Abstract
In this review, we consider the evidence that a reduction in neurogenesis underlies aging-related cognitive deficits, and impairments in disorders such as Alzheimer's disease (AD). The molecular and cellular alterations associated with impaired neurogenesis in the aging brain are discussed. Dysfunction of presenilin-1, misprocessing of amyloid precursor protein and toxic effects of hyperphosphorylated tau and β-amyloid likely contribute to impaired neurogenesis in AD. Since factors such as exercise, enrichment and dietary energy restriction enhance neurogenesis, and protect against age-related cognitive decline and AD, knowledge of the underlying neurogenic signaling pathways could lead to novel therapeutic strategies for preserving brain function. In addition, manipulation of endogenous neural stem cells and stem cell transplantation, as stand-alone or adjunct treatments, seem promising.
Introduction
There is a progressive decline in the regenerative capacity of most organs with increasing age, resulting in functional decline and poor repair from injury and disease. Once thought to exist only in high turnover tissues, such as the intestinal lining or bone marrow, it now appears that most tissues harbor stem cells that contribute to tissue integrity throughout life. In many cases, stem cell numbers decrease with age, suggesting stem cell aging may be of fundamental importance to the biology of aging (for review, see Ref. [1]). Therefore, understanding the regulation of stem cell maintenance and/or activation is of considerable relevance to understanding the age-related decline in maintaining tissue integrity, function, and regenerative response.
The adult brain contains neural stem cells (NSCs) that self-renew, proliferate and give rise to neural progenitor cells (NPC) that exhibit partial lineage-commitment. Following several cycles of proliferation, NPC differentiate into new neurons and glia. NSCs are increasingly acknowledged to be of functional significance and harbor potential for repair of the diseased or injured brain. The dramatic decline in neurogenesis with age is thought to underlie impairments in learning and memory, at least in part. Aging is also the greatest risk factor for Alzheimer's disease (AD), a neurodegenerative disease characterized by progressive loss of memory and cognitive decline. Alterations in neurogenesis have been described extensively in animal models of AD, and key proteins involved in AD pathogenesis are shown to regulate neurogenesis. By understanding the molecular mechanisms underlying neurogenesis and its decline with aging, it may be possible to manipulate NSCs, to contribute to repair and provide effective treatments for brain disorders.
Neurogenesis in the adult mammalian brain
There are two neurogenic areas in the adult brain: the subventricular zone (SVZ) abutting the lateral ventricles, which contains NSCs that give rise to neurons in the olfactory bulb, and the subgranular layer (SGL) in the dentate gyrus (DG) of the hippocampus, in which NSCs become new granule cell neurons (Figure 1). Thus, the adult brain has more capacity for plasticity at the cellular level than previously thought. The prevailing hypothesis holds that the putative NSCs of the SVZ are quiescent, glial fibrillary acidic protein (GFAP)-positive cells that share properties of astrocytes, referred to as type B cells (for review, see Ref. [2]). Type B cells give rise to transit-amplifying type C cells that are GFAP-negative. These intermediate progenitor cells (IPs or NPCs) give rise to polysialated neural cell adhesion molecule (PSA-NCAM)- and doublecortin (DCX)-expressing neuroblasts that migrate in chains through the rostral migratory stream (RMS) toward the olfactory bulb where they differentiate into either granule cells or periglomerular neurons [3](Figure 1). The migration and maturation of adult neuroblasts has been classified based on the electrophysiological properties they possess [4, 5].
Figure 1. Neurogenesis in the adult rodent brain.
A sagital section of mouse brain shows the neurogenic microenvironments in the adult brain: the subventricular zone (SVZ) and the subgranular layer (SGL) of the dentate gyrus (DG). Stages of morphological and physiological development of neural stem cells (NSCs) in the SVZ (left) and SGL (right) are illustrated in inserts. Specifically, the SGL contains Type I NSCs, type II and type III cells which can be identified by distinct morphological and molecular markers. Type I have radial processes extending into the inner molecular layer. These cells express nestin, glial fibrillary acidic protein (GFAP), mammalian hairy and Enhancer-of-split homologues (Hes5), brain lipid binding protein (BLBP) and Sex determining region Y-box 1,2 (Sox1 and Sox2). This pool of NSCs stays relatively stable throughout life. Type II [neural progenitor cells (NPCs) or intermediate progenitors (IP)] have only short processes if any, and do not express GFAP. Type II cells may arise from type I cells. Type II cells can be divided to two subpopulations: (i) type IIa, which express Mash1 (Ascl1) as well as retaining expression of NSC markers such as Sox2, and (ii) type IIb cells, which are early-committed neuronal progenitors expressing the transcription factors Prospero homeobox protein 1 (Prox1), Neurogenic differentiation 1 (NeuroD1), as well as Doublecortin (Dcx). They continue to proliferate and may give rise to type III cells, which are migratory neuroblasts that express DCX and polysialated neural cell adhesion molecule (PSA-NCAM)-. After a limited number of cell divisions, type III cells exit the cell cycle and become mature granule neurons. Multiple signals in the local niche determine the fate of NSCs. These signals include: soluble factors (purple ovals) such as brain derived neurotrophic factor (BDNF), cell surface signals (yellow squares) such as Notch1, and extracellular matrix (ECM) factors such as laminin (pink triangles). Endothelial cells (EC) and astrocytes (AS) are thought to be supporting cells in the neurogenic niche, providing signals that are important for maintaining and mobilizing the NSC populations [2, 74]. In the SVZ, type B cells resemble SGL type I cells. They express GFAP, nestin, sox1, sox2, BLBP and the astrocyte-specific L-glutamate L-aspartate transporter (GLAST). They give rise to GFAP-negative transit-amplifying type C cells, which then give rise to type A cells. Type A are PSA-NCAM- and DCX-expressing neuroblasts that migrate radially on “glial tubes” in the rostral migratory stream (RMS) to layers in the olfactory bulb (OB) before terminal differentiation [107]. Abbreviations: cc, corpus collosum; hipp, hippocampus).
Within the DG, newly formed neurons populate the inner third of the granule cell layer (GL). Two types of NSCs can be identified in the SGL according to their specific morphologies and expression of unique sets of molecular markers. Type I cells are similar to type B in the SVZ [6-8] and type II (nonradial) cells are similar to type C in the SVZ (Figure 1, [8]). Type I and II cells can be identified by distinct morphological and molecular markers (Figure 1). Newly formed neurons in the GCL send axonal projections to the CA3 subfield of the hippocampus and spineless dendrites to the molecular layer [9]. Both in the SGL and the SVZ, newly formed granule neurons have distinct characteristics that may uniquely contribute to brain and behavioral plasticity (see Refs. [10-12] for reviews).
