Abstract
Ligands acting at the same receptor can differentially activate distinct signal transduction pathways, which in turn, can have diverse functional consequences. Further, receptors expressed in different tissues may utilize intracellular signaling proteins in response to a ligand differently as well. The mu opioid receptor (MOR), which mediates many of the pharmacological actions of opiate therapeutics, is also subject to differential signaling in response to diverse agonists. To study the effect of diverse agonists on MOR signaling, we examined the effects of chronic opiate treatment on two distinct physiological endpoints, antinociceptive tolerance and physical dependence, in mice lacking the intracellular regulatory molecule, βarrestin2. While βarrestin2 knockout (βarr2-KO) mice do not become tolerant to the antinociceptive effects of chronic morphine in a hot plate test, tolerance develops to the same degree in both wild type and βarr2-KO mice following chronic infusion with methadone, fentanyl, and oxycodone. Studies here also assess the severity of withdrawal signs precipitated by naloxone following chronic infusions at three different doses of each opiate agonist. While there are no differences in withdrawal responses between genotypes at the highest dose of morphine tested (48 mg/kg/day), the βarr2-KO mice display several less severe withdrawal responses when the infusion dose is lowered (12 or 24 mg/kg/day). Chronic infusion of methadone, fentanyl, and oxycodone all lead to equivalent naloxone-precipitated withdrawal responses in both genotypes at all doses tested. These results lend further evidence that distinct agonists can differentially impact on opioid-mediated responses in vivo in a βarrestin2-dependent manner.
Keywords: opioid, G protein-coupled receptor, mu opioid receptor, antinociception, withdrawal, functional selectivity
1. Introduction
Opioid analgesics are commonly used to treat moderate to severe pain. Long-term administration, however, is associated with the development of undesirable side effects including analgesic tolerance and physical dependence. A number of studies have demonstrated that the physiological actions of morphine and other clinically used opiates are mediated primarily through activation of the mu opioid receptor (MOR), a G protein-coupled receptor (Matthes et al., 1996; Sora et al., 1997; Roy et al., 1998; Kieffer et al., 1999). While diverse signaling components and complex neuronal adaptations contribute to the development of analgesic tolerance and physical dependence, considerable evidence suggests that regulation of MOR signaling can affect these adaptive responses.
Previously, we have shown that βarrestin2, an important G protein-coupled receptor regulatory protein, differentially regulates opiate effects in a manner that is determined by the agonist. In βarrestin2 knockout (βarr2-KO) mice, acute morphine and heroin-induced antinociception is enhanced and prolonged, while acute antinociceptive responses to etorphine, methadone, and fentanyl are similar to those observed in their wild type (WT) counterparts (Bohn et al., 1999, 2004). Further, βarr2-KO mice also do not develop antinociceptive tolerance in a hot plate test following treatment with a single high dose of morphine or after chronic treatment using either repeated injections of morphine for 9 days or implantation with a 75 mg morphine pellet for 3 days (Bohn et al., 2000, 2002). However, the extent of physical dependence produced following a 72 hr treatment with the 75 mg morphine pellet is equivalent between both WT and βarr2-KO mice (Bohn et al., 2000). These earlier studies demonstrate a complex role for βarrestin2 in the regulation of morphine-induced antinociceptive tolerance and physical dependence; however, its role in MOR regulation with other agonists following chronic administration has not been previously investigated.
In this study, we have evaluated the development of tolerance and dependence in WT and βarr2-KO mice following chronic opiate infusion using implanted osmotic pumps. Since patients suffering from moderate to severe pain are commonly treated with sustained released formulations of morphine (MS Contin®), fentanyl (Duragesic®), and oxycodone (Oxycontin®), a continuous drug infusion paradigm used in these studies may closely mimic the exposure to opiate drugs in a clinical setting. Furthermore, while prior studies suggest that βarrestin2 does not play a significant role in morphine-induced physical dependence or in the display of somatic withdrawal signs, morphine was previously administered using a 75 mg pellet implantation (Bohn et al., 2000). Since this high dosing regimen may have occluded βarrestin2 contributions to this adaptive response, the effects of lower doses of morphine, as well as several doses of methadone, fentanyl and oxycodone, are assessed in this current study.
2. Methods
2.1. Animals
Male WT and βarr2-KO mice were generated from heterozygous breeding as previously described (Bohn et al., 1999). Mice were age-matched (3-6 months old), weighed between 20 and 35 grams, were group housed in a temperature-controlled room, and were maintained on a 12 hr reversed light/dark cycle. All behavioral studies were conducted during the light phase of the animal's circadian cycle. Mice had free access to food and water prior to experiments. Both genotypes were tested in parallel and each mouse was used only once for each experimental assay. All studies were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and with approval by The Ohio State University and The Scripps Research Institute Animal Care and Use Committees.
2.2. Drugs
Morphine sulfate pentahydrate and (±)-methadone hydrochloride were generously provided by the National Institute on Drug Abuse Drug Program (Bethesda, MD) or purchased from Sigma-Aldrich (St Louis, MO). Fentanyl citrate salt and oxycodone hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO), and naloxone hydrochloride dihydrate was purchased from Tocris Bioscience (Ellisville, MO). Drugs were dissolved in phosphate buffered saline for acute injections or in sterile distilled water when delivered using osmotic pumps. Opiate agonists were administered subcutaneously (s.c.) either by injection or osmotic pump, while the opiate antagonist naloxone was given intraperitoneally (i.p.). All drugs were injected at a volume of 10 μL/g body weight and freshly prepared prior to use. All studies were performed in parallel such that age matched WT and βarr2-KO mice received the same drug treatment at the same time. In order to avoid a day effect, we performed the studies in small cohorts of mice (2-5 WT, 2-5 KO) at a time. Upon comparison, the results obtained were uniform across sampling days and were combined for analysis.
2.3 Osmotic Pump Implantation
A single osmotic pump was subcutaneously implanted on the back of each mouse while under light isoflurane anesthesia. A small incision was made in the skin between the mouse's scapulae with scissors and a small pocket was formed just beneath the skin. The pump was then inserted and the incision was closed using 9-mm wound clips (Clay Adams Co., NY). To ensure immediate and optimal drug delivery, osmotic pumps were submersed in a 0.9% NaCl solution and incubated at 37°C for a minimum of 6 hrs prior to implantation for the tolerance studies to promote immediate release of drug upon implantation and to facilitate the cumulative dosing studies as described below.
