Abstract
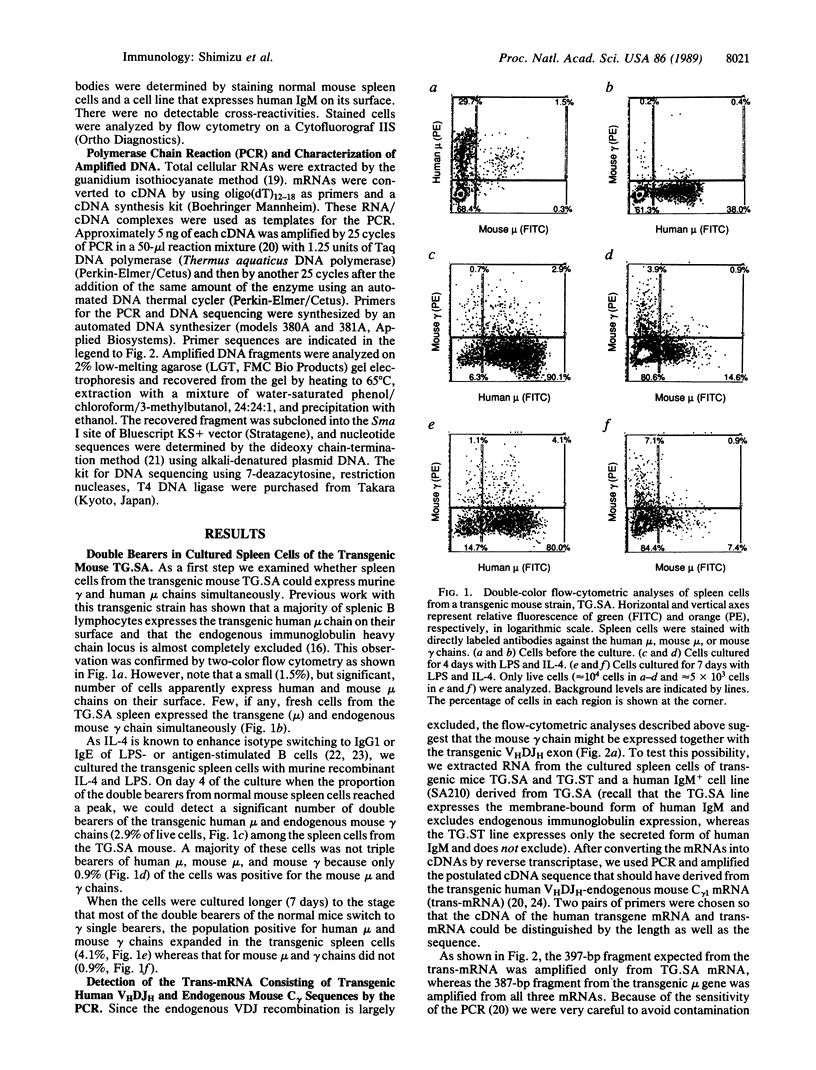
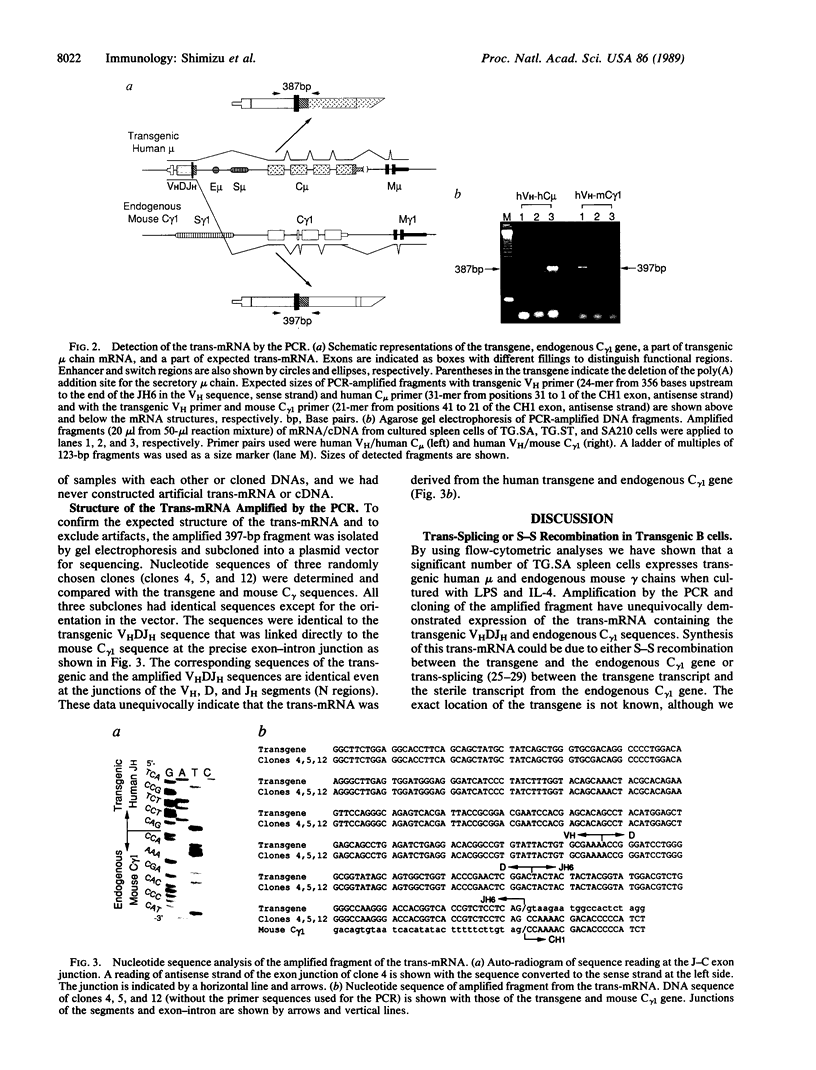
We have studied immunoglobulin double-isotype expression in a transgenic mouse (TG.SA) in which expression of the endogenous immunoglobulin heavy chain locus is almost completely excluded by a nonallelic rearranged human mu transgene. By flow-cytometric analyses, we have shown that a small, but significant, portion (about 4%) of transgenic spleen cells expresses human mu (transgene) and mouse gamma (endogenous) chains when cultured in vitro with bacterial lipopolysaccharide and interleukin 4. By using amplification of cDNA by the polymerase chain reaction, followed by cloning and sequencing of the amplified cDNA fragment, we have demonstrated expression of trans-mRNA consisting of the transgenic variable and endogenous constant (gamma 1) region sequences. Such trans-mRNA could be produced by either switch recombination or trans-splicing between the transgene and endogenous sterile gamma 1-gene transcripts. These results indicate that trans-splicing might be a possible mechanism for the immunoglobulin double-isotype expression in normal B lymphocytes that have not rearranged the second expressed constant region gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. W., Word C., Dev V., Uhr J. W., Vitetta E. S., Tucker P. W. Double isotype production by a neoplastic B cell line. II. Allelically excluded production of mu and gamma 1 heavy chains without CH gene rearrangement. J Exp Med. 1986 Aug 1;164(2):562–579. doi: 10.1084/jem.164.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cory S., Jackson J., Adams J. M. Deletions in the constant region locus can account for switches in immunoglobulin heavy chain expression. Nature. 1980 Jun 12;285(5765):450–456. doi: 10.1038/285450a0. [DOI] [PubMed] [Google Scholar]
- Durdik J., Gerstein R. M., Rath S., Robbins P. F., Nisonoff A., Selsing E. Isotype switching by a microinjected mu immunoglobulin heavy chain gene in transgenic mice. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2346–2350. doi: 10.1073/pnas.86.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T. Immunoglobulin genes. Annu Rev Immunol. 1983;1:499–528. doi: 10.1146/annurev.iy.01.040183.002435. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Coleclough C., Cebra J. J. CH gene rearrangements in IgM-bearing B cells and in the normal splenic DNA component of hybridomas making different isotypes of antibody. Cell. 1980 Nov;22(2 Pt 2):349–359. doi: 10.1016/0092-8674(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Kim H. S., Smithies O. Recombinant fragment assay for gene targetting based on the polymerase chain reaction. Nucleic Acids Res. 1988 Sep 26;16(18):8887–8903. doi: 10.1093/nar/16.18.8887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinashi T., Godal T., Noma Y., Ling N. R., Yaoita Y., Honjo T. Human neoplastic B cells express more than two isotypes of immunoglobulins without deletion of heavy-chain constant-region genes. Genes Dev. 1987 Jul;1(5):465–470. doi: 10.1101/gad.1.5.465. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Malek T. R., Hanly W. C. Recombinant rabbit secretory immunoglobulin molecules: alpha chains with maternal (paternal) variable-region allotypes and paternal (maternal) constant-region allotypes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1169–1173. doi: 10.1073/pnas.71.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B., Fromm H., Galun E., Edelman M. Evidence for in vivo trans splicing of pre-mRNAs in tobacco chloroplasts. Cell. 1987 Jan 16;48(1):111–119. doi: 10.1016/0092-8674(87)90361-8. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Campos-Torres J., Leder P. Allelic exclusion in transgenic mice carrying mutant human IgM genes. J Exp Med. 1988 Jun 1;167(6):1969–1974. doi: 10.1084/jem.167.6.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Shaw A. C., Sinn E., Danner D. B., Holmes K. L., Morse H. C., 3rd, Leder P. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987 May 15;236(4803):816–819. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- Perlmutter A. P., Gilbert W. Antibodies of the secondary response can be expressed without switch recombination in normal mouse B cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7189–7193. doi: 10.1073/pnas.81.22.7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernis B., Forni L., Dubiski S., Kelus A. S., Mandy W. J., Todd C. W. Heavy chain variable and constant region allotypes in single rabbit plasma cells. Immunochemistry. 1973 May;10(5):281–285. doi: 10.1016/0019-2791(73)90023-2. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Luzzati A. L. Synthesis of multiple immunoglobulin classes by single lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):175–183. doi: 10.1101/sqb.1977.041.01.023. [DOI] [PubMed] [Google Scholar]