Neurogenesis and learning and memory
Newly formed neurons are thought to play a role in brain function. In particular, the role of neurogenesis in olfaction- and hippocampal-dependent learning and memory seems to be multifaceted. Several approaches have been taken to elucidate the role of hippocampal neurogenesis in learning and memory (for a summary of methods used, see Box 1). It is critical for the interpretation of the data obtained in these studies to consider the method of intervention (chemical, genetic, environmental), the type of neurogenic pathway involved and its outcome on memory function (Box 2, Table S1 in the supplementary material online). For example, genetic manipulation of the neurotrophin-3 (NT-3)-dependent neurogenic pathway affects spatial memory [13], while manipulation of the presenilin-1 (PS1)-dependent neurogenic pathway affects contextual fear conditioning [14]. Environmental enrichment [15] and running [16] each produce increases in neurogenesis and enhanced performance on a spatial memory task in multiple strains of mice [17]. Conversely, stress paradigms [18], irradiation [19-21] and the DNA methylating agent methylazoxy-methanol acetate (MAM; [22]) all decrease neurogenesis and impair different aspects of hippocampal-dependent memory (Table S1 in the supplementary material online; for review see Ref. [23]).
While the role of neurogenesis in the SVZ is less clear, pioneering studies suggest that SVZ neurogenesis regulates synaptic plasticity in the olfactory bulb [24] and plays a functional role in olfaction (for review, see Ref. [25]). For example, a recent study suggests that both hippocampal- and SVZ- neurogenesis play a role in offspring recognition. Newly generated paternal olfactory interneurons were preferentially activated by adult offspring odors through prolactin signaling [26]. Likewise, male pheromone signatures induce neurogenesis in the SVZ and hippocampus of female mice [27]. Reinforcing an experience-based role for SVZ-neurogenesis in olfaction, another study suggests that exposure to male-soiled bedding, but not to its volatile compounds, increases the number of new neurons in the accessory olfactory bulb in mice [28]. Infusion of the antimitotic drug cytosine arabinoside (AraC) to the lateral ventricle abolished the arrival of newly born neurons into the olfactory bulb, resulting in decreased synchronized activity of mitral cells and impaired short-term olfactory memory [29]. In addition, long-term olfactory memory was found to be affected by focal irradiation of the SVZ, which decreased the rate of production of new olfactory bulb neurons [30]. Another study found that preventing cell death in the olfactory bulb (using a broad-spectrum caspase inhibitor) resulted in impaired odorant exploration and discrimination [34]), thus revealing an important role for neuronal turnover in the olfactory bulb.
Neurogenesis and Aging
Both germinal centers, the SVZ and the SGL, exhibit an age-related decline in the production of new neurons [31]. The age-related decline in cell proliferation and new neurons in the SVZ has been linked to functional decline in olfaction in mice [32], and in the SGL it is associated with decline in hippocampal-dependent spatial memory [31, 33, 34]. Despite an age-related reduction in the formation of new hippocampal neurons, the neurons that are added appear functionally equivalent to those in young brain [35, 36]. This observation suggests that neurogenesis in the aged brain is not aberrant, but simply downregulated. In support of that, reduction in cell proliferation in the DG and retarded neuronal maturation was observed in the aging SGL [37]. An interesting insight is provided by another study suggesting that the number of NSCs does not decline with aging, but that these cells exhibit increased quiescence that may be due to decreased volume of the vascular niche [38]. Further support for the notion that changes in the neurogenic milieu take place in aging is provided by the observation that the level of key neurotrophic factors such as fibroblast growth factor-2 (FGF-2), insulin growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF) are reduced in the aging hippocampus [39].
Maintenance of stem cell activity appears to be tightly controlled to prevent unregulated growth that could lead to cancer. Keeping stem cells out of the active cell cycle phase and minimizing the risk of DNA damage may be especially important in aging and has led to a stem cell hypothesis of aging [1, 40]. Tumor suppressor proteins, particularly p16INK4a, have been shown to suppress stem cell activity in many aging stem cell populations [41-43]. Curiously, p16INK4a regulates stem cell activation in the SVZ, but not in the hippocampus [42], suggesting a fundamentally different regulatory mechanism may be involved in the latter population. At this point, it is not known whether a different stem cell autonomous regulatory mechanism is at work or whether control of activation is mediated through signals in the neurogenic niche within the aged hippocampus. An important question about the age-related decline in neurogenesis that remains unanswered is whether there is intrinsic suppression of NSC proliferation and maturation, or whether the decline is due to lack of trophic support and deficits in the neurogenic niche. Resolving which of these two regulatory mechanisms is involved, or whether there is a relative contribution of both elements, will be necessary for advancing therapeutic stimulation of aging NSCs.
Neurogenesis impairments in AD
Progressive memory loss and cognitive decline are the fundamental characteristics of AD. In addition to memory loss and cognitive deterioration, individuals afflicted with the disease experience difficulties in learning, speed of performance, recall accuracy and problem solving (for review, see Ref. [44]). Impaired olfactory function (deficits in olfactory sensitivity, odor discrimination, and odor identification) appears to be one of the earliest detectable functional alterations in AD, and olfactory sensitivity and olfactory discrimination may prove to be useful as predictors of cognitive decline [45, 46].
Numerous studies employing different paradigms suggest that familial Alzheimer's disease (FAD)-linked transgenic mice exhibit impairments in learning and memory (for review, see Ref. [47]). These deficits include impairments in spatial reversal learning, acquisition of social recognition memory, acquisition of long-term spatial memory, utilization of spatial working memory, object recognition memory and contextual fear conditioning. Deficits in olfaction in mouse models of AD have been also described (for example, see Refs. [48-50]) One working hypothesis is that impaired adult neurogenesis may exacerbate memory deficits and impair olfactory sensory perception in AD, possibly by impairing hippocampal and olfactory neural circuits that support spatial memory and olfactory processing, respectively (for review, see Ref. [51]).