2.4. Hot plate Procedure
Opiate effects on paw withdrawal latencies to a thermal nociceptive stimulus were assessed using a hot plate analgesia meter maintained at 54°C (Columbus Instruments, Columbus, OH) (Bohn et al., 1999; Raehal et al., 2009). The time required for the mouse to either flick or lick its fore- or hindpaw(s) was measured to the nearest 0.1 s. A maximal possible response latency of 30 s was used to prevent tissue damage. Antinociception was calculated as the percentage of maximum possible effect (% MPE) using the following formula: % MPE = 100% × [(drug response latency − basal latency)/(30 s− basal latency)].
2.5. Tolerance Paradigms
Antinociceptive tolerance was assessed using two different treatment paradigms with the hot plate test. In both paradigms, basal latencies were measured immediately prior to pump implantation. In the first paradigm, mice were then chronically infused with morphine (48 mg/kg/day, s.c.), methadone (96 mg/kg/day, s.c.) or oxycodone (25 mg/kg/day, s.c.) at a rate of 1 μl/hr using Alzet mini-osmotic pumps (Model 2001, Durect Corporation, Cupertino, CA). Fentanyl (3.2 mg/kg/day, s.c.) was delivered at a rate of 0.5 μl/hr using Alzet micro-osmotic pumps (Model 1007D, Durect Corporation, Cupertino, CA). Response latencies were measured at 1, 3, and 5 days following pump implantation.
In the second tolerance paradigm, mice were treated with morphine, methadone, fentanyl, or oxycodone using a cumulative dosing regimen prior to pump implantation. Response latencies were measured following each dose at the time of peak effect: this is 30 min for morphine (Bohn et al., 1999, 2004) and methadone (Bohn et al., 2004); 10 min for fentanyl (Bohn et al., 2004); and 15 min for oxycodone (Yoburn et al., 1995; Madia et al., 2009). Two hours after the final injection, mice were implanted with an osmotic pump containing the same drug that was used in the cumulative dosing at concentrations and volumes described in the first paradigm. On day 7, mice were again treated using a cumulative dosing regimen and response latencies were assessed at the same time points as on day 1. To ensure that multiple exposures to the hot plate on day 1 did not influence morphine responses on day 7, 3-4 mice/genotype were treated with a single 20 mg/kg dose (the final cumulative dose) of morphine and were tested 30 min later on day 1. Chronic morphine administration and cumulative dosing were then assessed as described above on day 7. Statistical analysis of this group revealed that there were no differences in the two groups and therefore, these results were combined and analyzed collectively. For some of the experiments, another member of the lab who was blind to genotype and dose observed the hot plate responses and confirmed measurements.
2.6. Physical Dependence Studies
To induce physical dependence, mice were chronically treated with morphine (12, 24 or 48 mg/kg/day), methadone (48, 60 or 72 mg/kg/day), fentanyl (0.8, 1.6 or 3.2 mg/kg/day) or oxycodone (12.5, 25 or 75 mg/kg/day) via subcutaneous implantation of osmotic pumps. The extent of physical dependence that developed was assessed following 7 days of continuous drug infusion by precipitating withdrawal with an acute injection of naloxone (0.5 mg/kg, i.p). Mice were individually placed in Plexiglas cylinders (14.5 cm × 40.5 cm) and were observed and scored for the manifestation of different withdrawal signs including the total number of jumps, wet dog shakes, paw tremors which were counted in 5 min intervals. All plexiglass stations were labeled by non-identifier labels (i.e. A, B, C) and the response to naloxone-precipitated withdrawal was videotaped. The values obtained were verified by a lab member that was blind to genotype and dose. The percent occurrence of diarrhea and mastication was also measured at 5 min intervals by assigning a score of 0 if the behavior was absent and a score of 1 if the behavior was present. The percent occurrence was calculated by dividing the number of observed occurrences by 6 (the total number of intervals in the session) and then multiplying by 100%. Weight loss was also determined by subtracting measured body weight after withdrawal from body weight prior to precipitating withdrawal. A global withdrawal score was calculated based upon the method described by the Koob laboratory (Maldonado et al., 1992). It is calculated by assigning a constant that proportionally the individual withdrawal responses thereby giving more credence to measures that have been tightly correlated with physical dependence, such as jumps and wet dog shakes, and minimizing more variable, and less directly correlative measures, such as paw tremors. The signs were weighted as follows: jumps × 0.8; wet dog shakes × 1; paw tremors × 0.35; diarrhea score × 1.5; mastication score × 1.5; the sum of each of these weighted signs produces a global score for each mouse. The global scores were averaged to obtain a mean for each genotype at each dose of agonist tested (Maldonado et al., 1992; Berrendero et al., 2003; Raehal et al., 2009).
2.7. Statistical Analysis
Results for each experiment were expressed as mean ± S.E.M. Time-course and dose-response effects between genotypes were analyzed using a two-way analysis of variance (ANOVA) followed by Bonferroni post-hoc analysis. Time-course effects within a genotype were analyzed using a one-way ANOVA followed by Bonferroni post-hoc analysis. Dose response curves were compared by two-way ANOVA between genotypes except for the comparison WT vs. βarr2-KO on Day 7 of figure 2A where the doses tested were not the same between the genotypes. The ED50 values and 95% confidence intervals were calculated for cumulative dose-response curves using nonlinear regression analysis. To compare the ED50 values obtained in Table 1, a one-way ANOVA was used to compare the logED50± SEM generated for each curve followed by Bonferroni post-hoc analysis. For all tests, the criterion for significance was set at P<0.05. All analyses were performed using GraphPad Prism software (GraphPad Software, Inc., San Diego, CA).