- Radbruch A., Sablitzky F. Deletion of Cmu genes in mouse B lymphocytes upon stimulation with LPS. EMBO J. 1983;2(11):1929–1935. doi: 10.1002/j.1460-2075.1983.tb01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman P., Lutzker S., Cook W., Coffman R., Alt F. W. Mitogen plus interleukin 4 induction of C epsilon transcripts in B lymphoid cells. J Exp Med. 1988 Dec 1;168(6):2385–2389. doi: 10.1084/jem.168.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Honjo T. Immunoglobulin class switching. Cell. 1984 Apr;36(4):801–803. doi: 10.1016/0092-8674(84)90029-1. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988 Jan 1;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi S. L., Tosi R. M. Recombinant IgG molecules in rabbits doubly heterozygous for group a and group e allotypic specificities. Immunochemistry. 1973 Feb;10(2):65–71. doi: 10.1016/0019-2791(73)90232-2. [DOI] [PubMed] [Google Scholar]
- Tycko B., Palmer J. D., Sklar J. T cell receptor gene trans-rearrangements: chimeric gamma-delta genes in normal lymphoid tissues. Science. 1989 Sep 15;245(4923):1242–1246. doi: 10.1126/science.2551037. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J Exp Med. 1979 Sep 19;150(3):580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Ploeg L. H. Discontinuous transcription and splicing in trypanosomes. Cell. 1986 Nov 21;47(4):479–480. doi: 10.1016/0092-8674(86)90608-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., DePinho R. A., Zimmerman K. A., Lutzker S. G., Rosenberg N., Alt F. W. Secondary genomic rearrangement events in pre-B cells: VHDJH replacement by a LINE-1 sequence and directed class switching. EMBO J. 1986 Dec 1;5(12):3259–3266. doi: 10.1002/j.1460-2075.1986.tb04637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaoita Y., Honjo T. Deletion of immunoglobulin heavy chain genes from expressed allelic chromosome. Nature. 1980 Aug 28;286(5776):850–853. doi: 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Kumagai Y., Okumura K., Honjo T. Expression of lymphocyte surface IgE does not require switch recombination. Nature. 1982 Jun 24;297(5868):697–699. doi: 10.1038/297697a0. [DOI] [PubMed] [Google Scholar]