Abstract
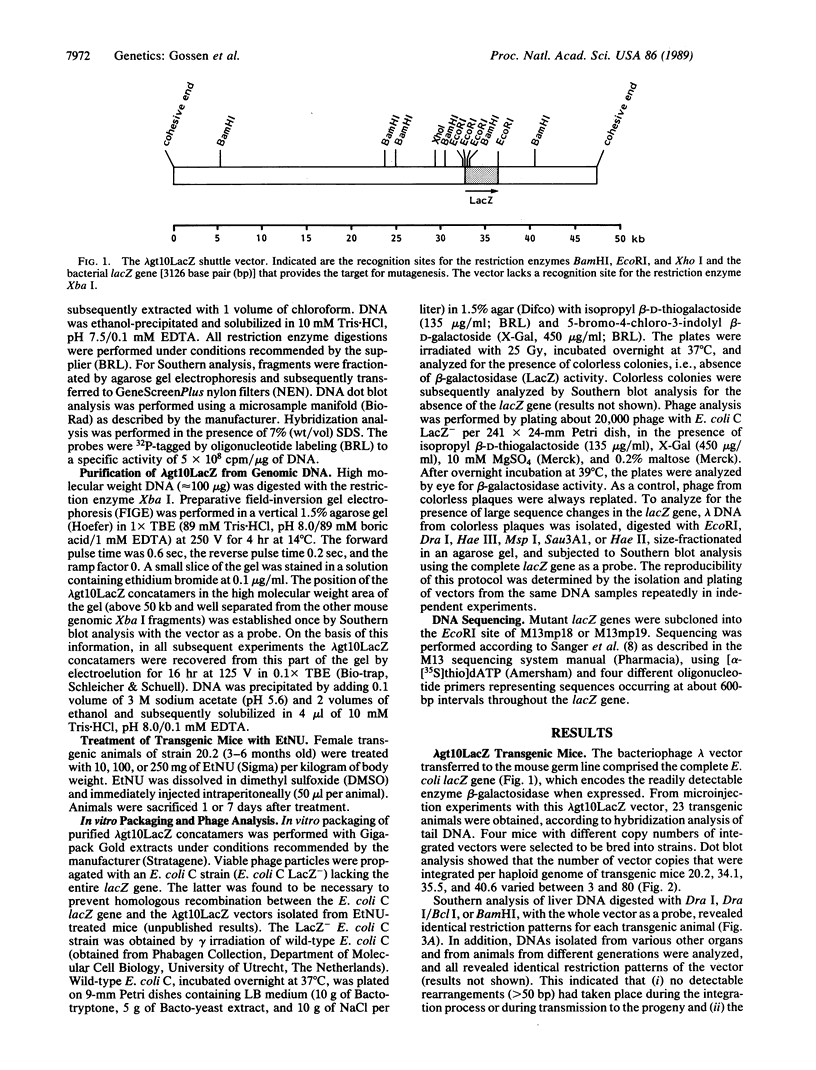
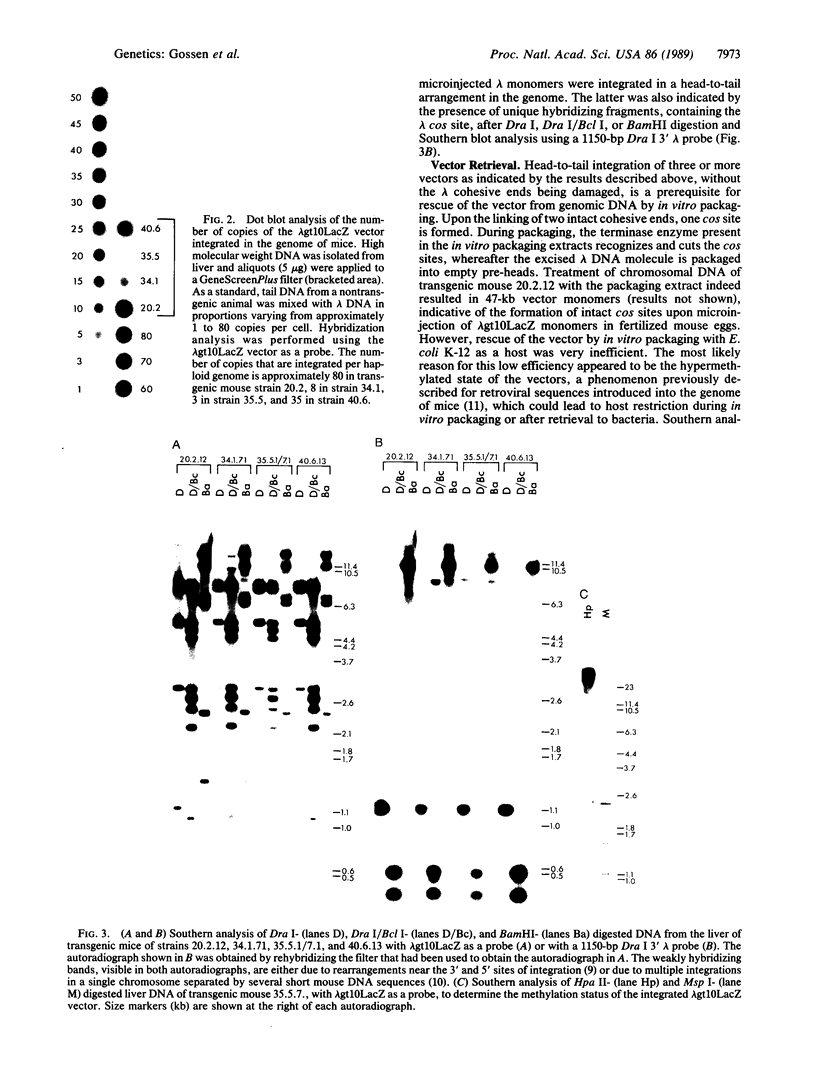
To study gene mutations in different organs and tissues of an experimental animal, we produced transgenic mice harboring bacteriophage lambda shuttle vectors integrated in the genome in a head-to-tail arrangement. As a target for mutagenesis, the selectable bacterial lacZ gene was cloned in the vector. The integrated vectors were rescued from total genomic DNA with high efficiency by in vitro packaging and propagation of the phages in a LacZ- strain of Escherichia coli C. The background mutation frequencies in brain and liver DNA appeared to be low, as was indicated by the absence of colorless plaques among 138,816 and 168,160 phage isolated from brain and liver DNA, respectively. Treatment of adult female transgenic mice with N-ethyl-N-nitrosourea resulted in a dose-dependent increase of the frequency of mutated vectors isolated from brain DNA, up to 7.4 x 10(-5) at 250 mg of the alkylating agent per kilogram of body weight. At this dose, in liver DNA of the same mice, mutation frequencies were approximately 3 x 10(-5). DNA sequence analysis of four mutant vectors isolated from brain DNA indicated predominantly G.C----A.T transitions. These results demonstrate the value of this transgenic mouse model in studying gene mutations in vivo. In addition to its use in fundamental research, the system could be used as a sensitive, organ-specific, short-term mutagenicity assay.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Burns P. A., Gordon A. J., Kunsmann K., Glickman B. W. Influence of neighboring base sequence on the distribution and repair of N-ethyl-N-nitrosourea-induced lesions in Escherichia coli. Cancer Res. 1988 Aug 15;48(16):4455–4458. [PubMed] [Google Scholar]
- Drinkwater N. R., Klinedinst D. K. Chemically induced mutagenesis in a shuttle vector with a low-background mutant frequency. Proc Natl Acad Sci U S A. 1986 May;83(10):3402–3406. doi: 10.1073/pnas.83.10.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBridge R. B., Calos M. P. Recombinant shuttle vectors for the study of mutation in mammalian cells. Mutagenesis. 1988 Jan;3(1):1–9. doi: 10.1093/mutage/3.1.1. [DOI] [PubMed] [Google Scholar]