The neuropathological hallmarks of AD, senile plaques and neurofibrillary tangles, appear throughout the hippocampal formation and some regions of the cerebral cortex, and include both the SGL and SVZ neurogenic areas. Senile plaques are extracellular aggregates of amyloid β-peptide (Aβ) surrounded by dystrophic neurites. Aβ is liberated from a larger integral membrane protein APP, by the concerted action of β- and γ-secretase (for review, see Ref. [44]). The vast majority of AD cases are the sporadic form of the disease. While the genetic cause for AD is not known, homozygosity for apolipoprotein E4 (APOE4) is the greatest risk factor after aging. Rare, familial, early-onset autosomal dominant FAD is caused by mutations in genes encoding amyloid precursor protein (APP), presenilins (PS1 and PS2). Presenilins, as components of the aspartyl protease γ-secretase complex, cleave numerous membrane proteins within their membrane-spanning domains. Transgenic animals harboring mutant forms of APP and PS1 exhibit impaired neurogenesis in both SVZ and SGL early in life, that begins prior to Aβ deposition and memory impairment [52]. In addition to Aβ, hyperphosphorylation and aggregation of tau within neurons may underlie some of these impairments [52]. Decreased neurogenesis is also observed in older FAD mice (for example, see Refs. [53, 54]). Nevertheless, the extent of neurogenesis may either increase or decrease during the progression of symptoms, as a response to the neurodegenerative process [55]. Studies examining neurogenesis in transgenic mice expressing single mutant variants of APP or PS1 have yielded conflicting observations (see below), possibly due to the different roles that APP and PS1 play in the regulation of adult neurogenesis (for review, see Ref. [51]).
Physiological roles of APP and PS1 in neurogenesis
Although several studies have shown that transgenic mice expressing mutant APP or PS show impaired adult neurogenesis, little is known of the physiological role of either protein in neurogenesis. For example, APP is processed into three main fragments and all three proteolytic products have been shown to modulate neurogenesis differently. A soluble fragment of APP generated by α-secretase (sAPPα), exerts proliferative effects on embryonic NSC and also stimulates proliferation of progenitor cells in the adult SVZ [56]. The APP intracellular domain (AICD) generated by γ-secretase cleavage negatively modulates embryonic neurogenesis (possibly via binding to the Fe65 adapter protein) [57]. AICD also inhibits adult neurogenesis [58] by inducing proinflammatory changes in AICD overexpressing transgenic mice [59]. Studies on the effect of Aβ, the third fragment produced by APP, on adult neurogenesis have produced conflicting results. Some studies have found that Aβ negatively modulates neurogenesis, while others investigations have reported that Aβ42 (or oligomeric Aβ) stimulates adult SVZ neurogenesis in young but not old animals (for a detailed discussion, see Ref. [51]). Recent studies suggest that an imbalance between inhibitory γ-aminobutyric (GABAergic) and excitatory glutamatergic neurotransmission may differentially impair neurogenesis in animal models of AD. Thus, in APOE4-knockin mice, presynaptic GABAergic input-mediated maturation of newborn neurons is diminished and potentiation of GABAergic signaling restores neurogenesis in these mice to normal levels [60]. In mice expressing mutant forms of APP (hAPP-J20), early inhibition of GABAA receptors to suppress GABAergic signaling or late inhibition of calcineurin for the enhancement of glutamatergic signaling normalizes neurogenesis [61].
The mechanisms underlying impaired neurogenesis in FAD mice expressing PS1 variants remain unclear. For example, conditional knockout of PS1 in the forebrains of adult mice resulted in decreased hippocampal neurogenesis in mice that were housed in an enriched environment [14]. This also caused subtle deficits in the performance of spatial memory behaviorial tasks, such as the water maze [62]. Other studies confirmed that PS1 regulates the profileration in adult neurogenesis that is triggered by enriched environment [63], possibly via a mechanism involving microglia [64]. Overexpression of wild type PS1 was shown to promote adult neurogenesis, but FAD mutant PS1 failed to do so [65]. Similarly, mutant PS1 knockin (PS1-KI) mice exhibit decreased neurogenesis and impaired associative learning compared to wild type mice [66]. By contrast, PS1-KO mice rescued with mutant PS1 exhibited increased proliferation of newly-born cells in the DG compared to mice rescued with wild type PS1 [67]. This discrepancy might be attributed to the different genetic background of these mouse models. Nevertheless, these models are not fully adequate for examining the role of PS1 in adult neurogenesis, as PS1 is either mutated or knocked down in large populations of neurons throughout the brain and not just in NPC populations. Such experimental limitations raise concerns about the relevance of these observations for neurogenesis specifically, and calls for further examination of the role of PS1 in adult neurogenesis.
Modulation of Neurogenesis by the Environment in Aging and in AD
Hippocampal neurogenesis can be regulated by environmental factors. In particular, environmental enrichment has been shown to be a positive regulator of adult neurogenesis [15]. Subsequent research revealed that the main neurogenic component of the enriched environment is physical activity ([68], Figure 2). Exercise-induced neurogenesis is correlated with improved learning and memory [69]. Bone morphogenetic protein (BMP) signaling has been suggested to mediate the effects of exercise on hippocampal neurogenesis and improved cognitive performance [70]. Age-dependent reduction in neurogenesis can be partially prevented when animals are housed with a running wheel over a six months period [71]. In addition, the decline in neurogenesis and cognition associated with normal aging can also be partially reversed by exercise (using a running wheel) that is initiated in 18-month old mice [72], but not when initiated in very old mice (22 months old) [73], possibly due to loss of plasticity of rapid amplifying Type II progenitor cells [74].
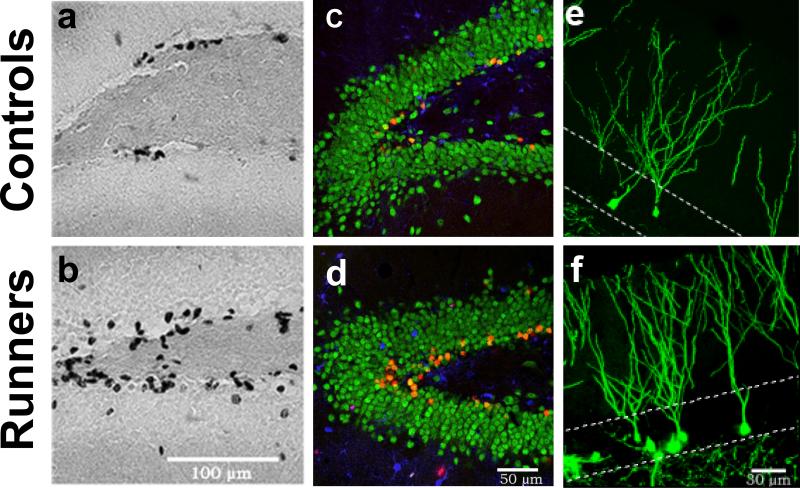
Figure 2. Regulation of cell proliferation and neurogenesis in the hippocampus by exercise.
