Functional overlap of retinoblastoma protein stability and activity reveals a novel conserved regulatory pathway during Drosophila development.
Abstract
The Retinoblastoma (RB) transcriptional corepressor and related family of pocket proteins play central roles in cell cycle control and development, and the regulatory networks governed by these factors are frequently inactivated during tumorigenesis. During normal growth, these proteins are subject to tight control through at least two mechanisms. First, during cell cycle progression, repressor potential is down-regulated by Cdk-dependent phosphorylation, resulting in repressor dissociation from E2F family transcription factors. Second, RB proteins are subject to proteasome-mediated destruction during development. To better understand the mechanism for RB family protein instability, we characterized Rbf1 turnover in Drosophila and the protein motifs required for its destabilization. We show that specific point mutations in a conserved C-terminal instability element strongly stabilize Rbf1, but strikingly, these mutations also cripple repression activity. Rbf1 is destabilized specifically in actively proliferating tissues of the larva, indicating that controlled degradation of Rbf1 is linked to developmental signals. The positive linkage between Rbf1 activity and its destruction indicates that repressor function is governed in a manner similar to that described by the degron theory of transcriptional activation. Analogous mutations in the mammalian RB family member p107 similarly induce abnormal accumulation, indicating substantial conservation of this regulatory pathway.
INTRODUCTION
Originally identified as an important player in juvenile retinal cancer, and the first example of a tumor suppressor protein, the retinoblastoma (RB) gene product has been recognized as a key regulator of the eukaryotic cell cycle. RB is also inactivated in a significant proportion of adult onset human cancers (Knudson, 1978; Classon and Harlow, 2002) attesting to the centrally important role for RB in proliferation control. Further analyses in mammals have revealed that other RB related proteins, p130 and p107, contribute to cell cycle governance, but the partitioning of cell cycle duties among family members is not well defined. Nonetheless, the RB family and their cognate regulatory networks are well conserved among metazoans, substantiating the physiological significance of RB family function (van den Heuvel and Dyson, 2008).
As potent regulators of cellular proliferation, the activities of RB family proteins are tightly regulated. The canonical pathway for RB family regulation is mediated by cyclin/Cdk complexes that phosphorylate pocket proteins at key points during the cell cycle. In response, phospho-RB dissociates from E2F binding partners, and transcription of cell cycle–related genes such as PCNA can initiate at the G1/S phase transition (Dyson, 1998). In addition to phosphorylation control, RB protein activities are also regulated by proteolysis. During in vitro differentiation of 3T3-L1 adipocytes, p130 levels are transiently decreased relative to p107 by a proteasome-mediated pathway, and this switch is associated with successful differentiation (Prince et al., 2002). RB levels can be regulated by the Mdm2 ubiquitin ligase, better known for its control of levels of the p53 tumor suppressor, and in cancers overexpressing Mdm2, RB levels are diminished (Sdek et al., 2005; Uchida et al., 2005). The idea that altered RB protein levels contribute to disease etiology is further highlighted during infection by certain oncogenic viruses that hijack the proteolytic process and induce RB family member turnover to relieve host control of cellular proliferation (Boyer et al., 1996; Stubdal et al., 1997). Together, these examples demonstrate that regulation of RB family protein levels are important for normal cellular growth, but that these processes are often deregulated in disease.
In Drosophila, the RB family (Rbf) comprises two members, Rbf1 and Rbf2, and like their mammalian counterparts, these proteins function as transcriptional corepressors that interact with the E2F family of transcription factors (Sutcliffe et al., 2003). The Drosophila Rbf proteins provide canonical cell cycle control functions, and they are similarly regulated by phosphorylation involving cyclin/cdk complexes (Xin et al., 2002; Frolov et al., 2005; Swanhart et al., 2007). Rbf proteins are further subjected to influence of their turnover rates. Our recent studies indicated that proteasome-mediated turnover of both Rbf1 and Rbf2 is prevented through an association with the COP9 signalosome (Ullah et al., 2007). This linkage may contribute to COP9 control of cell cycle and development in plants and animals (Wei et al., 2008). The COP9 signalosome consists of eight subunits (CSN1-8), many of which exhibit limited similarity to subunits of the 19S regulatory lid of the proteasome, suggesting that the COP9 signalosome may play a direct role in modulating protein stability, possibly via interactions with the catalytic 20S core proteasome (Su et al., 2003; Chang and Schwechheimer, 2004). The COP9 signalosome may also control protein degradation through interactions with and subsequent deneddylation of the cullin subunits of SCF ubiquitin E3 ligase complexes (Wei et al., 2008). Multiple subunits of the COP9 signalosome were found to physically associate with Rbf proteins, and the depletion of any of these subunits lead to destabilization of both Rbf1 and Rbf2 in cultured cells and embryos (Ullah et al., 2007), suggesting that the entire complex is involved in stabilizing Rbf proteins. However, it is not known whether the COP9 regulation of Rbf proteins is a constitutive process or whether this control is regulated during development. The CSN4 subunit of the COP9 signalosome cooccupies cell cycle regulated genes simultaneously with Rbf proteins, suggesting that processes affecting repressor stability are spatially and temporally linked to repressor function during gene regulation (Ullah et al., 2007).
