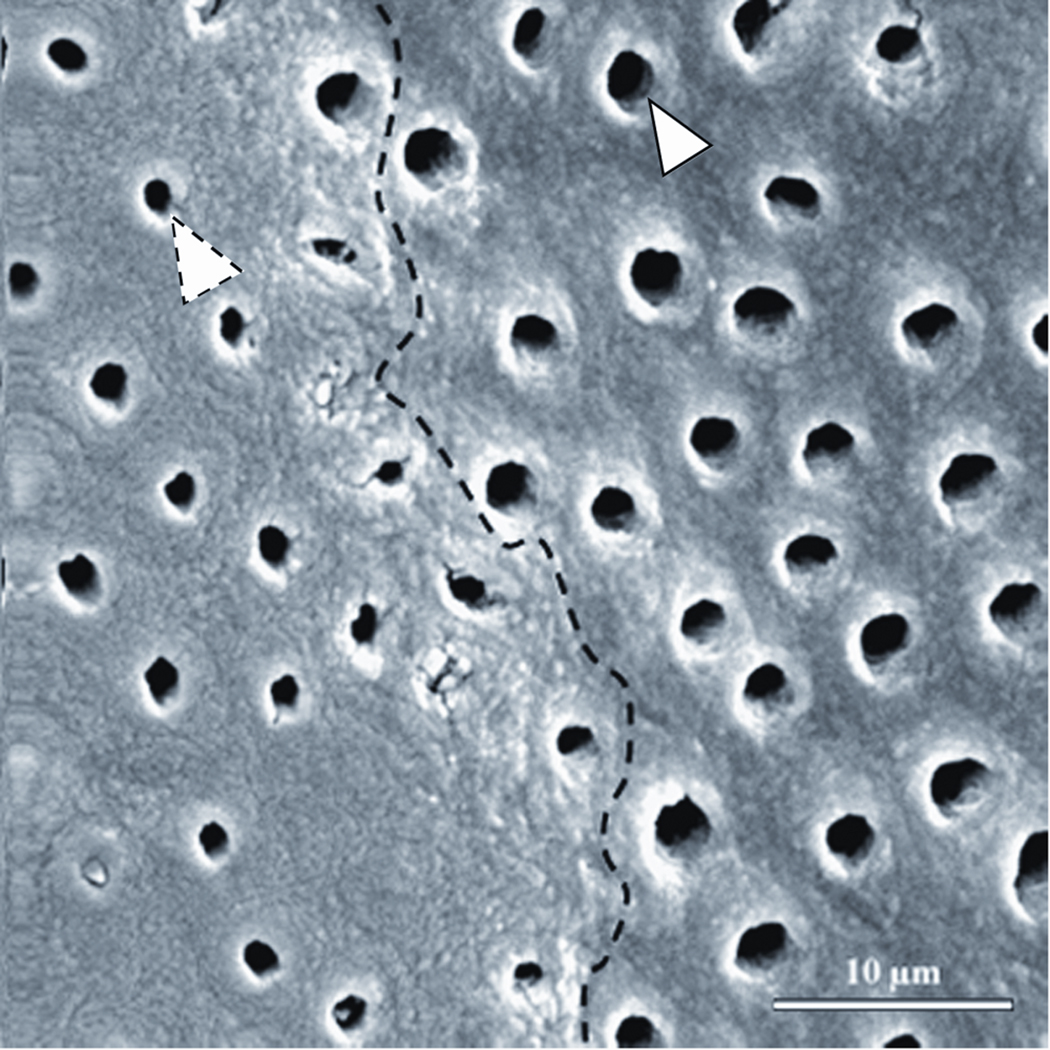
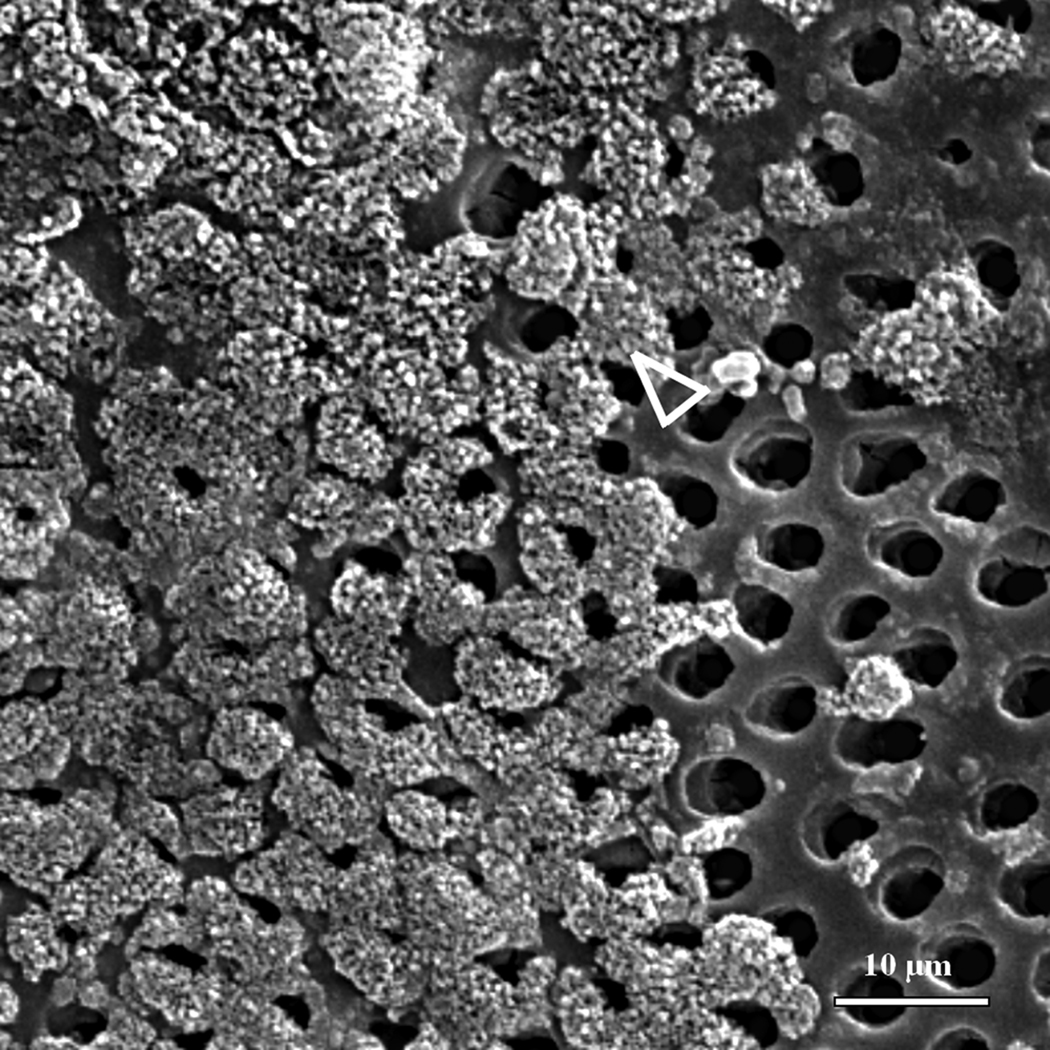
SEM images suggested that the artificial caries simulation protocol induced a demineralization of the surface layer of dentin indicated by the increased size of tubules due to the absence of peritubular mineral (arrowhead) (Fig. 3a). The interface between the normal (masked) and demineralized (unmasked) dentin is marked with a dotted line in Fig 3a. No significant differences were found between images of different treatment days, therefore only day 5 is presented here to illustrate the final results obtained with each remineralization treatment. Specimens treated with the continuous approach after five days did not exhibit an evident precipitation of mineral crystallites on the surface of the intertubular region (Fig. 3b), yet zones of mineral formation within the tubules were present (black pointers). The specimens remineralized with the static approach, on the other hand, exhibited random areas covered with calcium phosphate precipitates (white pointer) (Fig. 3c).
Figure 3.
SEM micrographs of normal-demineralized and remineralized specimens. (a) Dashed line suggests interface between normal (masked) on the left hand side and demineralized (unmasked) dentin on the right hand side. White arrowheads compare normal (dashed) and widened (full line) tubule lumens. Lumens are widened due to removal of peritubular dentin by demineralization. (b) Specimens remineralized with the continuous approach (5 days) did not show precipitation of mineral crystallites on the surface of intertubular dentin, however some tubules appeared filled with mineral (black pointers). (c) The static approach (5 days) suggested precipitation of mineral crystallites (white pointer) randomly distributed on the demineralized dentin.