(a,b) Photomicrographs of bromodeoxyuridine (BrdU) positive cells in the dentate gyrus (DG) of adult mice 1 day after the last of a series of 12 daily BrdU injections (50 mg/kg per day). Mice housed with a running wheel (b) have more BrdU+ cells than sedentary mice (a), showing that running increases cell proliferation. (c,d) Confocal images of sections that were immunofluorescent-triple-labeled for BrdU (red), NeuN (green; an indicator of neuronal phenotype), and S100β (blue, -selective for glial phenotype). Neurons double labeled for BrdU+ (red) and NeuN+ (green) appear orange. These images demonstrate that cell survival and neuronal differentiation is enhanced 4 weeks after the last BrdU injection in runners (d) relative to control (c) mice. (e,f) Labeling using a retrovirus (which only infects dividing cells) was used to identify new neurons [111]. Retrovirus expressing green fluorescent protein (GFP) was injected into the DG of sedentary (e) and running (f) mice. Confocal images show more GFP+ new neurons in running mice compared to sedentary mice 4 weeks after virus injection. The dashed lines represent the boundaries of the granule cell layer of the DG. Reproduced, with permission, from Ref. [112].
Although the benefits of exercise and enrichment on neurogenesis and learning are apparent in young mice, the data in mouse models of AD are not as clear. Environmental enrichment has been reported to improve cognition in mouse models of AD [51], and to enhance neurogenesis and ameliorate neuropathology in some mouse models of AD [75]. However, environmental enrichment did not enhance neurogenesis in transgenic mice harboring FAD-linked PS1 variants [64] and in mice with conditional ablation of PS1 in the forebrain [14]. Subsequent research suggests that soluble factors released from microglia may be responsible for the lack of an effect of enrichment in FAD-linked PS1 mutant mice [64]. In other AD mouse models, such as APOE4 transgenic mice, enrichment reduced neurogenesis [76], while in the APP23 mouse (which expresses a human mutation in the APP gene; hAPP751), it enhanced neurogenesis [77]. Taken together, these studies indicate that effects of environmental enrichment on adult neurogenesis vary greatly between the different mouse models of AD. Additional studies have reported beneficial effects of exercise on cognition, neurogenesis and amyloidosis in AD mouse models. In TgCRND8 mice (which express APP695swe,Ind), exercise improved spatial memory and reduced extracellular Aβ plaque load [78]. Exercise was also found to be beneficial in Tg2576 mice (which express APP695swe), even after the onset of pathology [79]. Similar to human studies on exercise, cognition, and APOE genotype [80], APOE4 mice have been shown to benefit from regular physical activity [81]. Future experiments should determine the effect of exercise on FAD animal models exhibiting different aspects of AD pathology.
Like increased exercise, moderation in dietary energy intake can lead to improved energy metabolism, the promotion of neurogenesis and the protection of the brain against the adversities of aging (Figure 3a). Overeating, and the obesity and diabetes that result from excessive energy intake, are a major cause of morbidity and premature death that is rapidly spreading throughout the industrialized world. Epidemiological and clinical studies have provided evidence that individuals who overeat are at increased risk for cognitive impairment, particularly when they reach their 5th and 6th decades of life [82]. Excessive dietary energy (calorie) intake can impair hippocampal neurogenesis in rat and mouse models [83], whereas dietary energy restriction (DR) enhances hippocampal neurogenesis [84]. A high-energy diet may adversely affect neurogenesis and cognitive function by increasing levels of systemic stress (hyperactivation of the hypothalamic - pituitary - adrenal axis) and intrinsic oxidative and inflammatory stress in neurons, and by reducing the production of brain derived neurotrophic factor (BDNF), protein chaperones and antioxidant enzymes [85, 86] (Figure 3b). These adverse effects of a high-energy diet may be counteracted, at least in part, by exercise, which has been demonstrated to stimulate production of BDNF [85].
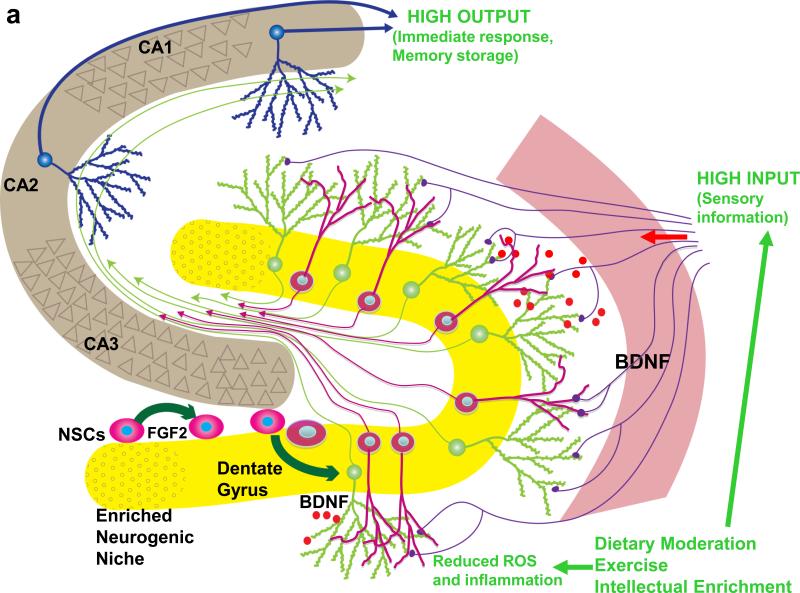
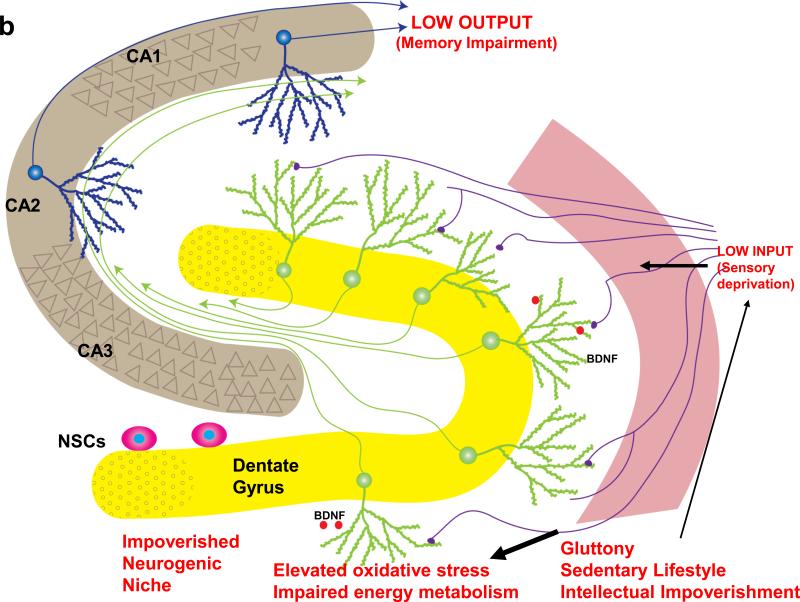
Figure3. Healthy and unhealthy lifestyles may differentially affect hippocampal plasticity and cognitive aging.