While proteasome-mediated destruction of cellular proteins is clearly linked to down-regulation of factor activity, the converse relationship has also been described, notably, that the potency of transcriptional regulatory proteins is directly linked to processes that mediate their destruction. This somewhat paradoxical relationship has been described for a variety of eukaryotic transcriptional activator proteins, including c-Jun, c-Fos, Myc, E2F1, and Gal4, all of which harbor degradation signals in regions closely overlapping with their activation domains (Salghetti et al., 1999; Salghetti et al., 2000; Salghetti et al., 2001). Synthetic constructs with multiple degradation domains exhibit higher levels of transcriptional activation, suggesting that the correspondence is not just coincidental (Salghetti et al., 1999; Salghetti et al., 2000). One proposed explanation for the tight correlation between protein lability and increased transcriptional potency posits that the proteasome, which is essential for turnover of ubiquitylated substrates, also mediates transcriptional activation functions directly (Gonzalez et al., 2002; Ferdous et al., 2007). A second mechanism suggests that activator ubiquitylation serves to recruit coactivator proteins, such as P-TEFb, to increase RNA polymerase elongation while simultaneously increasing the susceptibility of the activator to proteasome-mediated destruction (Muratani and Tansey, 2003; Lee et al., 2005; Collins and Tansey, 2006; Daulny et al., 2008). Although this effect has been observed for transcriptional activator proteins, no transcriptional repressor has been reported as potentiated by proteolytic susceptibility. In this study, we provide evidence that the lability of the Drosophila RB-related factor Rbf1 is tightly linked to its function as a transcriptional repressor, and that this evolutionarily conserved feature may provide an additional level of developmental control of the cell cycle.
MATERIALS AND METHODS
Expression Constructs and Transgenic Lines
To express Rbf1 proteins under control of the endogenous regulatory sequences, an 8.8-kbp genomic locus of Rbf1 was cloned, extending from 2.4 kb upstream of first exon to 2.4 kb downstream stop (2.1 kb downstream end of last exon) into pCaSpeR (Schejter and Shilo, 1989) between KpnI and XhoI sites, in three steps using PCR amplification of genomic DNA. Two Flag epitope tags were inserted immediately 5′ of the rbf1 stop codon into an XbaI site. The genomic construct of Rbf1 Δ728-786 was made by site-directed mutagenesis. For genes used in S2 cell culture transfection, rbf1 cDNA was PCR amplified and various mutants produced by site-directed mutagenesis were cloned from pLD02906 (Keller et al., 2005) into KpnI and XbaI sites of pAX vector (Ryu and Arnosti, 2003). Two Flag epitope tags were inserted 5′ of the stop codon. For misexpression in the fly, the constructs were cloned into KpnI and XbaI sites of pUAST (Brand and Perrimon, 1993). For bacterial expression of GST fusion proteins, the pRSF Duet-1 vector (Novagen, Darmstadt, Germany) was modified to introduce a GST ORF followed by a ligation independent cloning (LIC) site into its multiple cloning site I (MCS I) to generate the pRSF GST-Tb/LIC vector. rbf1 cDNA was PCR amplified and cloned into this LIC site to generate the pRSF GST-Rbf1 1-845 construct. The pRSF GST-Rbf1 Δ728-786 construct was generated by site-directed mutagenesis. For expression of human p107 in S2 cells, the cDNA and various mutants produced by site-directed mutagenesis were cloned into the pAX vector and modified with a C-terminal double Flag epitope. The pCaSpeR and pUAST plasmids were used to generate transgenic flies by P-element mediated germline transformation of yw flies. The transgenic flies were then balanced with SM2 CyO or TM3 Sb balancers.