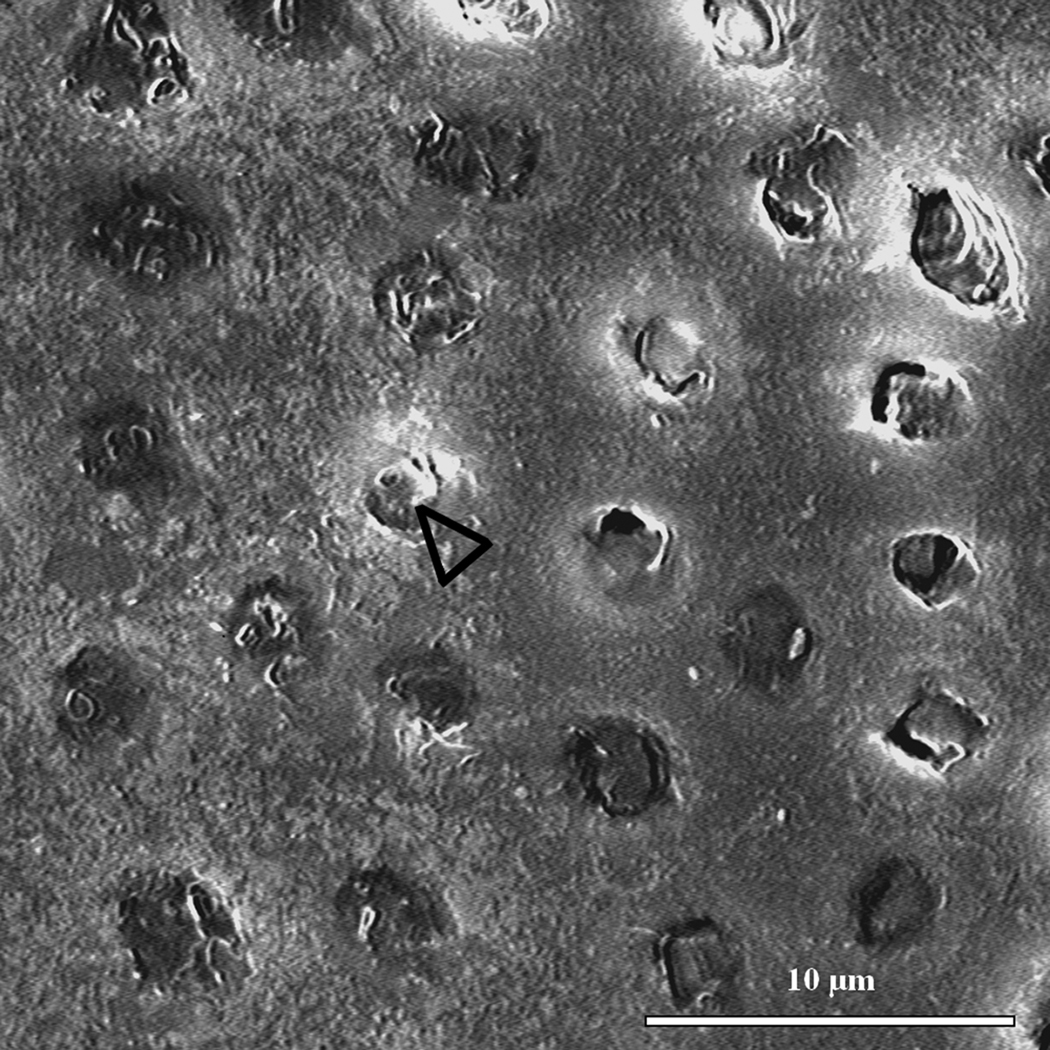
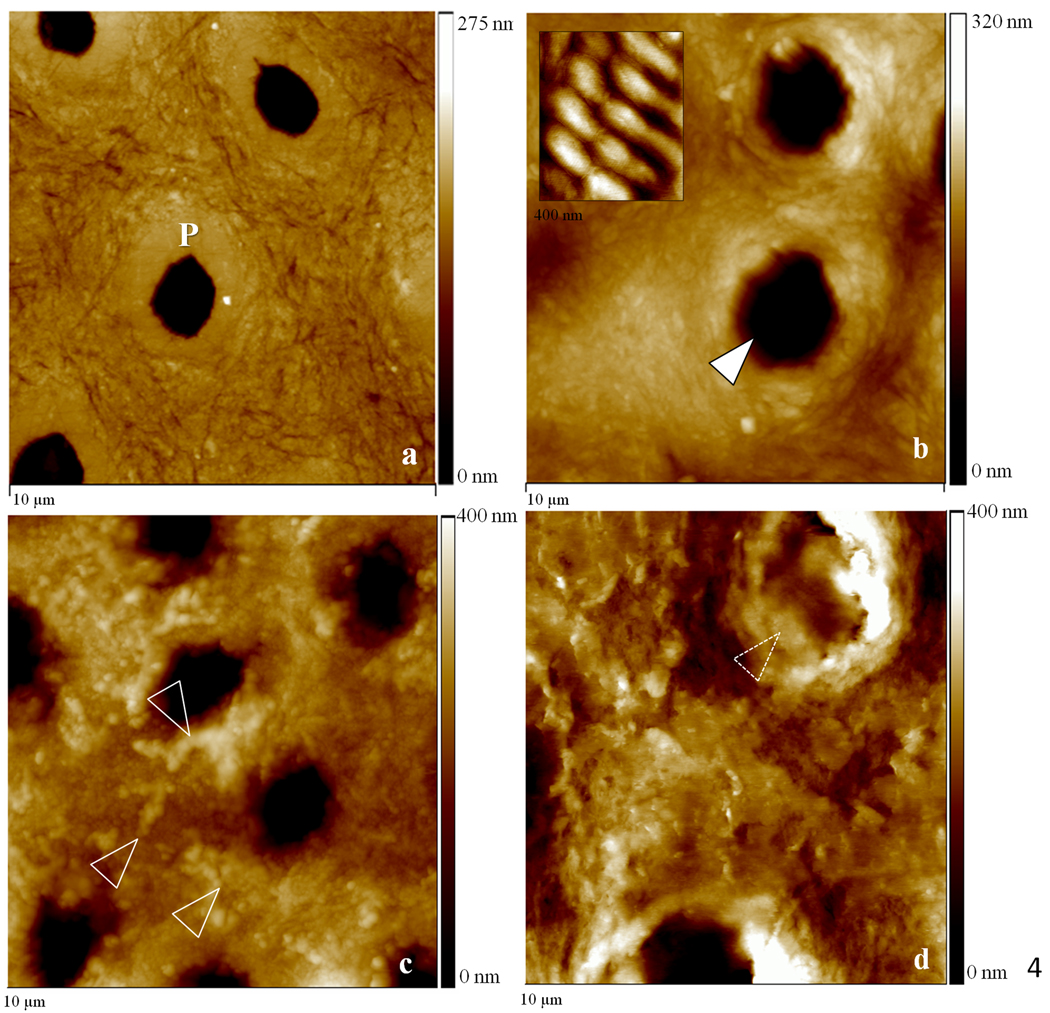
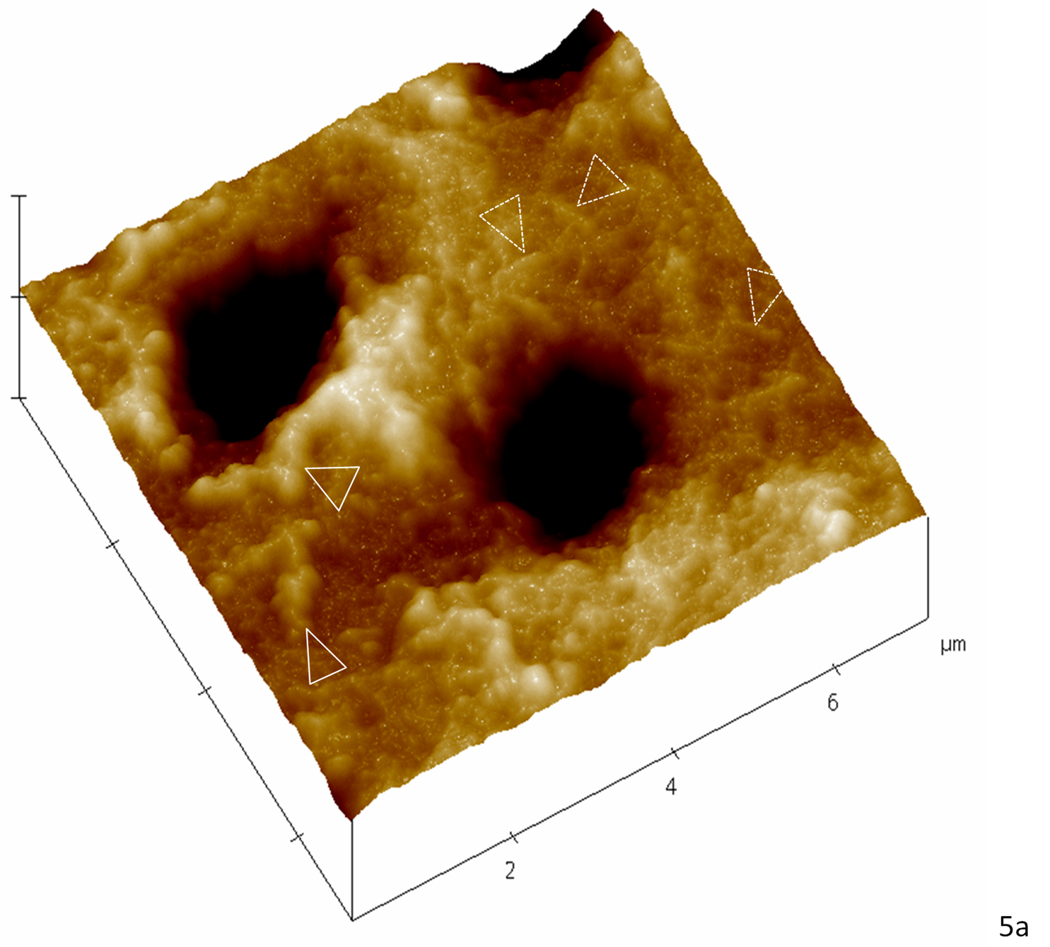
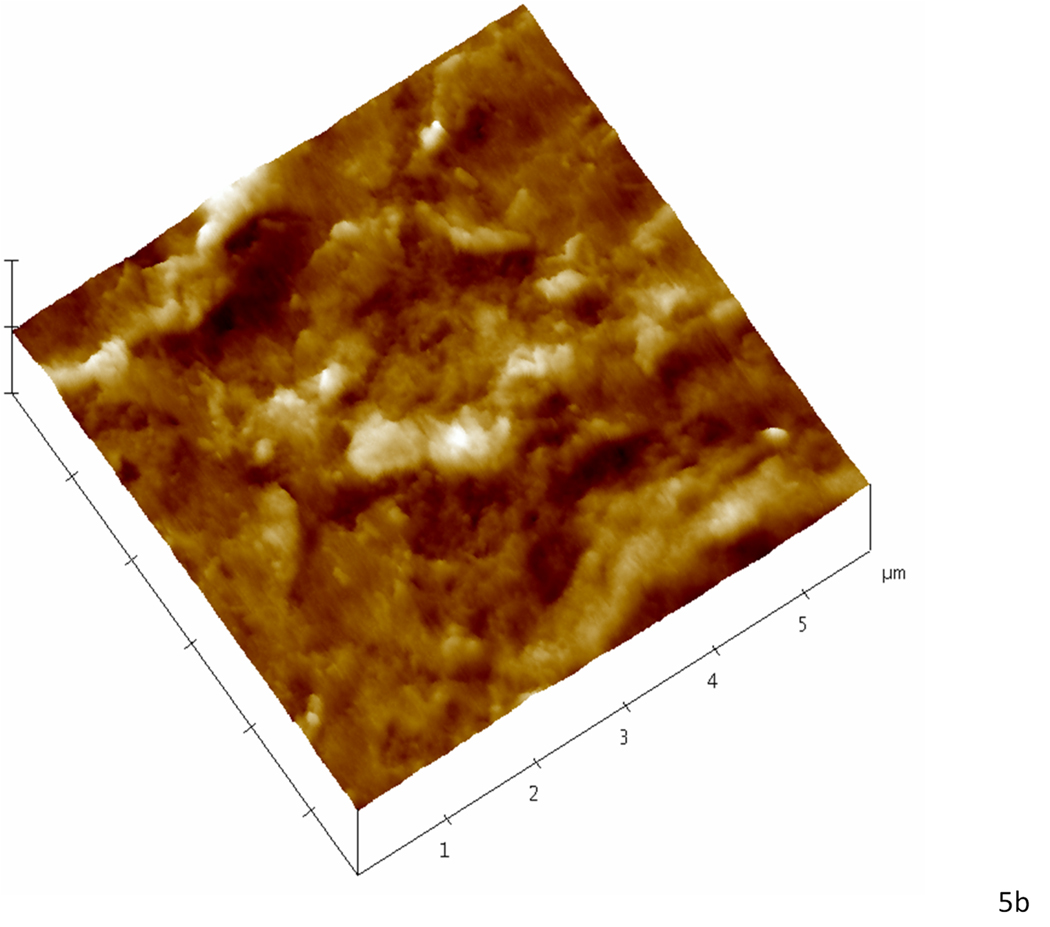
Contact mode AFM was used to analyze topographical features that were suggestive of mineral association with the organic matrix after remineralization. Although we attempted to obtain images in water for this study, we had difficulties in obtaining high quality and interpretable data, and therefore images shown are from the samples after drying under ambient conditions. These imaging difficulties were particularly evident in the static approach studies. We assume this difficulty might be due to the presence of the unbound mineral in the matrix as the specimens were scanned. Again, no major differences were found between images of different treatment days and only