(a) A working model illustrating mechanisms by which a moderation of dietary energy intake, exercise and a cognitively challenging lifestyle can enhance hippocampal plasticity and sustain cognitive performance into late life. Exercise, dietary energy restriction and intellectual challenges all increase the activation of excitatory input to dendrites of granule neurons (green neurons) in the dentate gyrus. This synaptic activity induces the expression of neurotrophic factors such as brain derived neurotrophic factor (BDNF) and fibroblast growth factor 2 (FGF-2), which have multiple actions on neural stem cells (NSCs) and differentiated neurons that enhance hippocampal functional capability. BDNF signaling increases the strength of potentiated synapses and also acts on NSCs to promote their differentiation into neurons. FGF-2 promotes the proliferation of stem cells to increase the NSC pool available to form new neurons and glial cells. By increasing neuronal network activity, healthy lifestyles can reduce levels of reactive oxygen species (ROS), hence reducing oxidative stress, and bolstering energy metabolism in hippocampal neurons and NSCs, thereby counteracting the aging process. (b) Mechanisms by which excessive energy intake and low levels of energy expenditure adversely affect neurogenesis and cognitive function. Overeating/gluttony, physical inactivity and a cognitively impoverished lifestyle all result in suboptimal levels of input to the hippocampus. The low neuronal network activity, in turn, results in reduced levels of neurotrophic factors such as BDNF and FGF-2, as well as lower levels of neuroprotective protein chaperones and antioxidants. Consequently, NSC proliferation and differentiation into dentate granule neurons is suppressed, and synapses may become dysfunctional and even degenerate. Elevated levels of oxidative and metabolic stress contribute to impaired synaptic plasticity and cognitive impairment. Diagram adapted from Ref. [113].
Modulation of Neurogenesis as a Therapeutic Approach: Minding the Neurogenic Niche
The studies described above imply that modulation of self-renewal, proliferation, migration and differentiation of endogenous neural stem and progenitor cells may hold great promise for the maintenance of brain plasticity, the preservation of learning and memory capabilities, the prevention of aging-linked decline in neurogenesis, and for the repair of the diseased brain. A prerequisite for the modulation of neurogenesis is the identification of molecular targets regulating these processes. The neurogenic niche is thought to be a specialized microenvironment within the adult brain, which has the capacity to sustain self-renewal of multipotent NSCs and promote their migration, as well as their differentiation into neurons and glia [87]. Adult progenitor cells derived from non-neurogenic areas exhibit self-renewal and multipotentiality once transplanted in a neurogenic brain area, and can differentiate in a region-specific context, suggesting that the microenvironment has a crucial role in providing and regulating fate-determining cues in the adult brain [88].
What makes the SVZ and SGL special in supporting the proliferation and differentiation of multipotent neural progenitors is an area of intensive investigation. It is postulated that endothelial cells and some specialized astrocytes provide a unique neurogenic niche and have the capability to promote proliferation and neuronal fate determination [89, 90]. In contrast, astrocytes from non-neurogenic regions, e.g., the adult spinal cord, do not promote neuronal differentiation [90]. In vivo hot spots of cell proliferation in the SGL are found to be in close proximity to capillaries and astrocytes [91, 92]. It is thought that astrocytes in the neurogenic niche have a broad diversity of functions. For example, some behave like stem cells [92, 93] and some provide neurogenic signals [90, 94]. The neurogenic niche is believed to play a regulatory role in all steps of NSC maturation [95]. It is thought that the niche is comprised of soluble, membrane-tethered and extracellular matrix signaling molecules expressed by endothelial cells, GFAP-expressing NSC, NPCs, as well as ependymal cells in the SVZ niche [96]. Progenitor cells actively interact with their microenvironment and have the capability to regulate it [90, 97]. Numerous signaling pathways, some of which are developmental signals, are implicated in the regulation of adult neurogenesis. For example, epidermal growth factor (EGF) and FGF-2 have been shown to play a major role in the proliferation of progenitor cells. Wingless-type MMTV integration site family, member 3 (Wnt3), TLX, Sonic Hedgehog (Shh), BMP antagonists, Notch, leukemia inhibitory factor, transforming growth factor-alpha and cytokines have also been shown to play a role in progenitor proliferation and maintenance. Additional neurotrophic and growth factors such as BDNF, VEGF and the neurotransmitters GABA, glutamate and serotonin contribute significantly to the proliferation, differentiation and integration of new neurons into the existing circuitry (for review, see Refs. [2, 98]), while DCX and NCAM have been specifically implicated as being important factors involved in neuroblast migration. It is noteworthy that extensive migration capacity in amphibians and reptiles is thought to play a role in the high regenerative capacity of these organisms [99]. In the mammalian brain, migration of progenitors is widespread neonatally, and dramatically ceases with adulthood. Understanding the molecular processes that support neurogenesis would enable the enhancement of specific neurogenic processes and facilitate successful transplantation of stem cells into the brain.
Stem Cell Therapy for the Aging Brain
Given the age-related increase in burden of neurological diseases and injury, such as stroke, the idea of transplanting NSCs into the impaired aging brain has great appeal. As discussed above, despite the reduction in neurogenesis in the aged brain and the delayed maturation of the newly generated neurons, the aged hippocampus appears to retain sufficient environmental niche signals to support the normal maturation of new neurons. Indeed, there are a few reports describing cognitive improvement following transplantation of NPC into the aged hippocampus in both rats [112] and humans [100, 101]. Another relevant model is the transplantation of NPCs into the injured, aged hippocampus. A successful differentiation of NPCs grafted into the aged hippocampus was achieved in an excitotoxic injury model in rats [102]. However, in a cautionary observation of the future challenges to therapeutic use of stem cells in the aged brain, a quite low rate of efficiency in this process was noted compared to experience with grafts transplanted into the young hippocampus. It remains to be determined if the efficiency of neuronal differentiation can be augmented by priming the environment to modulate expression of some of the signaling factors discussed above. Interestingly, grafting of NPCs appears to stimulate endogenous neurogenesis within the aged hippocampus [103]. Notably, recent studies show that transplantation of NPCs into a mouse model of FAD rescues cognitive deficits in these mice [104] via BDNF signaling [105].