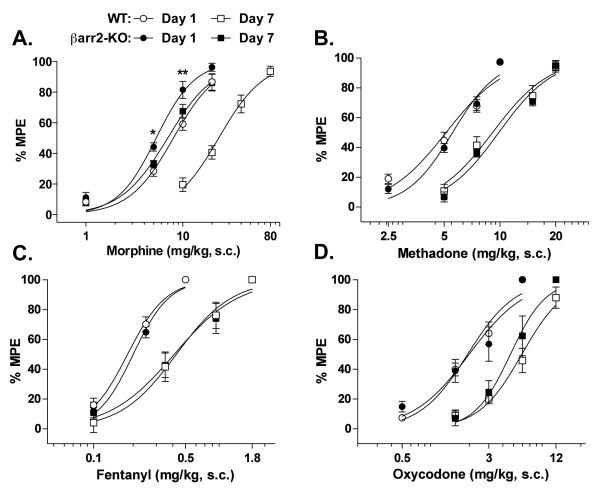
Figure 2.
Cumulative-dose response curves in WT and βarr2-KO mice in response to chronic (A) morphine (48 mg/kg/day, s.c.; two-way ANOVA analysis for genotype effect compared at day 1: P<0.0001, WT vs KO: *P<0.05, **P<0.01, Bonferroni post-hoc analysis, n=8-11 WT, 8-12 KO), (B) methadone (96 mg/kg/day, s.c., n=6 WT, 6 KO), (C) fentanyl (3.2 mg/kg/day, s.c., n=5 WT, 5 KO), or (D) oxycodone (25 mg/kg/day, s.c., n=5 WT, 5 KO) infusion over a 7 day period. Dose-response curves were determined using a cumulative dosing scheme on day 1 before osmotic pump implantation, and then again after 7 days of chronic drug infusion. Data are presented as the mean ± S.E.M. For B-D, two-way ANOVA analysis revealed no difference for genotype effect (P>0.05).
Table 1.
Summary of opiate ED50 values (mg/kg) (± 95% confidence intervals) for hot plate dose response curves obtained in WT and βarr2-KO mice presented in Figure 1.
| Drug | Genotype | Day 1 ED50 (95% CI) |
Day 7 ED50 (95% CI) |
|---|---|---|---|
| Morphine | WT βarr2-KO |
8.00 (6.96-9.20) 5.32 (4.65-6.07) |
23.46 (20.44-26.93)*** 6.98 (6.12-7.96)* |
| Methadone | WT βarr2-KO |
5.12 (4.59-5.72) 5.55 (5.15-5.99) |
9.13 (8.05-10.34)*** 9.86 (9.08-10.71)*** |
| Fentanyl | WT βarr2-KO |
0.18 (0.16-0.21) 0.20 (0.18-0.23) |
0.43 (0.33-0.55)*** 0.41 (0.32-0.53)*** |
| Oxycodone | WT βarr2-KO |
1.93 (1.65-2.27) 2.00 (1.15-2.64) |
5.92 (5.00-7.00)*** 4.68 (3.81-5.76)*** |
One-way ANOVA was used to compare ED50 values between: WT: Day 1 vs Day 7 and KO: Day 1 vs Day 7 where
P<0.05
P<0.001, as determined by Bonferroni post-hoc analysis.
3. Results
Antinociceptive tolerance in WT and βarr2-KO mice was evaluated using two different treatment paradigms. In the first paradigm, mice were assessed for their response latencies to a thermal hot plate stimulus every other day during 5 days of chronic agonist treatment. While the half-life of morphine is approximately 2 hrs in mice (Aceto et al., 1997), the half-lives of methadone, fentanyl, and oxycodone are much shorter at 60, 45 and 30 min respectively (Duttaroy and Yoburn, 1995; Kalvass et al., 2007; Boström et al., 2008). Therefore, in an attempt to overcome these differences, we utilized subcutaneously implanted osmotic pumps to deliver the drugs at a constant rate over time. Furthermore, to compare the drugs, we chose doses of each drug for infusion that produced a similar percentage of the maximum possible effect (% MPE) in the WT mice.
As shown in Figure 1A, WT mice display significantly reduced antinociceptive responses with chronic morphine infusion over time (one-way ANOVA: F(2,24)=13.54, P=0.0001), while the βarr2-KO mice continued to display a similar degree of responsiveness to morphine on days 3 and 5 as they did on day 1 (one-way ANOVA: F(2,21)=3.412, P=0.0521). The βarr2-KO mice also displayed enhanced antinociception at each time point assessed compared to their WT littermates (two-way ANOVA for genotype: F(1,45)=75.28, P<0.0001; time: F(2,45)=13.27, P<0.0001; interaction of genotype × time: F(2,45)=0.54, P=0.5885). These results are very similar to what was originally observed in these animals upon 75 mg morphine pellet implantation over 3 days (Bohn et al., 2000).
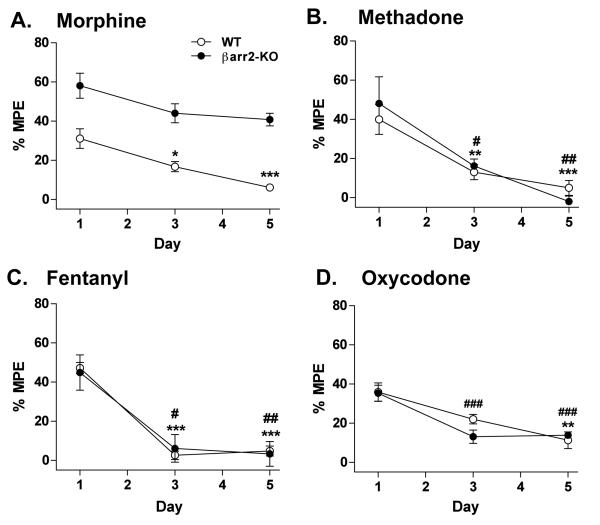
Figure 1.
Thermal (54°C hot plate) antinociceptive response s in WT and βarr2-KO mice in response to chronic infusion over a 5 day period with (A) morphine (48 mg/kg/day, s.c.; two-way ANOVA with Bonferroni post-hoc analysis for genotype effect compared at at each day: P<0.0001, n=9 WT, 8 KO); (B) methadone (96 mg/kg/day, s.c; n=7 WT, 6 KO); (C) fentanyl (3.2 mg/kg/day, s.c., n=5 WT, 5 KO); or (D) oxycodone (25 mg/kg/day, s.c., n=6 WT, 6 KO). Responses were measured on day 1, 3, and 5 following pump implantation. Data are presented as the mean ± S.E.M. For B-D, two-way ANOVA revealed no differences for genotype effect (P>0.05) and for A-D, one-way ANOVA for day effect reveals differences for WT: *P<0.05, **P<0.01, ***P<0.001; for KO: #P<0.05, ##p<0.01, ###P<0.001, Bonferroni post-hoc analysis.