day 5 is represented. Baseline normal dentin presented the expected smooth surface after polishing (Fig. 4a); images revealed the slightly elevated (lighter region) peritubular dentin (P) surrounding each tubule lumen, while the slightly lower (darker region) intertubular region showed evidence of the collagen network as indicated by the slight variations in topography. After demineralization (Fig.4b) the dentin surface appeared roughened, as compared to the normal tissue, and the peritubular mineral vanished, thus widening the tubule lumens (arrowhead). Note that the typical staggered pattern of collagen fibrils due to the characteristic 67 nm periodicity (inset of fully demineralized dentin) was not visible after demineralization, which is consistent with fibrils containing remnant intra- or extrafibrillar mineral. Images of the specimens remineralized with the continuous approach revealed fibrillar structures with significant topographical changes that suggested a preferential organization of mineral on the surface of the collagen fibrils (arrowheads) (Fig. 4c). Specimens remineralized with the static approach, on the other hand, exhibited a random precipitation of mineral on the surface of the dentin matrix entirely covering the intertubular region and part of a tubule (dotted arrowheads) (Fig. 4d). Higher magnification images of specimens treated with the continuous approach suggested areas with more (arrowheads) or less (dotted arrowheads) mineral attached to the collagen (Fig. 5a), whereas in the static approach (Fig. 5b), the collagen network seen in figures 4a,b,c and 5a is covered by a calcium and phosphate precipitate
Figure 4.
