Abstract
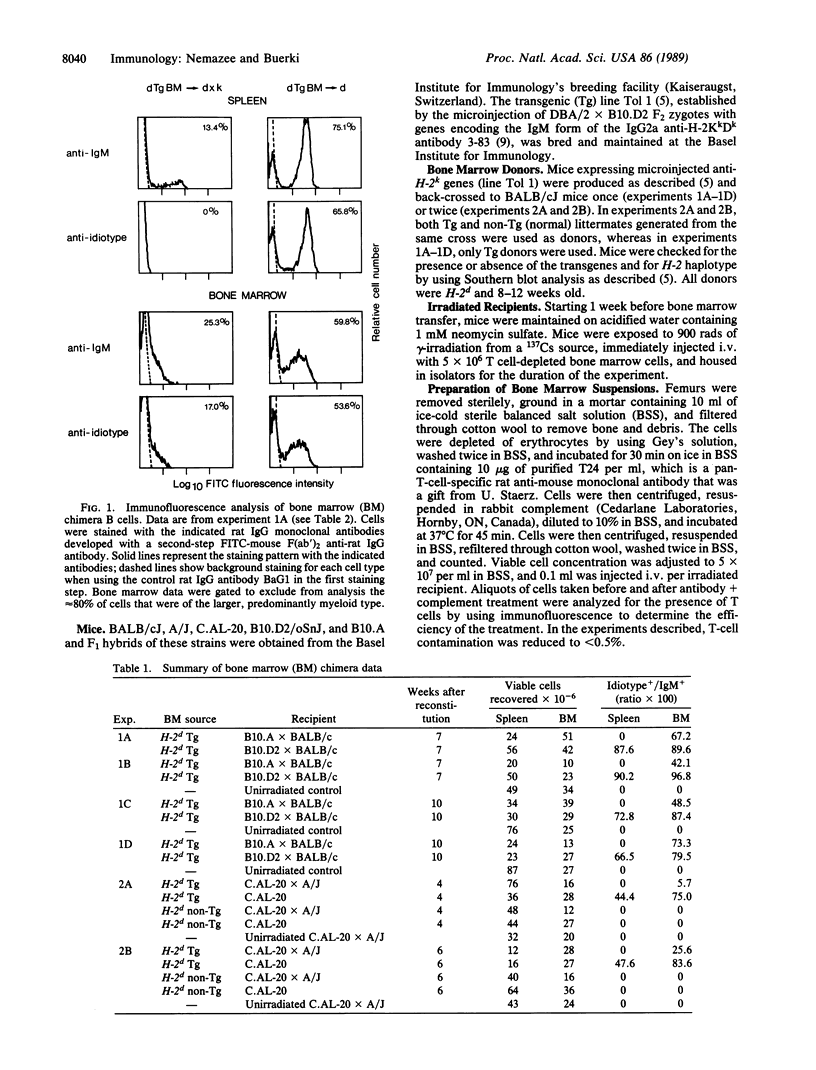
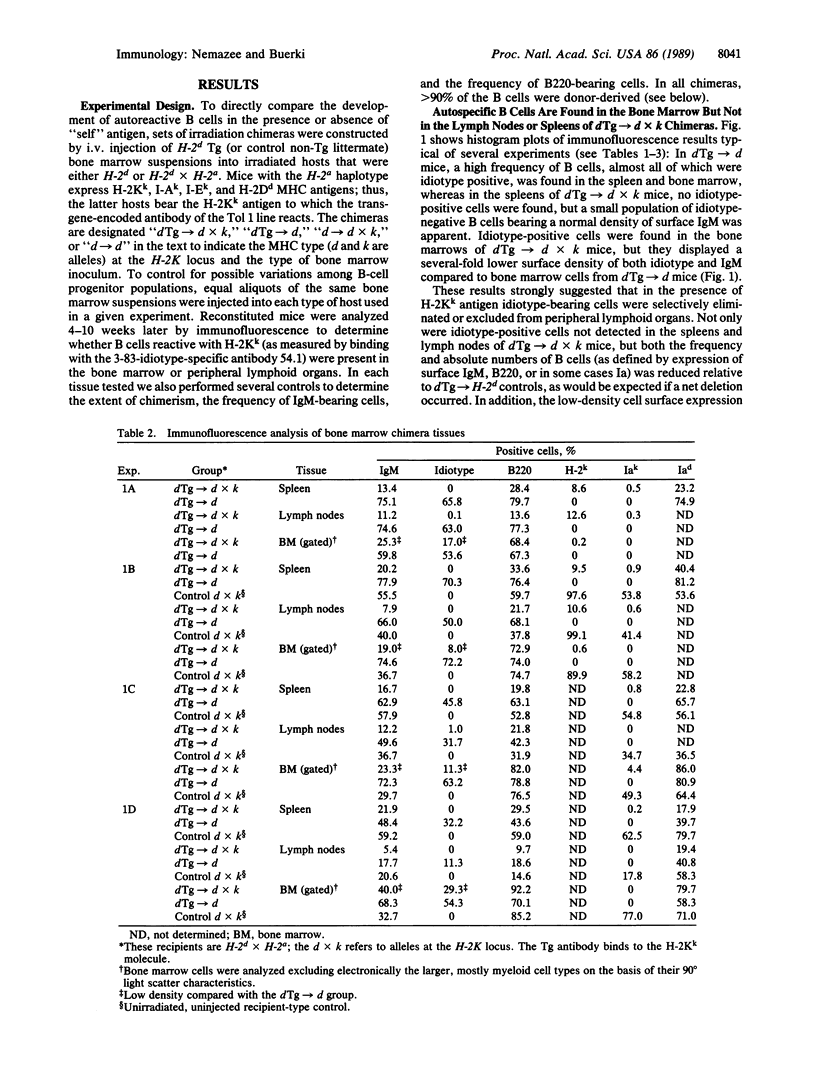
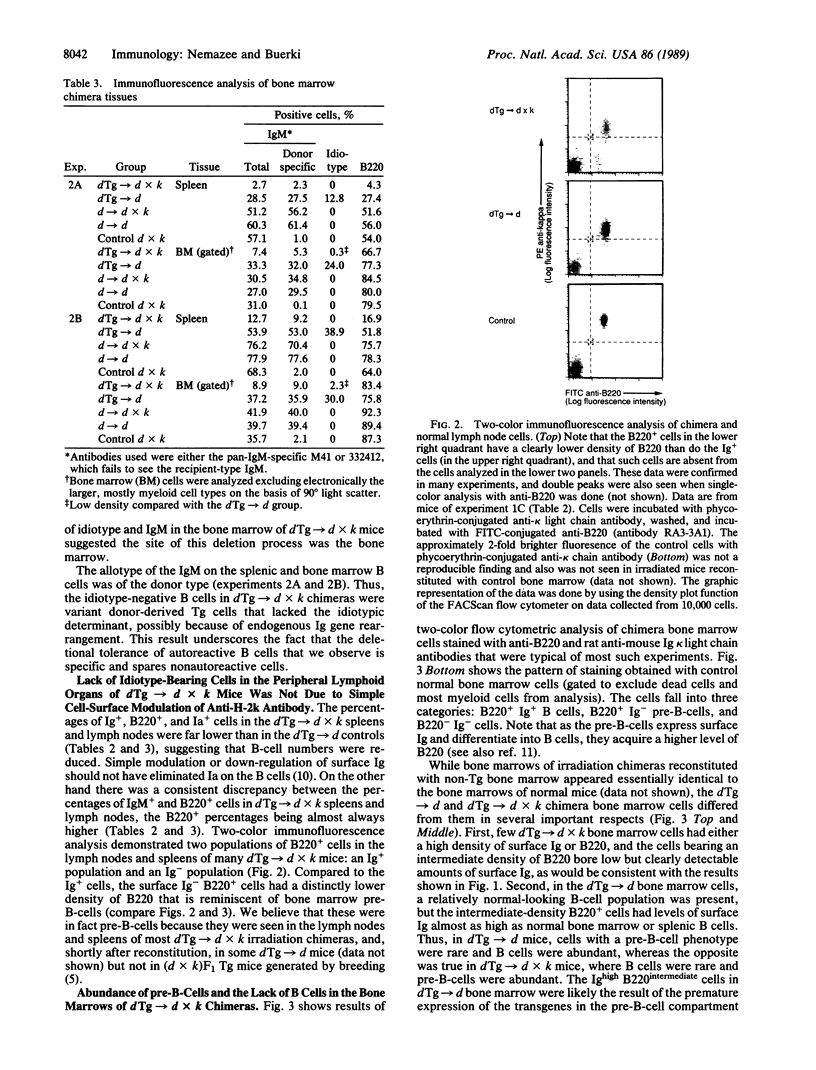
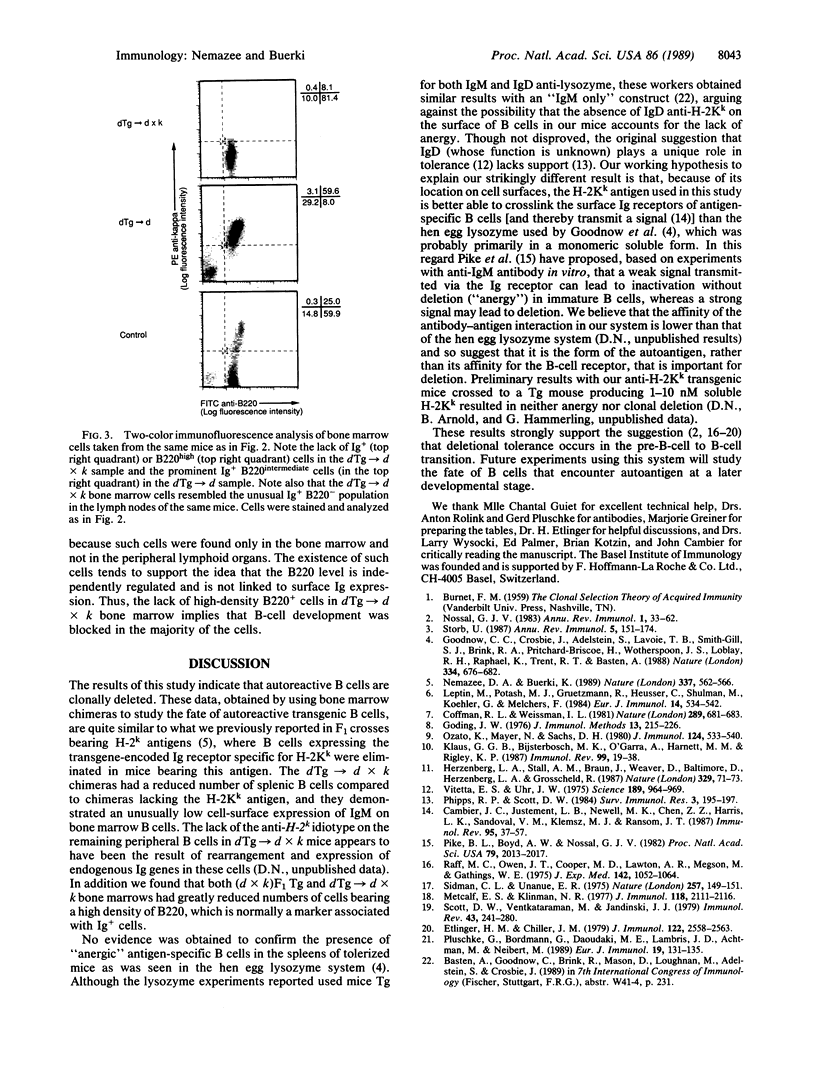
To study the fate of developing B cells in the presence and absence of the autoantigens to which they react, chimeric mice were constructed by injecting bone marrow cells from mice transgenic for rearranged immunoglobulin genes encoding an anti-H-2Kk antibody into irradiated recipients that did or did not express the H-2Kk antigen. In the presence of H-2Kk, the anti-H-2Kk-specific B cells were deleted from the spleen and lymph nodes, whereas in its absence, anti-H-2Kk cells were abundant. B cells bearing a low level of membrane immunoglobulin with the anti-H-2Kk idiotype were found in the bone marrows of H-2Kk recipients, suggesting that clonal deletion of autoreactive cells was occurring in the pre-B-cell to B-cell transitional stage of B-cell development.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cambier J. C., Justement L. B., Newell M. K., Chen Z. Z., Harris L. K., Sandoval V. M., Klemsz M. J., Ransom J. T. Transmembrane signals and intracellular "second messengers" in the regulation of quiescent B-lymphocyte activation. Immunol Rev. 1987 Feb;95:37–57. doi: 10.1111/j.1600-065x.1987.tb00499.x. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Etlinger H. M., Chiller J. M. Maturation of the lymphoid system. I. Induction of tolerance in neonates with a T-dependent antigen that is an obligate immunogen in adults. J Immunol. 1979 Jun;122(6):2558–2563. [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Goodnow C. C., Crosbie J., Adelstein S., Lavoie T. B., Smith-Gill S. J., Brink R. A., Pritchard-Briscoe H., Wotherspoon J. S., Loblay R. H., Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988 Aug 25;334(6184):676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Bijsterbosch M. K., O'Garra A., Harnett M. M., Rigley K. P. Receptor signalling and crosstalk in B lymphocytes. Immunol Rev. 1987 Oct;99:19–38. doi: 10.1111/j.1600-065x.1987.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Leptin M., Potash M. J., Grützmann R., Heusser C., Shulman M., Köhler G., Melchers F. Monoclonal antibodies specific for murine IgM I. Characterization of antigenic determinants on the four constant domains of the mu heavy chain. Eur J Immunol. 1984 Jun;14(6):534–542. doi: 10.1002/eji.1830140610. [DOI] [PubMed] [Google Scholar]
- Metcalf E. S., Klinman N. R. In vitro tolerance induction of bone marrow cells: a marker for B cell maturation. J Immunol. 1977 Jun;118(6):2111–2116. [PubMed] [Google Scholar]
- Nemazee D. A., Bürki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989 Feb 9;337(6207):562–566. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Phipps R. P., Scott D. W. Function of IgD in B cell triggering and tolerance: an overview of recent advances. Surv Immunol Res. 1984;3(2-3):195–197. doi: 10.1007/BF02918793. [DOI] [PubMed] [Google Scholar]
- Pike B. L., Boyd A. W., Nossal G. J. Clonal anergy: the universally anergic B lymphocyte. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2013–2017. doi: 10.1073/pnas.79.6.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Bordmann G., Daoudaki M. E., Lambris J. D., Achtman M., Neibert M. Isolation of rat IgM to IgG hybridoma isotype switch variants and analysis of the efficiency of rat Ig in complement activation. Eur J Immunol. 1989 Jan;19(1):131–135. doi: 10.1002/eji.1830190121. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Owen J. J., Cooper M. D., Lawton A. R., 3rd, Megson M., Gathings W. E. Differences in susceptibility of mature and immature mouse B lymphocytes to anti-immunoglobulin-induced immunoglobulin suppression in vitro. Possible implications for B-cell tolerance to self. J Exp Med. 1975 Nov 1;142(5):1052–1064. doi: 10.1084/jem.142.5.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Venkataraman M., Jandinski J. J. Multiple pathways of B lymphocyte tolerance. Immunol Rev. 1979;43:241–280. doi: 10.1111/j.1600-065x.1979.tb00424.x. [DOI] [PubMed] [Google Scholar]
- Sidman C. L., Unanue E. R. Receptor-mediated inactivation of early B lymphocytes. Nature. 1975 Sep 11;257(5522):149–151. doi: 10.1038/257149a0. [DOI] [PubMed] [Google Scholar]
- Storb U. Transgenic mice with immunoglobulin genes. Annu Rev Immunol. 1987;5:151–174. doi: 10.1146/annurev.iy.05.040187.001055. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Uhr J. W. Immunoglobulin-receptors revisited. Science. 1975 Sep 19;189(4207):964–969. doi: 10.1126/science.1083069. [DOI] [PubMed] [Google Scholar]