In contrast, the continuous infusion of methadone produced an equivalent degree of antinociception in both WT and βarr2-KO mice at every time point tested (two-way ANOVA for genotype: F(1,33)=0.07, P=0.7973; time: F(2,33)=20.63, P<0.0001; interaction of genotype × time: F(2,33)=0.65, P=0.5297), and response latencies significantly decreased in both genotypes at the same rate, and to the same extent, over the 5 day test period (one-way ANOVA for WT: F(2,18)=11.58, P=0.0006; for KO: F(2,15)=9.403, P=0.0023) (Figure 1B). WT and βarr2-KO mice also displayed a similar extent of antinociception with chronic fentanyl infusion (two-way ANOVA for genotype: F(1,24)=0.00, P=0.9844; time: F(2,24)=36.96, P<0.0001; interaction of genotype × time: F(2,24)=0.16, P=0.8550), and exhibited decreased antinociceptive responsiveness at a similar rate, with their responses returning to baseline 3 days after pump implantation (one-way ANOVA for WT: F(2,12)=99.35, P<0.0001; for KO: F(2,12)=9.502, P=0.0034) (Figure 1C). As shown in figure 1D, both genotypes also exhibited comparable antinociceptive responses with continuous oxycodone treatment (two-way ANOVA for genotype: F(1,30)=0.62, P=0.4380; time: F(2,30)=22.82, P<0.0001; interaction of genotype × time: F(2,30)=1.38, P=0.2673), which significantly declined in both WT (one-way ANOVA: F(2,15)=9.807, P=0.0019) and βarr2-KO mice (one-way ANOVA: F(2,15)= 15.56, P=0.0002) over the 5 day period.
To further characterize differences in agonist-induced antinociceptive tolerance in the βarr2-KO mice, the potency of each drug was examined using a cumulative dose-response study wherein responsiveness to opiate treatment on day 1 was compared to that measured after 7 days of continuous drug infusion. Morphine produces more antinociception on day 1 in the βarr2-KO mice compared to WT controls (two-way ANOVA for genotype: F(1,63)=20.62, P<0.0001; dose: F(3,63)=168.08, P<0.0001; interaction of genotype × dose: F(3,63)=2.22, P=0.0944) (Figure 2A). For statistical comparisons, the half-maximal effective doses (ED50 values) for each drug were calculated from the nonlinear regression analysis of each curve and genotype and chronic treatment impacts on drug potency and are compared in Table 1. Following 7 days of continuous morphine infusion, the morphine dose-response curve is significantly shifted rightward in the WT (~300%) and to a lesser extent, in the βarr2-KO mice (~30%) (Table 1).
Methadone produces the same effects in both genotypes on day 1 (two-way ANOVA for genotype: F(1,40)=0.96, P=0.3340; dose: F(3,40)=190.38, P<0.0001; interaction of genotype × dose: F(3,40)=0.64, P=0.5914) and after 7 days of chronic methadone treatment, the dose-response curves for both genotypes are significantly shifted rightward to the same degree (Figure 2B, Table 1). The cumulative dosing of fentanyl also reveals no difference between genotypes (two-way ANOVA for genotype: F(1,24)=0.91, P=0.3486; dose: F(2,24)=244.96, P<0.0001; interaction of genotype × dose: F(2,24)=0.25, P=0.7810) and both genotypes exhibited significant rightward shifts in their dose-response curves on day 7 in response to chronic fentanyl administration (Figure 2C, Table 1). The genotypes do not display differential responses to cumulative dosing of oxycodone on day 1 (two-way ANOVA for genotype: F(1,32)=0.00, P=0.9680; dose: F(3,32)=79.21, P<0.0001; interaction of genotype × dose: F(3,32)=0.51, P=0.6754) and both genotypes exhibit a significant rightward shift in their dose-response curves in response to continuous exposure to oxycodone (Figure 2D, Table 1).
Prolonged exposure to opioids leads to the development of physical dependence, which in rodents, can be modeled by assessing antagonist-precipitated somatic signs of withdrawal. Administration of a 0.5 mg/kg dose of naloxone (i.p.) precipitates withdrawal in both WT and βarr2-KO mice following chronic morphine treatment at several doses. As shown in figure 3A, the βarr2-KO mice display significantly fewer jumps compared to their WT counterparts (two-way ANOVA for genotype: F(1,49)=5.99, P=0.0180; dose: F(2,49)= 6.97, P=0.0022; interaction of genotype × dose: F(2,49)=1.78, P=0.1795) and paw tremors (two-way ANOVA for genotype: F(1,49)=10.18, P=0.0025; dose: F(2,49)= 3.32, P=0.0443; interaction of genotype × dose: F(2,49)=1.87, P=0.1647). A global withdrawal score was calculated wherein the individual responses are weighted and then summed to give a “global score” for each animal. For morphine, the global withdrawal score is significantly less in the βarr2-KO mice across doses (two-way ANOVA for genotype: F(1,47)=14.45, P=0.0004; dose: F(2,47)= 15.47, P<0.0001; interaction of genotype × dose: F(2,47)=2.94, P=0.0629). Individual withdrawal signs measured are presented in Table 2, and while jumps and paw tremors are diminished in βarr2-KO mice, other signs of withdrawal do not differ between the genotypes (two-way ANOVA for genotype: P>0.05).
Figure 3.
Naloxone-precipitated (0.5 mg/kg, i.p.) withdrawal jumps and global scores following 7 days of chronic infusion with (A) morphine (12, 24, 48 mg/kg/day, s.c.; two-way ANOVA analysis for genotype effect for jumps (P=0.0180) and global score (P=0.004), WT vs KO: *P<0.05, ***P<0.001, Bonferroni post-hoc analysis, n=8-11 WT, 7-10 KO), (B) methadone (48, 60, 72 mg/kg, s.c., n=8-10 WT, 8-10 KO), (C) fentanyl (0.8, 1.6, 3.2 mg/kg, s.c., n=7-11 WT, 6-10 KO) or, (D) oxycodone (12.5, 25, 75 mg/kg, s.c., n=5-6 WT, 5 KO). Immediately following naloxone administration, withdrawal signs were observed and scored over a 30 min period. Data are presented as the mean ± S.E.M. For methadone, fentanyl, and oxycodone, two-way ANOVA analysis revealed no differences for genotype effect for both jumps (P>0.05) and global score (P>0.05).