The goal of stem cell therapy would be to introduce new neurons that could contribute to functional enhancement or reconstruction of impaired neuronal circuitry. In this regard, it may not be necessary to transplant exogenous neural stem/progenitor cells to achieve this aim. An alternative therapeutic strategy may be to enhance endogenous neurogenesis. As discussed above, neurogenesis in the aged brain can be stimulated by a variety of factors and the modulation of the neurogenic niche may be accomplished by elevating levels of the various proliferative and differentiation signals mentioned above. Such a recruitment strategy can be envisioned for the aged neurogenic regions within the hippocampus and olfactory bulb, but may prove more difficult for the greater portion of the cortical and subcortical structures where neurodegeneration can also occur during the aging process and in neurological disorders such as AD and stroke. Recruitment of endogenous neural stem/progenitor cells would then rely upon directed migration from the neurogenic regions or mobilization of the rare progenitor-like cells distributed throughout the brain parenchyma [106]. Given the overall reduction of neurogenic signals in the aged brain, such recruitment strategies for these other brain regions may be difficult to achieve.
Conclusion/ Future Directions
The existence of neurogenic niches in the adult mammalian brain has initiated much hope for the use of NSCs for the therapy of the aging and diseased brain. Enhancement of brain plasticity, learning and memory, improved cognition and attenuation of neurodegeneration are only some of the high expectations of this therapy. Whether exogenous neural stem/progenitor cells are transplanted or locally recruited, their successful survival, differentiation, and functional integration will be undertaken on a background of neurodegenerative pathology. Therapeutic stem cells will provide little benefit if they fall prey to the same pathology they are intended to reverse. Future experimental strategies will need to ascertain the extent of this risk and consider approaches to conditioning the environment into which the cells are placed to protect the new cells. Alternatively, the therapeutic cells might be themselves genetically modified to produce the appropriate conditioning signals. Successful implementation of these strategies will require a more complete understanding of the environmental signals necessary to foster successful neuronal differentiation, functional integration into neuronal circuits and protection against pathology (Box 3).
Box 2 Figure I.
Increased neurogenesis is associated with improved discrimination between the locations of two adjacent identical stimuli. (a,b,c) A mouse learns to choose between two similar objects when only one of the objects is reinforced with a food reward. (a) During the training phase mice learn to touch a screen for a food pellet reward. (b) Thereafter, mice were housed with or without a running wheel and learned to differentiate between a rewarded and non-rewarded icon. (c) The mouse chooses the correct (reinforced) icon and is rewarded with a food pellet. (d) Subsequently, sedentary and running mice were tested with either a large or small distance between two identical touchscreen icons. Mice were trained to reach a criterion of 7 correct choices of the food-pellet rewarded trials out of a total of 8 trials. Mice that were housed with a running wheel in their cage (run) performed better than controls (con) in the acquisition of the small separation (*p<0.05), but not the big separation condition (p>0.24). (e) A trend towards a correlation between new neuron density in the DG and perfomance in the acquisition of the small separation was observed (p=0.13). Photomicrographs of bromodeoxyuridine (BrdU)-positive cells .in the DG of (f) control and (g) exercising mice ten weeks after the last injection of BrdU. BrdU is commonly used as a marker for dividing cells, since it incorporates into the newly synthesized DNA of dividing cells during the S phase, substituting for thymidine. Scale bar=50 μm. Reproduced, with permission, from Ref. [73].
Box 1. Neurogenesis and learning and memory: How do we make the connection?
Attempts to examine the relationship between neurogenesis and learning and memory have so far included:
- Modulation or ablation of neurogenesis and examination of the implications for learning and memory processes:
- Anti-mitotic drugs- would destroy proliferating neural progenitor cells, and any other proliferating cells in the brain (e.g. astrocytes). These drugs may affect quiescent NSCs to a lesser extent, as well as having potential secondary toxic effects on the parenchymal environment and its cellular residents (eg. see Ref. [22]).
- Irradiation- low dose irradiation would eliminate proliferating cells, while affecting quiescent NSCs to a lesser extent (eg. see Ref. [24]). Thus, days or weeks after the irradiation, NPC populations would be replenished. Irradiation causes an inflammatory reaction that lasts for several weeks. This may cause secondary damage to neuronal populations and may affect the neurogenic microenvironments.
- Expression of toxins driven by specific promoters- toxins driven by the promoters of genes that are active in NSC or neural progenitor cells (NPCs) would enable a selective elimination of a subpopulation of these cells. Examples of such gene promoters are, nestin-, Sox2+ and GFAP (eg. see Ref. [114]).
- Expression of different genes driven by specific promoters- this would affect specific signaling pathways regulating NSC biology and may change the course of neurogenesis (eg. see Ref. [115]).
- Manipulation of the environment, such as running, environmental enrichment. See for example, Ref. [69].
Tracing alterations in neurogenesis following behavioral training.
Tracing of NSCs, NPCs and/or their subpopulations, by, for example, nucleotide analogs, expression of retrovirally-expressed fluorescent proteins etc. Animals are then subject to learning and memory-related task or behavioral manipulation See for example, Ref. [116].
Box 2. Insight into the functional significance of adult hippocampal neurogenesis.
It has long been known that the hippocampus is important for the acquisition of new memories [108]. However, the concept that different hippocampal subfields (dentate gyrus (DG), Area CA1, Area CA3) may make specific contributions to memory formation has been investigated only more recently [109]. The DG is considered important for spatial pattern separation, the ability to make fine spatial distinctions. Adult neurogenesis may be closely linked to this proposed function of the DG [73, 110]. Using either a physiological model of newly born cell ablation, the natural decline of neurogenesis to very low levels in old mice [73], or by x-irradiation in young mice, deficits in distinguishing between narrowly separated stimuli have been observed [73, 110] .
Box 3. Outstanding Questions.
What is the significance of adult neurogenesis in relation to cognitive functions, and specifically in relation to the function of the DG and hippocampus, in humans?
What are the molecular signals underlying neurogenesis in the adult brain?
To what extent does deficient neurogenesis exacerbate cognitive impairments in humans affected with AD?
Can recruited or grafted progenitor cells functionally contribute to circuitry in non-neurogenic regions of the brain, particularly on a background of aging and neurodegeneration?
Is the aging-linked decline in neurogenesis comparable to stem cell decline in other organs?
Is there a peripheral biomarker that could be used to detect reduced neurogenesis?