- Featherstone T., Marshall P. D., Evans H. J. Problems and pitfalls in assessing human T-lymphocyte mutant frequencies. Mutat Res. 1987 Aug;179(2):215–230. doi: 10.1016/0027-5107(87)90312-5. [DOI] [PubMed] [Google Scholar]
- Frei J. V., Swenson D. H., Warren W., Lawley P. D. Alkylation of deoxyribonucleic acid in vivo in various organs of C57BL mice by the carcinogens N-methyl-N-nitrosourea, N-ethyl-N-nitrosourea and ethyl methanesulphonate in relation to induction of thymic lymphoma. Some applications of high-pressure liquid chromatography. Biochem J. 1978 Sep 15;174(3):1031–1044. doi: 10.1042/bj1741031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer P. M., Sarkar S. N., Summers W. C. Detection and analysis of UV-induced mutations in mammalian cell DNA using a lambda phage shuttle vector. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1041–1044. doi: 10.1073/pnas.83.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen J. A., Vijg J. E. coli C: a convenient host strain for rescue of highly methylated DNA. Nucleic Acids Res. 1988 Oct 11;16(19):9343–9343. doi: 10.1093/nar/16.19.9343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Chromosomal position and specific demethylation in enhancer sequences of germ line-transmitted retroviral genomes during mouse development. Mol Cell Biol. 1985 Sep;5(9):2212–2220. doi: 10.1128/mcb.5.9.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R. Use of recombinant DNA techniques in cloning DNA repair genes and in the study of mutagenesis in mammalian cells. Mutat Res. 1985 Jun-Jul;150(1-2):61–67. doi: 10.1016/0027-5107(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Michalova K., Bucchini D., Ripoche M. A., Pictet R., Jami J. Chromosome localization of the human insulin gene in transgenic mouse lines. Hum Genet. 1988 Nov;80(3):247–252. [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tennant R. W., Margolin B. H., Shelby M. D., Zeiger E., Haseman J. K., Spalding J., Caspary W., Resnick M., Stasiewicz S., Anderson B. Prediction of chemical carcinogenicity in rodents from in vitro genetic toxicity assays. Science. 1987 May 22;236(4804):933–941. doi: 10.1126/science.3554512. [DOI] [PubMed] [Google Scholar]
- Vijg J., Uitterlinden A. G. A search for DNA alterations in the aging mammalian genome: an experimental strategy. Mech Ageing Dev. 1987 Nov;41(1-2):47–63. doi: 10.1016/0047-6374(87)90053-4. [DOI] [PubMed] [Google Scholar]