Table 2.
Summary of naloxone-precipitated (0.5 mg/kg, i.p.) withdrawal signs (except for jumps) in WT and (βarr2-KO mice chronically infused with morphine, methadone, fentanyl, and oxycodone for 7 days as described for Figure 3. Immediately following naloxone administration, withdrawal signs were observed and scored over a 30 min period.
| Dose (mg/kg/day) |
Wet Dog Shakes Number ± S.E.M. |
Paw Tremors Number ± S.E.M. |
Diarrhea % Occurrence ± S.E.M. |
Mastication % Occurrence ± S.E.M. |
Weight Loss Amount (g) ± S.E.M. |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| WT | βarr2-KO | WT | βarr2-KO | WT | βarr2-KO | WT | βarr2-KO | WT | βarr2-KO | |
|
Morphine 12 24 48 |
4.1 ± 0.9 5.2 ± 1.1 8.5 ± 1.4 |
3.9 ± 0.6 5.8 ± 1.1 8.8 ± 1.0 |
40.8 ± 10.8 41.2 ± 9.0 45.4 ± 7.1 |
13.0 ± 3.6 10.9 ± 2.6* 42.4 ± 8.6 |
31.3 ± 4.9 48.3 ± 3.9 46.9 ± 3.0 |
35.7 ± 8.5 38.9 ± 5.6 53.3 ± 4.1 |
58.3 ± 7.7 73.3 ± 6.7 69.7 ± 5.9 |
71.4 ± 4.8 71.9 ± 6.8 73.3. ± 8.7 |
1.0 ± 0.1 1.0 ± 0.1 1.4 ± 0.1 |
1.0 ± 0.1 0.8 ± 0.2 1.2 ± 0.1 |
|
Methadone 48 60 72 |
6.0 ± 0.8 12.3 ± 3.4 8.7 ± 1.5 |
10.8 ± 2.3 18.0 ± 4.2 10.2 ± 1.9 |
72.9 ± 20.0 83.4 ± 29.3 71.5 ± 15.4 |
20.6 ± 5.3 127 ± 29.4 47.4 ± 16.1 |
31.3 ± 7.3 50.0 ± 3.1 40.0 ± 3.7 |
35.4 ± 6.6 46.7 ± 4.2 46.7 ± 5.4 |
60.4 ± 7.0 62.5 ± 11.3 60.6 ± 7.7 |
50.0 ± 6.3 75.5 ± 4.6 63.8 ± 9.7 |
1.5 ± 0.2 0.7 ± 0.1 1.2 ± 0.2 |
1.5 ± 0.2 0.6 ± 0.1 1.3 ± 0.2 |
|
Fentanyl 0.8 1.6 3.2 |
7.0 ± 2.6 11.0 ± 2.2 11.6 ± 4.3 |
6.0 ± 1.2 10.7 ± 1.9 14.6 ± 2.0 |
41.3 ± 12.0 37.0 ± 27.7 17.9 ± 8.0 |
19.2 ± 6.4 50.0 ± 7.1 5.4 ± 1.8 |
16.7 ± 5.5 35.7 ± 6.7 31.3 ± 10.2 |
16.7 ± 6.2 30.6 ± 8.0 40.0 ± 6.7 |
75.8 ± 4.7 69.1 ± 5.7 50.0 ± 8.9 |
76.7 ± 5.7 83.3 ± 0.1 60.0 ± 8.3 |
0.9 ± 0.2 0.8 ± 0.1 1.4 ± 0.2 |
0.9 ± 0.2 1.2 ± 0.2 1.3 ± 0.3 |
|
Oxycodone 12.5 25 75 |
7.2 ± 2.1 5.7 ± 1.0 4.4 ± 0.6 |
8.8 ± 3.6 7.2 ± 2.1 8.0 ± 2.6 |
64.0 ± 23.3 35.0 ± 22.1 10.6 ± 2.5 |
29.0 ± 10.2 16.0 ± 6.6 18.6 ± 8.8 |
36.7 ± 9.7 27.5 ± 3.5 40.0 ± 4.1 |
41.0 ± 13.3 36.7 ± 3.3 36.7 ± 9.7 |
83.3 ± 5.3 66.7 ± 6.1 76.7 ± 6.7 |
83.3 ± 9.1 80.0 ± 3.3 80.0 ± 3.3 |
0.4 ± 0.1 0.6 ± 0.1 1.0 ± 0.2 |
0.4 ± 0.1 0.9 ± 0.2 1.0 ± 0.1 |
Data are presented as the mean ± S.E.M. Two-way ANOVA analysis for genotype effect reveal differences for morphine-induced paw tremors, P=0.0025
WT vs,KO: P<0.05, Bonferroni post-hoc analysis.
Unlike morphine, chronic exposure to methadone (Figure 3B), fentanyl (Figure 3C), and oxycodone (Figure 3D) lead to no discernable differences in the number of jumps (two-way ANOVA for genotype with methadone: F(1,48)=0.66, P=0.4215; dose: F(2,48)=8.82, P=0.0005; interaction of genotype × dose: F(2,48)=2.77, P=0.0725; genotype with fentanyl: F(1,46)=0.00, P=0.9555; dose: F(2,46)=8.68, P=0.0006; interaction of genotype × dose: F(2,46)=0.22, P=0.8019; genotype wtih oxycodone: F(1,25)=0.00, P=0.9531; dose: F(2,25)=8.37, P=0.0016; interaction of genotype × dose: F(2,25)=0.14, P=0.8713) precipitated by naloxone. No differences in the individual withdrawal signs were seen between genotype for any of the drugs tested (two-way ANOVA for genotype: P>0.5) (Table 2). Further, comparison of the global withdrawal scores also reveal no differences between genotypes at any of the doses tested (two-way ANOVA for genotype with methadone: F(1,48)=0.12, P=0.7326; dose: F(2,48)=4.64, P=0.0143; interaction of genotype × dose: F(2,48)=3.49, P=0.0383; genotype with fentanyl: F(1,46)=0.01, P=0.9059; dose: F(2,46)=7.78, P=0.0012; interaction of genotype × dose: F(2,46)=0.85, P=0.4334; genotype with oxycodone: F(1,25)=0.17, P=0.6855; dose: F(2,25)=2.77, P=0.0822; interaction of genotype × dose: F(2,25)=0.73, P=0.4918).