10 µm AFM scans in the contact mode of specimens remineralized for five consecutive days as well as normal and demineralized specimens. (a) Normal dentin showing peritubular mineral surrounding tubules (P); (b) demineralized dentin exhibited surface roughened topography and widened tubular lumens (arrowhead). Fully demineralized collagen from dentin is shown in the detail for comparison purposes (400 nm scan); (c) remineralization with continuous approach suggested mineral in association with the demineralized organic network, white arrowheads show zones suggestive of mineral attached to the collagen network. (d) Remineralization using static approach showed random precipitation of mineral onto the surface of dentin with no preferential organization;
Figure 5.
Higher magnification AFM scans in the contact mode of demineralized dentin after remineralization for five consecutive days. (a) Specimen treated with the continuous approach; white arrowheads show zones suggestive of higher mineral content attached to the collagen network while dotted arrowheads show zones suggestive collagen with less mineral attached. (b) Specimen treated with the static approach; the precipitate completely covers the intertubular dentin hiding any structure that resembled the collagen network seen in ‘a’.
Discussion
The results of this study confirmed the hypothesis that a controlled and continuous mineral formation facilitates remineralization and surface mechanical recovery of demineralized dentin. The following discussion will address possible mechanisms that might be responsible for the mechanical and structural changes we found.
Mineral matrix binding vs. surface precipitation
The method used in the continuous approach has been suggested to enable the formation of a stable mineral phase that grows in a controlled manner by keeping the solution saturation constant throughout the experiment (Tomson and Nancollas, 1978; Wang and Nancollas, 2008). It has also been suggested (ten Cate, 2008) that if precipitation is maintained as a slow and controlled process, as in the continuous approach, a constant concentration of calcium and phosphate will be maintained homogeneously throughout the matrix, hence the mineral is more likely to grow through the body of the lesion and less likely to form a surface precipitate. Although this study only evaluated surface structural and mechanical changes following the remineralization treatments we contend that this might be a possible mechanism. More studies are needed to provide definitive conclusions on this matter. In the static experiments, on the other hand, it is known that the calcium and phosphate ionic concentrations are likely to vary during reaction, thus the solution becomes saturated with respect to different calcium and phosphate phases that can form and dissolve as changes occur (Tomson and Nancollas, 1978). In the latter, since there is no compensation of ions or pH, the controlled and continued mineralization that allows a distributed nucleation and growth is less likely to occur, thus a surface precipitate is more likely to form. This is a possible explanation for the results found in our static experiments.
These hypotheses are supported by our imaging results, which showed a clear distinction between specimens treated with the static and the continuous approaches. Accordingly, while SEM and AFM images of the static approach (Fig. 3c, 4d and 5b) showed a clear formation of a surface precipitate, SEM images of the continuous approach showed no mineral crystallites on the surface of the specimens (Fig. 3b) and AFM revealed topographical changes suggestive of mineral association with the organic matrix (Fig. 4c and 5a). This suggests that the mineral precursors were more homogeneously distributed through the matrix allowing for a finer interaction with the organic network, as opposed to precipitated on the surface.
Additionally, it has been suggested that in remineralization of dentin, once precipitation occurs the concentration gradient needed for a homogeneously distributed ionic diffusion (ten Cate, 2001) is reduced, and therefore the calcium and phosphate free in solution tend to grow on top of existing crystallites (Wang and Nancollas, 2008). Therefore, on the specimens treated with the static approach, once surface crystallization took place the mineral precursors were likely to be sequestered by existing crystallites and thus precipitate, as suggested by our SEM and AFM results. These crystals, in turn, covered the lesion surface and limited the ionic diffusion into the matrix and led to a lower interaction with the organic matrix. This was reflected in the lower mechanical properties obtained with the static approach. In the continuous approach, conversely, the mineral was likely to grow both within the lesion and more evenly distributed across the outer regions, thus reinforcing not only the surface but also subsurface areas that were beyond our detection.