Luciferase Reporter Assay
Drosophila S2 cells were transfected using Effectene transfection reagent (Qiagen, Valencia, CA) according to the manufacturer's protocol. Typically, 1.5 million cells were transfected with 1 μg of PCNA-Luciferase reporter, 0.25 μg of pRL-CMV Renilla luciferase reporter (Promega, Madison, WI) and 0.2 μg of one of pAX-rbf1 constructs. Cells were harvested 72 h after transfection, and luciferase activity was measured using the Dual-Glo Luciferase assay system (Promega) and quantified using the Veritas microplate luminometer (Turner Biosystems, Sunnyvale, CA). Firefly luciferase activity was normalized to renilla luciferase activity.
Immunocytochemistry
Drosophila S2 cells were transfected with 400 ng of each rbf mutant using the Effectene transfection reagent (Qiagen) according to the manufacturer's protocol. Cells were grown directly on cover slips pretreated with 0.01% poly-L-Lysine (Sigma, St. Louis, MO). Three days after transfection, cells were washed once in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4) and fixed in 4% paraformaldehyde (in PBS) for 30 min at room temperature. Cells were then washed four times in PBS, permeabilized in PBS+Triton-X-100 (0.4% vol/vol) for 10 min at room temperature, and blocked with 1% bovine serum albumin (in PBS). Cells were then incubated with M2 anti-Flag antibody (Sigma; final concentration 20 g/ml) in 1% wt/vol BSA in PBS buffer, washed three times in TBST (10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 0.05% Tween-20) for 5 min at room temperature, and incubated for 1 h at room temperature with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G (1:500 dilution) (Boehringer Mannheim, Mannheim, Germany, and Invitrogen, Carlsbad, CA). Cells were then washed three times in TBST and mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing 1.5 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) and incubated overnight at room temperature. Cells were visualized using an Olympus BX51 fluorescent microscope.
Western Blot Analysis
To measure protein expression in larval tissue, third-instar larvae were collected from transgenic lines expressing Flag-tagged Rbf1 and Rbf1 Δ728-786, mashed with a plastic pestle, and sonicated (3 cycles of 12 pulses each) in lysis buffer (50 mM HEPES, pH 7.9, 150 mM NaCl, 10% glycerol, 0.1 mM EDTA, 12.5 mM MgCl2, Complete mini-EDTA free protease inhibitor cocktail, Roche, Indianapolis, IN). Imaginal discs were dissected out from ten third-instar larvae and extracts were prepared in lysis buffer. Extracts were run on 10% SDS-PAGE gels and analyzed by Western blotting using M2 anti-Flag (mouse monoclonal, 1:10,000, 5 mg/ml Sigma; F3165). Antibody incubation was performed in TBST (20 mM Tris-Cl, pH 7.5, 120 mM NaCl, 0.1% Tween-20) with 5% nonfat dry milk. Blots were developed using HRP-conjugated secondary antibodies (Pierce, Rockford, IL) and SuperSignal West Pico chemiluminescent substrate (Pierce). To measure protein expression in cell culture, 50 μg S2 cell lysates were resolved by SDS-PAGE, transferred to a PVDF membrane, and probed with M2 anti-Flag mouse monoclonal at 1:10,000 dilution, mouse monoclonal anti-tubulin (Iowa Hybridoma Bank) at 1:20,000 dilution, anti-Groucho (mouse mAb obtained from the Developmental Studies Hybridoma Bank and used at 1:50 dilution) and anti-Rbf1 antibody as described previously (Keller et al., 2005).
Treatments with MG132 Proteasome Inhibitor and Cycloheximide
For proteasome inhibitor treatments, S2 cells were transfected with 0.5 μg of pAXrbf1 constructs using the calcium phosphate transfection method. The cells were grown for 5 d then treated with 50 μg/ml MG132 (Sigma-Aldrich) or the vehicle DMSO for the indicated times. For determination of Rbf1 protein half-life, 1.5 million S2 cells were transfected using Effectene transfection reagent (Qiagen) with 10 ng of pAXrbf1 1-845 or 4K-A.1 genes. Seventy-two hours post-transfection the cells were treated with 100 μM cycloheximide for the indicated times.