Supplementary Material
Acknowledgements
The authors’ work was supported by the NIH AG033570, AG036208Z (OL), AG20047 and AG22555 (DAP) and AG026146 (SWP); The Intramural Research Program of the National Institute on Aging (MM; HvP); Alzheimer's Association Young Investigator Award, Alzheimer's disease Research Fund, IDPH, and the Brain Research Foundation (OL). The authors thank Archana Gadadhar, Yuan-shih Hu and Michael Demars for producing images presented in this manuscript. The authors thank KC Alexander and David J. Creer for figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nature reviews Mol Cell Biol. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 2.Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 3.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22(14):6106–6013. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carleton A, et al. Becoming a new neuron in the adult olfactory bulb. Nat Neurosci. 2003;6(5):507–518. doi: 10.1038/nn1048. [DOI] [PubMed] [Google Scholar]
- 5.Platel JC, Lacar B, Bordey A. GABA and glutamate signaling: homeostatic control of adult forebrain neurogenesis. J Mol Histol. 2007;38(6):602–610. doi: 10.1007/s10735-007-9153-y. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda S, et al. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23(28):9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7(11):1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 8.Suh H, et al. In Vivo Fate Analysis Reveals the Multipotent and Self-Renewal Capacities of Sox2(+) Neural Stem Cells in the Adult Hippocampus. Cell Stem Cell. 2007;1(5):515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao C, et al. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- 11.Ge S, et al. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30(1):1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Kee N, et al. Preferential incorporation of adult-generated granule cells into spatial memory networks in the dentate gyrus. Nat Neurosci. 2007;10(3):355–362. doi: 10.1038/nn1847. [DOI] [PubMed] [Google Scholar]
- 13.Shimazu K, et al. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13(3):307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng R, et al. Deficient neurogenesis in forebrain-specific presenilin-1 knockout mice is associated with reduced clearance of hippocampal memory traces. Neuron. 2001;32(5):911–926. doi: 10.1016/s0896-6273(01)00523-2. [DOI] [PubMed] [Google Scholar]
- 15.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 16.van Praag H, et al. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16(1):129–136. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 18.Lemaire V, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97(20):11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rola R, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Madsen TM, et al. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119(3):635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- 21.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 22.Shors TJ, et al. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bizon JL, Gallagher M. More is less: neurogenesis and age-related cognitive decline in Long-Evans rats. Sci Aging Knowledge Environ. 2005;2005(7):re2. doi: 10.1126/sageke.2005.7.re2. [DOI] [PubMed] [Google Scholar]
- 24.Nissant A, et al. Adult neurogenesis promotes synaptic plasticity in the olfactory bulb. Nat Neurosci. 2009;12(6):728–730. doi: 10.1038/nn.2298. [DOI] [PubMed] [Google Scholar]
- 25.Doetsch F, Hen R. Young and excitable: the function of new neurons in the adult mammalian brain. Current opinion in neurobiology. 2005;15(1):121–128. doi: 10.1016/j.conb.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 26.Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13(6):753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- 27.Mak GK, et al. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10(8):1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 28.Oboti L, et al. Integration and sensory experience-dependent survival of newly-generated neurons in the accessory olfactory bulb of female mice. Eur J Neurosci. 2009;29(4):679–692. doi: 10.1111/j.1460-9568.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- 29.Breton-Provencher V, et al. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J Neurosci. 2009;29(48):15245–15257. doi: 10.1523/JNEUROSCI.3606-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarini F, et al. Cellular and behavioral effects of cranial irradiation of the subventricular zone in adult mice. PLoS One. 2009;4(9):e7017. doi: 10.1371/journal.pone.0007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal GM, Peterson DA. Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell. 2004;3(6):345–351. doi: 10.1111/j.1474-9728.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 32.Enwere E, et al. Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J Neurosci. 2004;24(38):8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3(4):227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- 34.Dupret D, et al. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3(4):e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morgenstern NA, Lombardi G, Schinder AF. Newborn granule cells in the ageing dentate gyrus. J Physiol. 2008;586(16):3751–3757. doi: 10.1113/jphysiol.2008.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toni N, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11(8):901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao MS, et al. Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur J Neurosci. 2005;21(2):464–476. doi: 10.1111/j.1460-9568.2005.03853.x. [DOI] [PubMed] [Google Scholar]
- 38.Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol Aging. 2008;29(1):129–147. doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51(3):173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 40.Beausejour CM, Campisi J. Ageing: balancing regeneration and cancer. Nature. 2006;443(7110):404–405. doi: 10.1038/nature05221. [DOI] [PubMed] [Google Scholar]
- 41.Janzen V, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443(7110):421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 42.Molofsky AV, et al. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443(7110):448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino J, et al. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135(2):227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001;81(2):741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki Y, et al. Smell identification test as an indicator for cognitive impairment in Alzheimer's disease. Int J Geriatr Psychiatry. 2004;19(8):727–733. doi: 10.1002/gps.1161. [DOI] [PubMed] [Google Scholar]
- 46.Wilson RS, et al. The relationship between cerebral Alzheimer's disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78(1):30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashe KH. Learning and memory in transgenic mice modeling Alzheimer's disease. Learn Mem. 2001;8(6):301–308. doi: 10.1101/lm.43701. [DOI] [PubMed] [Google Scholar]
- 48.Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2009;30(9):1430–1443. doi: 10.1016/j.neurobiolaging.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 49.Guerin D, et al. Early locus coeruleus degeneration and olfactory dysfunctions in Tg2576 mice. Neurobiol Aging. 2009;30(2):272–283. doi: 10.1016/j.neurobiolaging.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 50.Wesson DW, et al. Olfactory dysfunction correlates with amyloid-beta burden in an Alzheimer's disease mouse model. J Neurosci. 2010;30(2):505–514. doi: 10.1523/JNEUROSCI.4622-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: At the crossroads. Exp Neurol. 2010;223(2):267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demars M, et al. Impaired neurogenesis is an early event in the etiology of familial Alzheimer's disease in transgenic mice. J neurosci res. 2010;88(10):2103–2117. doi: 10.1002/jnr.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verret L, et al. Alzheimer's-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang C, et al. Long-lasting impairment in hippocampal neurogenesis associated with amyloid deposition in a knock-in mouse model of familial Alzheimer's disease. Exp Neurol. 2007;204(1):77–87. doi: 10.1016/j.expneurol.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Q, et al. Adult neurogenesis is functionally associated with AD-like neurodegeneration. Neurobiol Dis. 2008;29(2):316–326. doi: 10.1016/j.nbd.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caille I, et al. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131(9):2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 57.Ma QH, et al. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008;10(3):283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- 58.Ghosal K, Stathopoulos A, Pimplikar SW. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS One. 2010;5(7):e11866. doi: 10.1371/journal.pone.0011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosal K, et al. Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc Natl Acad Sci U S A. 2009;106(43):18367–18372. doi: 10.1073/pnas.0907652106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li G, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5(6):634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun B, et al. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer's Disease. Cell Stem Cell. 2009;5(6):624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31(5):713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 63.Wen PH, et al. The presenilin-1 familial Alzheimer disease mutant P117L impairs neurogenesis in the hippocampus of adult mice. Exp Neurol. 2004;188(2):224–237. doi: 10.1016/j.expneurol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Choi SH, et al. Non-cell-autonomous effects of presenilin 1 variants on enrichment-mediated hippocampal progenitor cell proliferation and differentiation. Neuron. 2008;59(4):568–580. doi: 10.1016/j.neuron.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wen PH, et al. Overexpression of wild type but not an FAD mutant presenilin-1 promotes neurogenesis in the hippocampus of adult mice. Neurobiol Dis. 2002;10(1):8–19. doi: 10.1006/nbdi.2002.0490. [DOI] [PubMed] [Google Scholar]
