Abstract
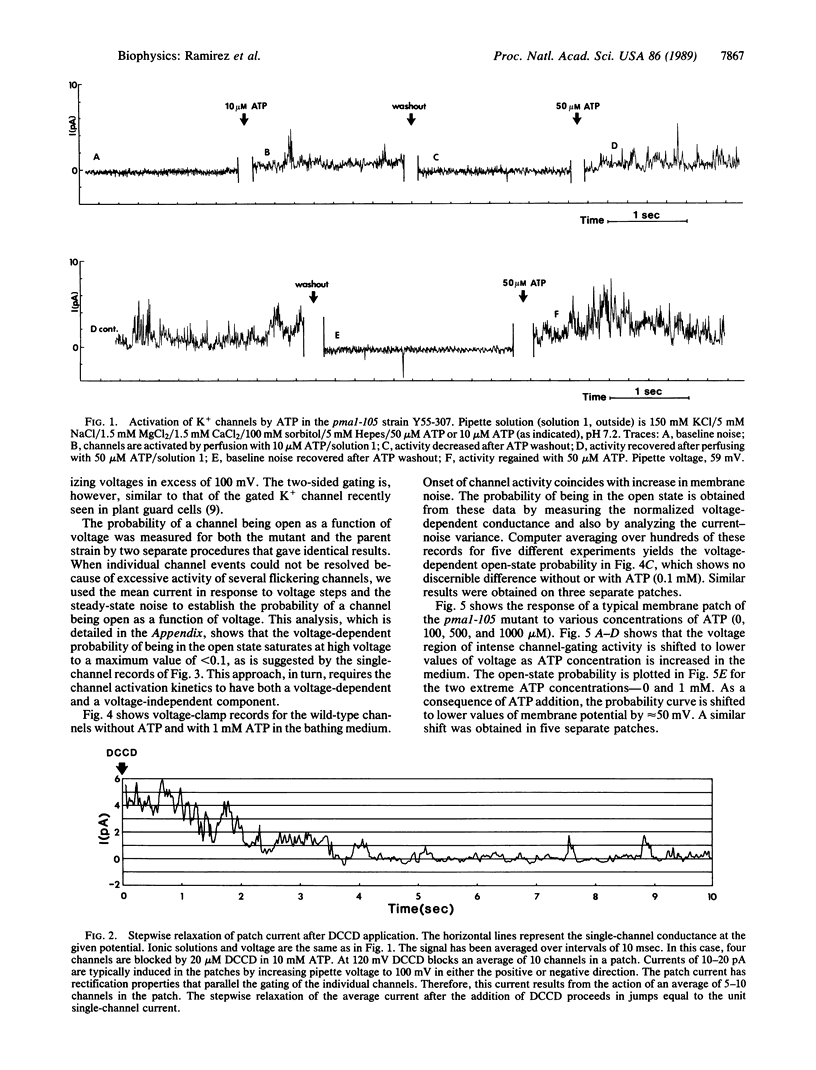
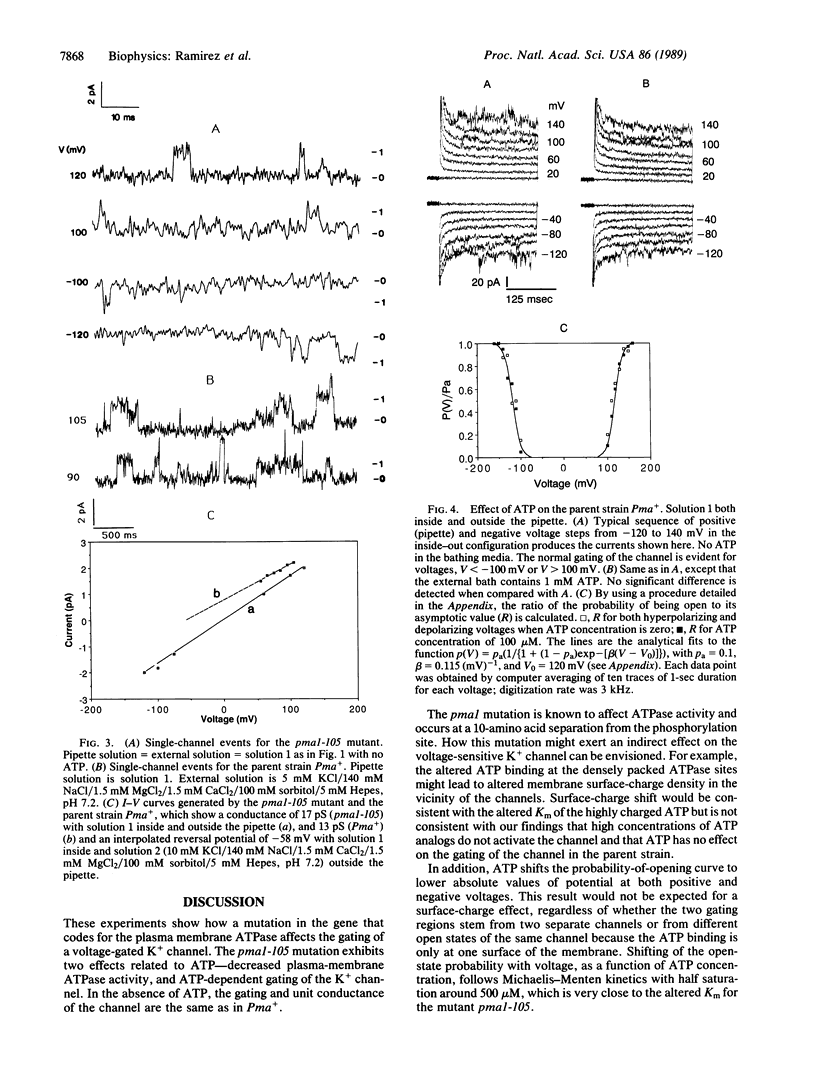
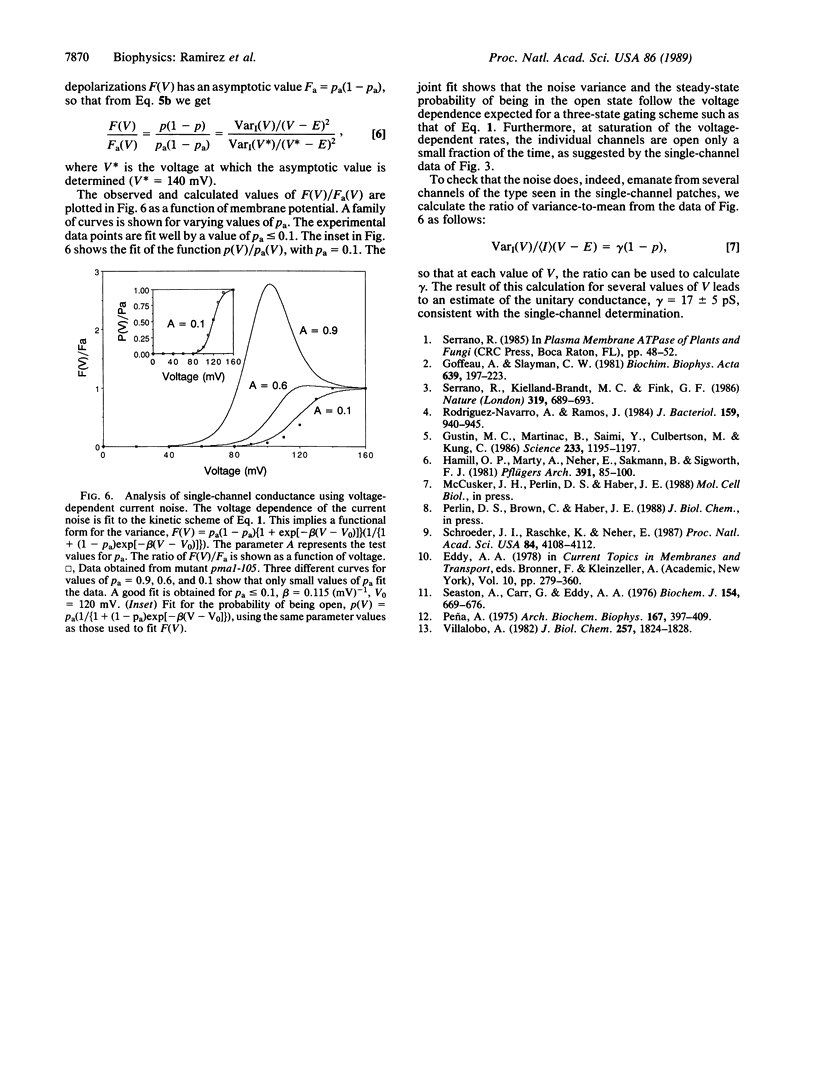
A mutant in the plasma membrane H+-ATPase gene of the yeast Saccharomyces cerevisiae with a reduced H+-ATPase activity, when examined at the single-channel level with the patch-clamp technique, was found to exhibit K+ channels activated by intracellular application of ATP. In the parent strain, the same channel, identified by its conductance and selectivity, is not activated by ATP. This activity in the mutant is blocked by the ATPase inhibitor N,N'-dicyclohexylcarbodiimide. ADP and the ATP analog adenosine 5'-[gamma-[35S]thio]triphosphate do not activate the channel. These findings suggest a tight physical coupling between the plasma membrane ATPase and the K+ channel.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Peña A. Studies on the mechanism of K+ transport in yeast. Arch Biochem Biophys. 1975 Apr;167(2):397–409. doi: 10.1016/0003-9861(75)90480-4. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Ramos J. Dual system for potassium transport in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):940–945. doi: 10.1128/jb.159.3.940-945.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaston A., Carr G., Eddy A. A. The concentration of glycine by preparations of the yeast Saccharomyces Carlsbergensis depleted of adenosine triphosphate: Effects of proton gradients and uncoupling agents. Biochem J. 1976 Mar 15;154(3):669–676. doi: 10.1042/bj1540669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Villalobo A. Potassium transport coupled to ATP hydrolysis in reconstituted proteoliposomes of yeast plasma membrane ATPase. J Biol Chem. 1982 Feb 25;257(4):1824–1828. [PubMed] [Google Scholar]