Protein–Protein Interaction Studies
For the expression of GST fusion proteins, the appropriate expression constructs were transformed into Rosetta2 (DE3) E. coli cells (Novagen). Protein expression was induced by 0.5 mM IPTG for 3 h at 37°C. The proteins were purified on Glutathione sepharose beads (GE Healthcare, Piscataway, NJ). The [35S]-Met labeled E2f proteins were generated using the TNT T7 Quick for PCR DNA Kit (Promega). In vitro translated proteins were bound to ∼1 μg of preincubated immobilized GST fusion proteins for 3 h at room temperature. The beads were washed three times with HEMGT-150 buffer (25 mM HEPES, 0.1 mM EDTA, 12.5 mM MgCl2, 10% Glycerol, 0.1% Tween-20, 150 mM KCl). Bound proteins were eluted by boiling in 1X Laemmli sample buffer and analyzed by SDS-PAGE and autoradiography. For the coimmunoprecipitation assays, 200 ng Myc-tagged E2f1 and 200 ng of various Flag-tagged Rbf1 constructs were cotransfected into S2 cells using Effectene transfection reagent (Qiagen). Cells were grown for 3 d after which whole cell extracts were prepared and Flag immunoprecipitation reactions were performed (Anti-Flag M2 affinity gel, Sigma) followed by anti-Myc Western blotting (mouse monoclonal, 1:3000 dilution, 5 mg/ml, Roche).
Chromatin Immunoprecipitation
Chromatin was prepared and analyzed from 0- to 20-h-old embryos as described previously (Martinez and Arnosti, 2008), except that the chromatin (1 ml) was incubated with 5 μl (5 μg) of Flag antibody (Sigma; F7425) or 2 μl H3 antibody (Abcam, Cambridge, MA; 0.4 μg/μl) overnight at 4°C. The recovered DNA was dissolved in 40 μl water. 2 μl of each ChIP sample was used for 28 cycles of PCR. The oligos used for PCR were 5′-CCGCAAGCATCGATAATGAGCAGA-3′ and 5′-AGTTGTGCGGGTACTTGGTTTC C-3′ for the DNA primase promoter; 5′-TGTGGGCTCTCTTCGTGTAGACTT-3′ and 5′-TGGTTTCTGATTCTCACACACGAC-3′ for the sloppy paired 1 promoter and 5′-GTTGAGAATGTGAGAAAGCGG-3′ and 5′-CGAAAAAGGAGAAGGCACAAAG-3′ for an intergenic region.
Fly Assays
Flies harboring the wild-type or mutant rbf1 forms in the pUAST vector were crossed with flies containing an eyeless-Gal4/CyO driver (Gilbert et al., 2006), and the offspring were screened for eye phenotypes. The rbf14 mutant (stock number 7435) was obtained from the Bloomington Stock Center.
Immunohistochemical Staining of Imaginal Discs
Imaginal discs were dissected in chilled PBS from third-instar larvae of rbf1 and rbf1Δ728-786 flies and fixed in 3.7% formaldehyde in 10 mM potassium phosphate, pH 6.8; 15 mM NaCl; 45 mM KCl; 2 mM MgCl2 for 30 min at room temperature. Antibody detection was performed by diaminobenzadine staining using the Vectastain kit (Vector Labs). Primary M2 α-Flag dilution was 1:1500. Following the horseradish peroxidase reaction, discs were mounted in 70% glycerol.
RESULTS
The Rbf1 C-Terminal Region Encodes an Instability Element
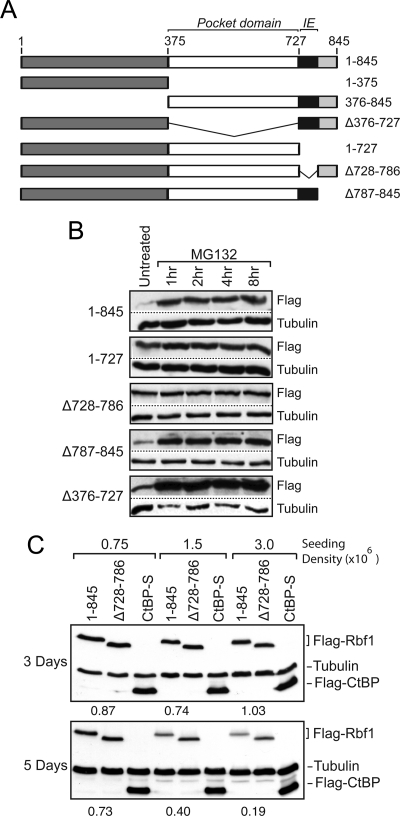
Our previous studies demonstrated that endogenous Rbf1 and Rbf2 proteins are dependent on the presence of the COP9 signalosome for stability; depletion of COP9 subunits resulted in a loss of Rbf protein, which was prevented by the addition of proteasome inhibitors, indicating the involvement of the 26S proteasome pathway (Ullah et al., 2007). To identify regions involved in Rbf turnover as first step toward understanding the process of Rbf stabilization, we examined the stability of epitope-tagged transfected Rbf1 proteins in S2 cells. We focused on Rbf1 because this protein represents the predominant functional RB family member in Drosophila; rbf1 null mutations are lethal, while rbf2 null mutants have only very modest phenotypes (Stevaux et al., 2005). Furthermore, previous data suggested that