4. Discussion
In this study several different opioid analgesics were compared for their ability to induce antinociceptive tolerance and physical dependence in WT and βarr2-KO mice following chronic infusion via osmotic pumps. Under these dosing conditions, morphine produces robust tolerance in WT mice, while this effect is greatly attenuated in the βarr2-KO mice. At high doses of morphine both WT and βarr2-KO mice display equivalent somatic signs of withdrawal, while at lower doses, the βarr2-KO mice display less severe symptoms. Methadone, fentanyl and oxycodone produce a robust and equivalent degree of tolerance and dependence in both genotypes. These findings underscore the prominent role that βarrestin2 plays in the adaptations following chronic morphine treatment and further demonstrates that diverse opioid ligands may differentially utilize βarrestin2 in vivo.
These observations are consistent with behavioral responses observed in the βarr2-KO mice as they have been shown to not develop morphine tolerance in the hot plate assays under several different testing conditions including: a daily 10 mg/kg repeated injection paradigm over 7 or 9 days, a 3 day implantation of a 75 mg morphine pellet, and an acute injection of 100 mg/kg 24 hours prior to a 10 mg/kg challenge (Bohn et al., 2000; 2002). In the current study, the continuous infusion of opioids via the osmotic pumps followed by a cumulative dosing curve reveals a substantial rightward shift (~300%) in morphine potency in the WT mice as well as a slight shift (~30%) in the βarr2-KO mice (Figure 2A, Table 1). While the shift in potency curves seen in the βarr2-KO mice is ~ 10 fold less than that seen in the WT group, the ED50 is significantly greater in the βarr2-KO group following morphine treatment (Table 1). While this could reflect some adaptations due to the experimental design (such as obtaining repeated response latencies on the hot plate for the cumulative dosing paradigm on day one and again on day 5 in the same group of mice) this shift could also reflect the contributions of other regulatory molecules such as protein kinase A (PKA) or protein kinase C (PKC) which have also been shown to play a role in the development of antinociceptive tolerance (Smith et al., 2006; Bailey et al., 2006; Gabra et al., 2008). Earlier studies in the βarr2-KO mice revealed a role for PKC in the development of morphine tolerance in the tail flick test that was made more apparent in the absence of βarrestin2 (Bohn et al., 2002). Collectively, the findings in this manuscript are in accord with the previous reports that βarrestin2 is highly involved in morphine-induced tolerance in the hot plate test.
In contrast to morphine, the loss of βarrestin2 does not impact on the acute response profiles determined by cumulative dosing or the development of tolerance when methadone, fentanyl, or oxycodone is administered chronically (Figures 1 & 2). Like morphine, oxycodone and methadone also have some affinity for delta and kappa opioid receptors, although their primary effects on hot plate latencies are most likely due to actions at the MOR. In the βarr2-KO animals, morphine-induced antinociception can be blocked by naloxone, but is not effected by antagonists to delta and kappa opioid receptors further implicating the disrupted regulation of MOR in determining the different response profiles between the genotypes (Bohn et al., 1999). The βarr2-KO mice also display an enhanced hypothermic response to morphine (Bohn et al., 1999), and while there have been studies demonstrating that lowered body temperature can increase tail flick response latencies, there is no evidence that a mild difference in body temperature could lead to a robust difference in paw withdrawal latencies in the hot plate test (Berge et al., 1988; Tjolsen et al., 1989). Nevertheless, the high temperature of the hot plate (54°C giving ~7 sec basal latencies for both genoty pes) and the 30 second cutoff time were chosen to minimize potential contribution of other physiological effects of opioids (such as changes in body temperature or exploratory behavior) that could differ between the genotypes and potentially confound the interpretation of the hot plate response (Dykstra, 1985; Mogil et al., 2001).
While morphine, methadone and fentanyl have been shown to activate MOR signaling (G protein coupling, cyclase inhibition, ERK activation), the effect they have on regulation of the receptor differs. In cell culture studies, morphine does not promote robust receptor phosphorylation (Zhang et al., 1998), βarrestin2 recruitment (Zhang et al., 1998; Bohn et al., 2004) or MOR internalization (Arden et al., 1995; Keith et al., 1998; Sternini et al., 1996; Zhang et al., 1998; Whistler and von Zastrow, 1998; Koch et al., 2005) while methadone and fentanyl do (Keith et al., 1998; Bohn et al., 2004; Koch et al., 2005). In locus coeruleus brain slices, similar differences between morphine, methadone and fentanyl desensitization and internalization profiles has also been observed (Virk and Williams, 2008; Arttamangkul et al., 2008).
βArrestins also mediate agonist-induced GPCR internalization, an event distinct from desensitization that removes receptors from the cell surface where they can then be recycled or degraded. Internalization can also serve as a compartmentalization event to facilitate signal transduction. It has been suggested that MOR internalization counteracts receptor desensitization by serving as a means to recycle receptors which can thereby promote their resensitization to a responsive state (Whistler et al., 1999; He and Whistler, 2005). Therefore, agonists that promote robust receptor internalization (such as fentanyl and methadone), are hypothesized to produce less antinociceptive tolerance than those that do not robustly internalize the MOR (such as morphine) (Whistler et al., 1999; Finn and Whistler 2001; He and Whistler, 2005). However, when these drugs are administered at equiefficacious doses via a chronic infusion model, all of the compounds produce antinociceptive tolerance to a similar extent in WT mice (Figure 2 and Table 1). A recent study by Kim et al., (2008) reported that methadone does not produce tolerance in mice when given twice daily at a dose of 4 mg/kg for 5 days. The difference in these two outcomes likely resides in the dosing regimen as intermittent drug dosing produces less tolerance than chronic infusion (Madia et al., 2009) and the fact that methadone is rapidly metabolized in mice (Kalvass et al., 2007).