Steps leading to mechanical recovery via continuous remineralization
A recent investigation (Balooch et al., 2008) has shown that in demineralization with 10 vol % citric acid, a much stronger acid than the one used here, the intrafibrillar mineral is removed from the dentin at a much slower rate than the extrafibrillar mineral, thus we hypothesize that intrafibrillar mineral crystallites remaining within the collagen after demineralization might have worked as sites for apatite growth. We suggest that this remaining mineral allowed the apatite to re-grow bound to the collagen fibrils thus yielding a significant reinforcement of the collagen structure, as expected (Bertassoni et al., 2009), which was reflected in the significantly higher mechanical properties obtained with the continuous remineralization. With the static approach, as mentioned earlier, this finer interaction of the mineral precursors with the collagen fibrils was prevented because the calcium and phosphate tended to react with existing crystallites on the specimen’s surface, as suggested by the SEM images.
Another interesting finding was that the properties of the demineralized dentin (0.3 GPa) were strikingly higher after the first day of remineralization (9.7 GPa) using the continuous approach. It is hypothesized that this relatively rapid increase occurred due to the remineralization of the intrafibrillar spaces of the collagen fibrils, which is critical for high mechanical properties (Kinney et al., 2003), during the early stages of the experiments. Additional mineral regained after the first day might have grown between the fibrils, thus not contributing as significantly to further mechanical recovery. However, our results do not allow a conclusive explanation for this phenomenon. An evaluation of mechanical changes occurring in the first 24 hours of remineralization could provide a more conclusive answer for this question, and forms the basis for future studies.
Continuous remineralization with the constant composition method
Remineralization using the constant solution composition method appears to be useful to study the effects of isolated changes in the thermodynamic driving forces on remineralization of dentin, however, its use remains far from the clinical setting, which makes our results limited in relation to eventual clinical applications. Nonetheless, this study gives insights into the steps leading to the association of mineral with the dentin organic matrix and also provides evidence that the mechanical recovery of demineralized dentin measured in near physiological conditions is attainable. The results also suggest that future clinical approaches for remineralization of dentin should focus on continued delivery of metastable concentrations of mineral precursors, such as calcium and phosphate, for prolonged periods of time.
Conclusion
In summary, this study provided evidence that a continuous delivery of calcium and phosphate favors remineralization and mechanical recovery of dentin by allowing a finer association of the mineral with the organic matrix. Conversely, the static approach appears to favor precipitation on the surface with little mechanical reinforcement, possibly due to poor association of mineral with the demineralized organic network.
Acknowledgements
This work was supported by USPHS NIH/NIDCR Grants R01 DE016849 and RO1 DE017529. The authors also thank Professor John D. B. Featherstone for helpful discussions regarding the artificial caries model.
Footnotes
Conflict of Interest
The authors have no conflict of interest.
References
- Angker L, Swain MV, Kilpatrick N. Characterising the micro-mechanical behaviour of the carious dentine of primary teeth using nano-indentation. Journal of Biomechanics. 2005;38:1535–1542. doi: 10.1016/j.jbiomech.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Balooch M, Habelitz S, Kinney JH, Marshall SJ, Marshall GW. Mechanical properties of mineralized collagen fibrils as influenced by demineralization. Journal of Structural Biology. 2008;162:404–410. doi: 10.1016/j.jsb.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW., Jr Biomechanical perspective on the remineralization of dentin. Caries Research. 2009;43:70–77. doi: 10.1159/000201593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MC, Ko CC, Liu CC, Douglas WH, DeLong R, Seong WJ, Hodges J, An KN. Elasticity of alveolar bone near dental implant–bone interfaces after one month's healing. Journal of Biomechanics. 2003;36:1209–1214. doi: 10.1016/s0021-9290(03)00113-1. [DOI] [PubMed] [Google Scholar]
- Doerner MF, Nix WD. A method for interpreting the data from depth-sensing indentation instruments. Journal of Materials Research. 1986;1:601–609. [Google Scholar]
- Hara AT, Karlinsey RL, Zero DT. Dentine remineralization by simulated saliva formulations with different Ca and Pi contents. Caries Research. 2008;42:51–56. doi: 10.1159/000111750. [DOI] [PubMed] [Google Scholar]