endogenous Rbf1 levels fluctuate during embryogenesis (Keller et al., 2005; Stevaux et al., 2005). We initially examined the importance of the conserved central pocket domain, as well as the less-conserved N- and C-terminal regions (Figure 1A; Table 1). In this process, we identified a region in the C terminus of the protein as an instability element (IE); proteins lacking residues 728-786 accumulated to high levels, and these levels were not further increased by treatment with the proteasome inhibitor MG132 (Figure 1B). In contrast, Rbf1 proteins containing the IE were expressed at lower levels, and these levels were enhanced by proteasome inhibition. Rbf1 stability was sensitive to growth conditions; Rbf1 ΔIE proteins were expressed at higher levels than proteins containing this domain under conditions of higher cell density, longer periods of cell culture, or with low amounts of transfected DNA (Figure 1C). This last observation suggested that the system for Rbf1 turnover can be saturated, and indeed we observed greater differences between the wild-type and mutant Rbf1 ΔIE proteins in cells expressing lower levels of each protein (not shown). Under these cell culture conditions we also observed that the endogenous Rbf1 protein was stabilized by addition of MG132 (Supplementary Figure 1). We conclude that the C-terminal region encompassing amino acids 728-786 harbors element(s) that contribute to Rbf1 instability and proteasome responsiveness.
Figure 1.
Identification of an instability element (IE) in Rbf1. (A) Schematic diagram of Rbf1 proteins expressed in Drosophila S2 cells. The N and C termini are indicated in dark and light gray, respectively; the black box represents the instability element; the E2f-binding pocket domain is in white. (B) Effect of proteasome inhibitor MG132 on Rbf1 protein levels. Cells were transfected to express the indicated proteins and treated for 1–8 h with MG132, and protein levels assayed by Western blot using antibodies to C-terminal Flag epitope tag. The wild-type 1-845 and mutants lacking the extreme C terminus (Δ787-845) or the pocket domain deletion mutant (Δ376-727) were expressed at lower levels and were strongly stabilized by this drug, while the mutants lacking the IE (Δ728-786 and 1-727) were expressed at higher levels and were not much further stabilized by MG132 treatment. (C) Effects of cell density and culture time on differential expression of wild-type Rbf1 and IE mutant. 400 ng of Rbf1 expression plasmid was transfected into S2 cells. At lower initial cell densities (0.75 × 106/ml) and shorter growth times (3 d), expression of wild-type Rbf1 (1-845) and a deletion mutant lacking the IE (Δ 728-786) accumulate to similar levels. Normalized protein levels are shown below the lanes containing Rbf1. Cells at higher initial densities (1.5–3 × 106/ml) grown for longer times (5 d) show higher levels of the mutant protein relative to the wild-type form. Levels of transfected CtBP protein, and endogenous tubulin protein, are shown as controls.
Table 1.
Rbf1 repression, stability, and localization
| Rbf1 construct | Repression activity ± SD | Protein stability | Nuclear localization |
|---|---|---|---|
| 1-845 | 100 ± 9 | + | |
| 1-375 | 12 ± 1 | − | |
| 376-845 | 42 ± 3 | + | |
| 1-727 | 16 ± 2 | + | − |
| Δ728-786 | 16 ± 4 | + | + |
| Δ787-845 | 107 ± 14 | − | |
| K754A | 65 ± 6 | + | |
| K754R | 81 ± 9 | + | |
| K774A | 151 ± 15 | + | |
| K774R | 125 ± 22 | + | |
| 3K-A.1 | 35 ± 11 | + | + |
| 3K-R.1 | 105 ± 26 | + | |
| 4K-A.1 | 22 ± 5 | + | + |
| 4K-R.1 | 86 ± 7 | + | |
| 6K-A.1 | 36 ± 9 | + | + |
| 6K-R.1 | 110 ± 9 | + |
Constructs marked (−) for nuclear localization were not exclusively nuclear.