Oxycodone, has been shown to produce less MOR internalization (Koch et al., 2005; Arttamangkul et al., 2008; Virk and Williams, 2008) and since this is a property it shares with morphine, we predicted that it would produce the same effects as morphine in βarr2-KO mice. However, unlike morphine, oxycodone produced the same extent of tolerance in both genotypes (Figure 1D and 2D and Table 1). Virk and Williams (2008) showed that oxycodone, unlike morphine, does not induce MOR desensitization in locus coeruleus neuron cultures. It is attractive to hypothesize that oxycodone may activate the MOR in a manner that is insensitive to βarrestin2 regulation, and data presented in our study may also support this interpretation, as its deletion has no impact on the onset of oxycodone-induced tolerance. However, it should also be considered that the in vivo studies could also reflect the actions of oxymorphone, an active metabolite of oxycodone (Kaiko et al., 1996, Lemberg et al., 2006), which may act differently than oxycodone at MOR (Arttamangkul et al., 2008; Virk and Williams, 2008).
Previously we reported that morphine induces the same degree of physical dependence in both WT and βarr2-KO mice following treatment with a 75 mg morphine pellet for 3 days (Bohn et al., 2000), a regimen that has been shown to produce significantly higher steady-state morphine levels than that seen for a 25 mg/kg/day pump infusion (Feng et al., 2006). Consistent with our previous study, we find that the highest infusion dose of morphine (48 mg/kg/day) used here does not produce differences between genotypes. This may be due to a ceiling effect wherein at a high doses of morphine, cellular adaptations may occur even in the absence of βarrestin2 which may overcome any βarrestin2-limited threshold for the differential display of jumping and other withdrawal signs with chronic morphine. The biochemical nature of such mechanisms remain to be elucidated.
When the dose of morphine is lowered (12 or 24 mg/kg/day), the severity of antagonist-precipitated withdrawal signs is significantly attenuated in the βarr2-KO mice compared to their WT counterparts (Figure 3 and Table 2). This is particularly evident in the jumping behaviors which is considered highly correlative with the severity of dependence (Kest et al., 2002). While these observations support the notion that βarrestin2 may play a role in the adaptations that underlie the onset of morphine dependence, another possible interpretation is that βarrestin2 could be playing a role in determining the overall extent of the display of certain somatic withdrawal signs (such as jumping or paw tremors). However, if this was the case, then one would expect that βarrestin's role would lie downstream of opiate actions at MOR, and therefore, that all opiate agonists would produce a diminished withdrawal response in the βarr2-KO mice. The display of withdrawal signs following chronic administration of methadone, fentanyl or oxycodone is equivalent between the genotypes across several doses during the infusion period (Figure 3, Table 2) suggesting that βarrestin2 is not a limiting factor in the adaptive responses induced by these analgesics and that the differences observed with morphine are likely not due to an inability of the βarr2-KO mice to express the full withdrawal response.
Therefore, the decreased severity of withdrawal signs in the βarr2-KO mice may support a model wherein βarrestin2 facilitates MOR signaling in neurons involved in the development of physical dependence and/or antagonist-precipitated withdrawal behaviors. While MOR signaling via βarrestins has not yet been demonstrated in vivo, a recent study by the Loh group suggests that MOR can utilize βarrestins to activate Map kinases in a ligand-dependent manner in cultured cell models (Zheng et al., 2008).
Ligand-directed signaling is likely due to conformations of the receptor imposed by binding of chemically distinct ligands and is influenced by the receptor's ability to engage with intracellular proteins; therefore, different agonists may induce specific receptor conformations that possess different affinities for βarrestins, resulting in different receptor desensitization, internalization, and signaling profiles which ultimately dictate physiological outcomes (Urban et al., 2007; Kenakin, 2007; Schmid and Bohn, 2009). This concept, referred to as functional selectivity, may explain the differences we see between the different opiates in the WT and βarr2-KO mice, whereby, in the absence of βarrestin2, βarrestin1 may compensate to induce receptor desensitization and antinociceptive tolerance when agonists that induce robust phosphorylation and βarrestin1 and 2 interactions are bound (Zhang et al., 1998; Bohn et al., 2004).
Overall, it is becoming evident that it is important to study receptor function in physiologically appropriate systems. Moreover, the neuronal environment in which the MOR is expressed may influence how the receptor is regulated, as MORs involved in antinociception and antinociceptive tolerance appear to be regulated differently than those involved in mediating physical dependence. Furthermore, diverse opiate agonists can reveal differential roles for βarrestin2 in regulating MOR responsiveness to produce functional consequences in vivo.