- Katz EP, Li ST. Structure and function of bone collagen fibrils. Journal of Molecular Biology. 1973;80:1–15. doi: 10.1016/0022-2836(73)90230-1. [DOI] [PubMed] [Google Scholar]
- Katz EP, Wachtel E, Yamauchi M, Mechanic GL. The structure of mineralized collagen fibrils. Connective Tissue Research. 1989;21:149–154. doi: 10.3109/03008208909050005. discussion 155-8. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. Journal of Dental Research. 2003;82:957–961. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]
- Koutsoukos PG, Nancollas GH. The kinetics of mineralization of human dentin in vitro. Journal of Dental Research. 1981;60:1922–1928. doi: 10.1177/00220345810600120101. [DOI] [PubMed] [Google Scholar]
- Landis WJ. Mineral characterization in calcifying tissues: atomic, molecular and macromolecular perspectives. Connective Tissue Research. 1996;34:239–246. doi: 10.3109/03008209609005267. [DOI] [PubMed] [Google Scholar]
- Larsen MJ. Ion Products and Solubility of Calcium Phosphates. Denmark: Royal Dental College; 2001. [Google Scholar]
- Mahoney E, Holt A, Swain M, Kilpatrick N. The hardness and modulus of elasticity of primary molar teeth: an ultra-micro-indentation study. Journal of Dentistry. 2000;28:589–594. doi: 10.1016/s0300-5712(00)00043-9. [DOI] [PubMed] [Google Scholar]
- Marshall GW, Jr, Marshall SJ, Kinney JH, Balooch M. The dentin substrate: structure and properties related to bonding. Journal of Dentistry. 1997;25:441–458. doi: 10.1016/s0300-5712(96)00065-6. [DOI] [PubMed] [Google Scholar]
- McIntyre JM, Featherstone JD, Fu J. Studies of dental root surface caries. 1: Comparison of natural and artificial root caries lesions. Australian Dental Journal. 2000;45:24–30. doi: 10.1111/j.1834-7819.2000.tb00238.x. [DOI] [PubMed] [Google Scholar]
- Oliver WC, Pharr GM. An Improved Technique for Determining Hardness and Elastic-Modulus Using Load and Displacement Sensing Indentation Experiments. Journal of Materials Research. 1992;7:1564–1583. [Google Scholar]
- Oyen ML. Nanoindentation hardness of mineralized tissues. Journal of Biomechanics. 2006;39:2699–2702. doi: 10.1016/j.jbiomech.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Rahiotis C, Vougiouklakis G. Effect of a CPP-ACP agent on the demineralization and remineralization of dentine in vitro. Journal of Dentistry. 2007;35:695–698. doi: 10.1016/j.jdent.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Shibata Y, He LH, Kataoka Y, Miyazaki T, Swain MV. Micromechanical property recovery of human carious dentin achieved with colloidal nano-beta-tricalcium phosphate. Journal of Dental Research. 2008;87:233–237. doi: 10.1177/154405910808700315. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Remineralization of caries lesions extending into dentin. Journal of Dental Research. 2001;80:1407–1411. doi: 10.1177/00220345010800050401. [DOI] [PubMed] [Google Scholar]
- ten Cate JM. Remineralization of deep enamel dentine caries lesions. Australian Dental Journal. 2008;53:281–285. doi: 10.1111/j.1834-7819.2008.00063.x. [DOI] [PubMed] [Google Scholar]
- Tomson MB, Tomazic B, Nancollas GH, Miller W, Everett M. The seeded growth of calcium phosphates on dentin and predentin. Journal of Dental Research. 1977;56:1369–1375. doi: 10.1177/00220345770560111501. [DOI] [PubMed] [Google Scholar]
- Tomson MB, Nancollas GH. Mineralization Kinetics: A Constant Composition Approach. Science. 1978;200:1059–1060. doi: 10.1126/science.200.4345.1059. [DOI] [PubMed] [Google Scholar]
- Vollenweider M, Brunner TJ, Knecht S, Grass RN, Zehnder M, Imfeld T, et al. Remineralization of human dentin using ultrafine bioactive glass particles. Acta Biomaterialia. 2007;3:936–943. doi: 10.1016/j.actbio.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Nancollas GH. Calcium Orthophosphates: Crystallization and Dissolution. Chemical Reviews. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Goodis HE, Marshall SJ, Marshall GW. Sterilization of Teeth by Gamma-Radiation. Journal of Dental Research. 1994;73:1560–1567. doi: 10.1177/00220345940730091201. [DOI] [PubMed] [Google Scholar]
- Wu W, Nancollas GH. The relationship between surface free-energy and kinetics in the mineralization and demineralization of dental hard tissue. Advances in Dental Research. 1997;11:566–575. doi: 10.1177/08959374970110042601. [DOI] [PubMed] [Google Scholar]
- Zaura E, Buijs MJ, ten Cate JM. Effects of ozone and sodium hypochlorite on caries-like lesions in dentin. Caries Research. 2007;41:489–492. doi: 10.1159/000109086. [DOI] [PubMed] [Google Scholar]
- Zysset PK, Guo XE, Hoffler CE, Moore KE, Goldstein SA. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. Journal of Biomechanics. 1999;32:1005–1012. doi: 10.1016/s0021-9290(99)00111-6. [DOI] [PubMed] [Google Scholar]