Critical Roles of Lysine Residues within Instability Element
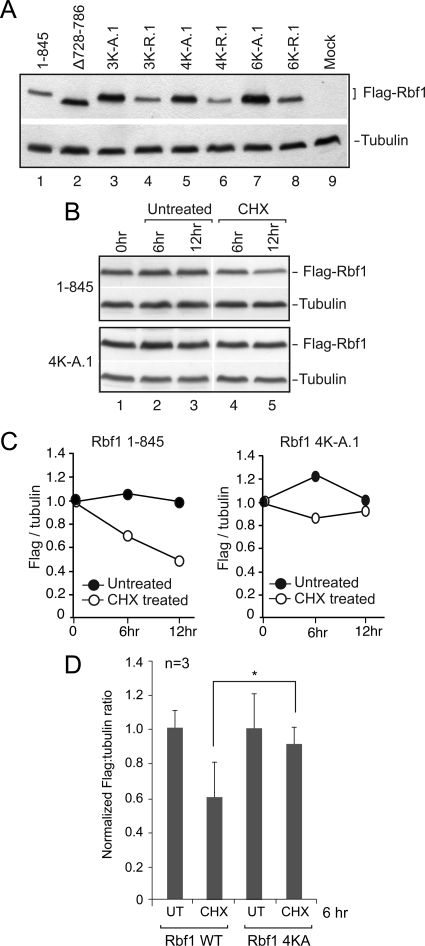
The striking accumulation of wild-type Rbf1 protein in cells treated with the proteasome inhibitor MG132 indicated that this protein, but not the mutant forms lacking the IE, is subject to active degradation. We hypothesized that the Rbf1 IE may serve as a target for protein ubiquitylation as one mechanism explaining the contribution of this region to proteasome-mediated turnover. Protein ubiquitylation of lysine residues often directs processing by the 26S proteasome, therefore we tested whether the lysine residues in the IE are involved in the stability of Rbf1 (Figure 2; Table 1). Mutant Rbf1 in which three, four, or all of the six lysines were converted to alanine (K to A) were assessed for expression. All three of these mutant forms accumulated to significantly higher levels than the wild-type protein. In contrast, mutant Rbf1 proteins harboring charge-conserving lysine-to-arginine substitutions in the same residues did not over accumulate, suggesting that the positive charge of the side chain, rather than its ability to be ubiquitylated, is important for low steady state levels (Figure 2A). To determine whether the change in steady state levels is due to altered stability, we next tested whether the half-life of wild-type and mutant (4KA) Rbf1 proteins differed by treating S2 cells with the translational inhibitor cycloheximide. Three days after transfection at a point when our previous data indicated that Rbf1 (4KA) mutant protein was expressed at higher levels than wild-type Rbf1, S2 cells were treated with cycloheximide and Rbf1 protein levels subsequently measured at 0, 6, and 12 h (Figure 2B, 2C). By 6 h, levels of the wild-type Rbf1 protein, but not the mutant Rbf1 (4KA), were significantly decreased, confirming that the heightened accumulation of Rbf1 proteins lacking the IE is caused by reduced rate of Rbf1 degradation (Figure 2D).
Figure 2.
Conserved lysine residues in IE play critical roles in accumulation and stability of Rbf1. (A) Mutation of multiple lysine residues within the IE leads to increased protein accumulation. Lysine residues were changed to alanine (K732A, K739A, K740A for 3K-A; also K754A for 4K-A; also K774A and K782A for 6K-A) or to arginine. Rbf1 overaccumulation is not observed with the lysine to arginine substitution. 1.5 × 106 S2 cells were transfected with 100 ng of Rbf1 expression plasmid and grown for five days. The data shown are representative of three biological experiments. (B and C) Half-life measurements of unstable wild-type and stable IE mutant Rbf1 proteins. Three days after transfection, cells were treated with cycloheximide and harvested at the indicated times. Rbf1 protein levels were quantitated by photon-capture analysis with a Fuji LAS-3000 Imager and normalized to tubulin levels. (D) Bar graphs showing averaged normalized flag:tubulin ratios for the Rbf1 wild-type and 4K-A mutant proteins at the 6-h time point from three biological replicates. At this time point, the difference between the wild-type and the 4K-A mutant protein levels was statistically significant (p = 0.05).