Acknowledgements
This work was supported by the National Institute on Drug Abuse grants DA021952 (K.M.R), DA14600 and DA18860 (L.M.B.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aceto MD, Bowman ER, Harris LS, May EL. Dependence Studies of New Compounds in the Rhesus Monkey, Rat and Mouse. In: Harris LS, editor. Problems of Drug Dependence, NIDA Research Monograph. Vol. 19997. NIDA; Washington D.C.: 1997. pp. 338–395. [Google Scholar]
- Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- Arttamangkul S, Quillinan N, Low MJ, von Zastrow M, Pintar J, Williams JT. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–565. doi: 10.1016/j.tips.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Berge O-G, Garcia-Cabrera I, Hole K. Response latencies in the tail-flick test depend on tail skin temperature. Neurosci Lett. 1988;86:284–288. doi: 10.1016/0304-3940(88)90497-1. [DOI] [PubMed] [Google Scholar]
- Berrendero F, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. Increase of morphine withdrawal in mice lacking A2a receptors and no changes in CB1/A2a double knockout mice. Eur J Neurosci. 2003;17:315–324. doi: 10.1046/j.1460-9568.2003.02439.x. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. doi: 10.1124/mol.66.1.106. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Caron MG. Differential mechanisms of morphine antinociceptive tolerance revealed in (beta)arrestin-2 knock-out mice. J Neurosci. 2002;22:10494–10500. doi: 10.1523/JNEUROSCI.22-23-10494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Bostrom E, Hammarlund-Udenaes M, Simonsson US. Blood-brain barrier transport helps to explain discrepancies in in vivo potency between oxycodone and morphine. Anesthesiology. 2008;108:495–505. doi: 10.1097/ALN.0b013e318164cf9e. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- Dykstra LA. Behavioral and pharmacological factors in opioid analgesia. In: Seiden LS, Balster RL, editors. Behavioral Pharmacology: The Current Status. Alan R. Liss, Inc.; New York: 1985. pp. 111–129. [Google Scholar]
- Feng P, Rahim RT, Cowan A, Liu-Chen LY, Peng X, Gaughan J, Meissler JJ, Jr., Adler MW, Eisenstein TK. Effects of mu, kappa or delta opioids administered by pellet or pump on oral Salmonella infection and gastrointestinal transit. Eur J Pharmacol. 2006;534:250–257. doi: 10.1016/j.ejphar.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- Gabra BH, Bailey CP, Kelly E, Smith FL, Henderson G, Dewey WL. Pre-treatment with a PKC or PKA inhibitor prevents the development of morphine tolerance but not physical dependence in mice. Brain Res. 2008;1217:70–77. doi: 10.1016/j.brainres.2008.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Whistler JL. An opiate cocktail that reduces morphine tolerance and dependence. Curr Biol. 2005;15:1028–1033. doi: 10.1016/j.cub.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Kaiko RF, Benziger DP, Fitzmartin RD, Burke BE, Reder RF, Goldenheim PD. Pharmacokinetic-pharmacodynamic relationships of controlled-release oxycodone. Clin Pharmacol Ther. 1996;59:52–61. doi: 10.1016/S0009-9236(96)90024-7. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Cassidy MP, Selley DE, Pollack GM. Pharmacokinetics and pharmacodynamics of seven opioids in P-glycoprotein-competent mice: assessment of unbound brain EC50,u and correlation of in vitro, preclinical, and clinical data. J Pharmacol Exp Ther. 2007;323:346–355. doi: 10.1124/jpet.107.119560. [DOI] [PubMed] [Google Scholar]
- Keith DE, Anton B, Murray SR, Zaki PA, Chu PC, Lissin DV, Monteillet-Agius G, Stewart PL, Evans CJ, von Zastrow M. mu-Opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in the mammalian brain. Mol Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity through protean and biased agonism: who steers the ship? Mol Pharmacol. 2007;72:1393–1401. doi: 10.1124/mol.107.040352. [DOI] [PubMed] [Google Scholar]
- Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–469. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kim JA, Bartlett S, He L, Nielsen CK, Chang AM, Kharazia V, Waldhoer M, Ou CJ, Taylor S, Ferwerda M, Cado D, Whistler JL. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch T, Widera A, Bartzsch K, Schulz S, Brandenburg LO, Wundrack N, Beyer A, Grecksch G, Hollt V. Receptor endocytosis counteracts the development of opioid tolerance. Mol Pharmacol. 2005;67:280–287. doi: 10.1124/mol.104.004994. [DOI] [PubMed] [Google Scholar]
- Lemberg KK, Kontinen VK, Siiskonen AO, Viljakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA. Antinociception by spinal and systemic oxycodone: why does the route make a difference? In vitro and in vivo studies in rats. Anesthesiology. 2006;105:801–812. doi: 10.1097/00000542-200610000-00027. [DOI] [PubMed] [Google Scholar]
- Madia PA, Dighe SV, Sirohi S, Walker EA, Yoburn BC. Dosing protocol and analgesic efficacy determine opioid tolerance in the mouse. Psychopharmacology (Berl) 2009;207:413–422. doi: 10.1007/s00213-009-1673-6. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Negus S, Koob GF. Precipitation of morphine withdrawal syndrome in rats by administration of mu-, delta- and kappa-selective opioid antagonists. Neuropharmacology. 1992;31:1231–1241. doi: 10.1016/0028-3908(92)90051-p. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Wan Y. Assessing nociception in murine subjects. In: Kruger L, editor. Methods in Pain Research. CRC Press, LLC; Boca Raton: 2001. pp. 11–39. [Google Scholar]
- Raehal KM, Schmid CL, Medvedev IO, Gainetdinov RR, Premont RT, Bohn LM. Morphine-induced physiological and behavioral responses in mice lacking G protein-coupled receptor kinase 6. Drug Alcohol Depend. 2009;104:187–196. doi: 10.1016/j.drugalcdep.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Liu HC, Loh HH. mu-Opioid receptor-knockout mice: the role of mu-opioid receptor in gastrointestinal transit. Brain Res Mol Brain Res. 1998;56:281–283. doi: 10.1016/s0169-328x(98)00051-5. [DOI] [PubMed] [Google Scholar]
- Schmid CL, Bohn LM. Physiological and pharmacological implications of beta-arrestin regulation. Pharmacol Ther. 2009;121:285–293. doi: 10.1016/j.pharmthera.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FL, Javed RR, Smith PA, Dewey WL, Gabra BH. PKC and PKA inhibitors reinstate morphine-induced behaviors in morphine tolerant mice. Pharmacol Res. 2006;54:474–480. doi: 10.1016/j.phrs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR. Opiate receptor knockout mice define mu receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci U S A. 1997;94:1544–1549. doi: 10.1073/pnas.94.4.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternini C, Spann M, Anton B, Keith DE, Jr., Bunnett NW, von Zastrow M, Evans C, Brecha NC. Agonist-selective endocytosis of mu opioid receptor by neurons in vivo. Proc Natl Acad Sci U S A. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjolsen A, Lund A, Berge O-G, Hole K. An improved method for tail-flick testing with adjustment for tail-skin temperature. J Neurosci Meth. 1989;26:259–265. doi: 10.1016/0165-0270(89)90124-6. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]
- Virk MS, Williams JT. Agonist-specific regulation of mu-opioid receptor desensitization and recovery from desensitization. Mol Pharmacol. 2008;73:1301–1308. doi: 10.1124/mol.107.042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc Natl Acad Sci U S A. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoburn BC, Shah S, Chan K, Duttaroy A, Davis T. Supersensitivity to opioid analgesics following chronic opioid antagonist treatment: relationship to receptor selectivity. Pharmacol Biochem Behav. 1995;51:535–539. doi: 10.1016/0091-3057(94)00375-s. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci U S A. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Loh HH, Law PY. Beta-arrestin-dependent mu-opioid receptor-activated extracellular signal-regulated kinases (ERKs) Translocate to Nucleus in Contrast to G protein-dependent ERK activation. Mol Pharmacol. 2008;73:178–190. doi: 10.1124/mol.107.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]