- 66.Wang R, et al. Presenilin 1 familial Alzheimer's disease mutation leads to defective associative learning and impaired adult neurogenesis. Neuroscience. 2004;126(2):305–312. doi: 10.1016/j.neuroscience.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 67.Chevallier NL, et al. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167(1):151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Praag H. Neurogenesis and exercise: past and future directions. Neuromolecular Med. 2008;10(2):128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 69.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 70.Gobeske KT, et al. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4(10):e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kronenberg G, et al. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27(10):1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 72.van Praag H, et al. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Creer DJ, et al. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci U S A. 2010;107(5):2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lugert S, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6(5):445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 75.Hu YS, et al. Complex environment experience rescues impaired neurogenesis, enhances synaptic plasticity, and attenuates neuropathology in familial Alzheimer's disease-linked APPswe/PS1ΔE9 mice. FASEB J. 2010;24(6):1667–1681. doi: 10.1096/fj.09-136945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levi O, Michaelson DM. Environmental enrichment stimulates neurogenesis in apolipoprotein E3 and neuronal apoptosis in apolipoprotein E4 transgenic mice. J Neurochem. 2007;100(1):202–210. doi: 10.1111/j.1471-4159.2006.04189.x. [DOI] [PubMed] [Google Scholar]
- 77.Mirochnic S, et al. Age effects on the regulation of adult hippocampal neurogenesis by physical activity and environmental enrichment in the APP23 mouse model of Alzheimer disease. Hippocampus. 2009;19(10):1008–1018. doi: 10.1002/hipo.20560. [DOI] [PubMed] [Google Scholar]
- 78.Adlard PA, et al. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25(17):4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf SA, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60(12):1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Etnier JL, et al. Cognitive performance in older women relative to ApoE-epsilon4 genotype and aerobic fitness. Med Sci Sports Exerc. 2007;39(1):199–207. doi: 10.1249/01.mss.0000239399.85955.5e. [DOI] [PubMed] [Google Scholar]
- 81.Nichol K, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5(4):287–294. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor VH, MacQueen GM. Cognitive dysfunction associated with metabolic syndrome. Obes Rev. 2007;8(5):409–418. doi: 10.1111/j.1467-789X.2007.00401.x. [DOI] [PubMed] [Google Scholar]
- 83.Stranahan AM, Mattson MP. Impact of energy intake and expenditure on neuronal plasticity. Neuromolecular Med. 2008;210(4):209–218. doi: 10.1007/s12017-008-8043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee J, Seroogy KB, Mattson MP. Dietary restriction enhances neurotrophin expression and neurogenesis in the hippocampus of adult mice. J Neurochem. 2002;80(3):539–547. doi: 10.1046/j.0022-3042.2001.00747.x. [DOI] [PubMed] [Google Scholar]
- 85.Stranahan AM, et al. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus. 2009;19(10):951–961. doi: 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Arumugam TV, et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ninkovic J, Gotz M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17(3):338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Shihabuddin LS, et al. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000;20(23):8727–8735. doi: 10.1523/JNEUROSCI.20-23-08727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doetsch F. A niche for adult neural stem cells. Curr Opin Genet Dev. 2003;13(5):543–550. doi: 10.1016/j.gde.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417(6884):39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- 91.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425(4):479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 92.Seri B, et al. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21(18):7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Doetsch F, et al. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97(6):703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 94.Lim DA, Alvarez-Buylla A. Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A. 1999;96(13):7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Seidenfaden R, et al. Glial conversion of SVZ-derived committed neuronal precursors after ectopic grafting into the adult brain. Mol and Cell Neurosci. 2006;32(1-2):187–198. doi: 10.1016/j.mcn.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 96.Lim DA, Huang YC, Alvarez-Buylla A. The adult neural stem cell niche: lessons for future neural cell replacement strategies. Neurosurg Clin N Am. 2007;18(1):81–92. ix. doi: 10.1016/j.nec.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 97.Shen Q, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304(5675):1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 98.Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58(6):865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaslin J, Ganz J, Brand M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2008;363(1489):101–22. doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodges H, et al. Conditionally immortal neuroepithelial stem cell grafts reverse age-associated memory impairments in rats. Neuroscience. 2000;101(4):945–955. doi: 10.1016/s0306-4522(00)00408-5. [DOI] [PubMed] [Google Scholar]
- 101.Qu T, et al. Human neural stem cells improve cognitive function of aged brain. Neuroreport. 2001;12(6):1127–1132. doi: 10.1097/00001756-200105080-00016. [DOI] [PubMed] [Google Scholar]
- 102.Shetty AK, Rao MS, Hattiangady B. Behavior of hippocampal stem/progenitor cells following grafting into the injured aged hippocampus. J Neurosci Res. 2008;86(14):3062–3074. doi: 10.1002/jnr.21764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park DH, et al. Increased neuronal proliferation in the dentate gyrus of aged rats following neural stem cell implantation. Stem Cells Dev. 2010;19(2):175–180. doi: 10.1089/scd.2009.0172. [DOI] [PubMed] [Google Scholar]
- 104.Yamasaki TR, et al. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J Neurosci. 2007;27(44):11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Blurton-Jones M, et al. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci U S A. 2009;106(32):13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hallbergson AF, Gnatenco C, Peterson DA. Neurogenesis and brain injury: managing a renewable resource for repair. J Clin Invest. 2003;112(8):1128–1133. doi: 10.1172/JCI20098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–184. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 109.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11(6):626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 110.Clelland CD, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Praag H, et al. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kobilo T, Potter MC, van Praag H. Neurogenesis and exercise. Encyclopedia of Behavioral Neuroscience. 2010;3:404–409. [Google Scholar]
- 113.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 114.Imayoshi I, et al. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11(10):1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 115.Zhang CL, et al. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451(7181):1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- 116.Trouche S, et al. Recruitment of adult-generated neurons into functional hippocampal networks contributes to updating and strengthening of spatial memory. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5919–5924. doi: 10.1073/pnas.0811054106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.