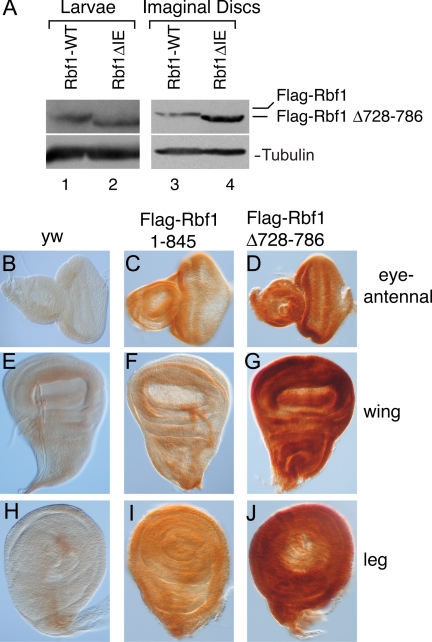
To assess whether the Rbf1 IE functions as an instability element in the context of normal Drosophila development, we devised a rescue construct that expresses epitope-tagged Rbf1 under the control of the endogenous rbf1 regulatory sequences. Developmental expression of the wild-type Rbf1 and Rbf1 ΔIE (Δ728-786) proteins was then assessed by Western blotting. As shown in Figure 3A (left panel), the overall levels of both proteins were similar in third-instar larval extracts, suggesting that the deletion mutant accumulated to wild-type levels. However, a very different picture emerged when we measured protein expression in imaginal disc tissue from third-instar larvae as shown through Western blots in Figure 3A (right panel) and imaginal disc staining in Figure 3, B–J. Steady state levels of wild-type Rbf1 were far lower than Rbf1 ΔIE in eye, wing, and leg imaginal discs; this observation was consistent for three independent lines of each construct (Figure 3, B–J). The relationship between this effect and previously characterized Rbf1 function is especially evident in the eye imaginal disc. The terminally differentiating cells of the posterior eye disc normally have no transcription of rbf1 and low or nonexistent levels of Rbf1 (Keller et al., 2005), but the Rbf1 ΔIE mutant also shows staining in these posterior cells, suggesting an abnormal perdurance of the protein (Figure 3C, D). The marked difference between the steady-state levels of the two proteins in these contexts indicates that the wild-type Rbf1 protein is specifically destabilized in the proliferating and differentiating tissue of the imaginal discs. The tissue-specific stability of the Rbf1 wild-type and mutant proteins suggests that turnover of Rbf1 is a regulated event and is likely triggered by developmental signals. The cell density–dependent difference in protein accumulation for wild-type and IE-deleted Rbf1 proteins as described in Figure 1C also supports this hypothesis.
Figure 3.
Expression of wild-type and IE mutant forms of Rbf1 in the Drosophila larva. Indicated proteins were expressed from the endogenous rbf1 promoter, and expression levels were assayed in total larval extracts as well as in imaginal discs. (A) Western blot showing expression of Flag-tagged Rbf1 from third-instar larvae (left panel) and pooled imaginal discs (right panel) carrying homozygous copies of rbf1 genomic constructs. Equivalent levels of proteins were noted in whole larval extracts whereas the mutant protein was found to accumulate to ∼fourfold of the wild-type protein in the imaginal discs. The Western blot of whole larval extracts is representative of four biological replicates for the two lines shown in C, F, I, and D, G, J; the average difference in protein levels in total larval extracts was 13% ± 2%. (B–J) Rbf1 expression in third-instar larval imaginal discs. (B–D) Eye discs, (E–G) wing discs, and (H–J) leg discs. Weak background staining was observed in nontransgenic yw flies (B, E, and H), and specific but weak staining was evident in discs expressing wild-type Rbf1 protein (C, F, and I). Strong expression was noted in flies expressing the inactive Rbf1 Δ 728-786 IE mutant protein (D, G, and J). The imaginal disc staining is representative of stainings of three different lines for each construct; in all cases, the IE mutant protein was expressed at higher levels.
The Rbf1 Instability Element Contributes to Repression Potency
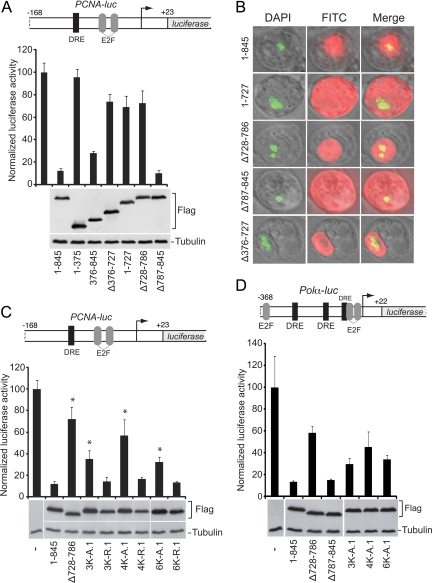
In the previous experiment, the rbf1-Flag transgene rescued an rbf14 null mutant, substituting for both zygotic and maternal Rbf1 protein as demonstrated by its ability to support viable flies for generations (Table 2 and data not shown). In contrast, the similar construct expressing Rbf1 (ΔIE)–Flag protein was not capable of rescuing the mutation, despite robust expression in imaginal discs and wild-type expression at the third-instar larval stage. We therefore hypothesized that the IE is required for Rbf1's role in regulating activity. To test this hypothesis, S2 cells were cotransfected with expression plasmids encoding wild-type or mutant Rbf1 proteins and the effect on repression potency was determined using PCNA-luciferase reporter construct, which is sensitive to repression by Rbf1 (Stevaux et al., 2002). As expected, proteins lacking the central pocket domain were inactive; this region of the protein is required for interaction with the E2F transcription factors that recruit Rbf1 to the promoter (Figure 4A). Removal of the N-terminal portion of the protein had only a mildly deleterious effect on repression, consistent with previous studies that suggested it is not required for transcriptional activity in vivo and in vitro (Hiebert et al., 1992). In contrast, removal of portions of the entire C terminus revealed multiple effects. First, deletion of the IE region alone had a strong inhibitory effect on transcriptional repression, and this effect was just as severe as removal of the critical pocket domain. The Rbf1 ΔIE and pocket deletion mutant proteins did not exhibit aberrant localization, but remained in the nucleus (Figure 4B). Second, loss of the adjacent C-terminal 59 amino acids (Δ787-845) did not abolish repression but did change its subcellular localization so that the protein was no longer strictly nuclear. These data indicate that this region harbors a nuclear targeting element governing Rbf1 cytoplasmic/nuclear distribution. As observed for deletion of the entire IE (Δ728-786), removal of portions of this 59-aa region in blocks of 20 was sufficient to inhibit repression activity, suggesting that the function of the IE is distributed over numerous residues throughout this region (data not shown).
Table 2.
rbf14 rescued by transgenic Rbf1
| Strain | Genotype (%) |
n | |||
|---|---|---|---|---|---|
| rbf14/Y | FM7/Y | rbf14/+ | FM7/+ | ||
| rbf14 mutant male flies rescued by rbf1 transgene | |||||
| Rbf1 L1 | 3.7 | 19.1 | 41.2 | 36.0 | 1116 |
| Rbf1 L2 | 3.6 | 22.6 | 39.8 | 34.0 | 1163 |
| Rbf1Δ728-786 | 0 | 30.0 | 37.4 | 32.6 | 697 |
| rbf14/Y | FM7/Y | rbf14/rbf14 | rbf14/FM7 | ||
|---|---|---|---|---|---|
| rbf14 mutant female flies rescued by rbf1 transgene | |||||
| Rbf1 L1 | 6.1 | 39.6 | 9.8 | 44.5 | 164 |
| Rbf1 L2 | 1.1 | 36.7 | 8.5 | 53.7 | 188 |
L1 and L2 are two independent transgenic lines expressing wild-type Rbf1 protein. Rbf1Δ728-786 expresses a nonfunctional proteolytically stabilized form of Rbf1. rbf14 is a complete deletion mutant of Rbf1. FM7 represents an X-chromosome balancer. rbf14/Y represents rescued males; rbf14/rbf14 represents rescued females. The larger percentage of flies carrying the wild-type (+) or balancer (FM7) X-Chromosome indicates that some flies are not rescued.
Figure 4.
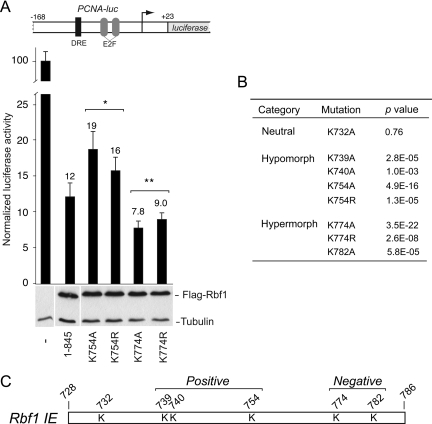
Rbf1 requires the IE for transcriptional repression. (A) Deletion of the IE (Δ 728-786) or E2f binding pocket (Δ376-727) compromises transcriptional repression activity of Rbf1 proteins measured on the PCNA-luciferase reporter gene (bar graph). Under these transfection conditions, proteins were expressed at similar levels (Western blot). (B) Subcellular localization of wild-type (1–845) and deletion mutants. DAPI staining indicates DNA in nucleus, and FITC staining the Rbf1 proteins. Proteins lacking residues 787–845, which include the presumptive nuclear localization signal, are found predominantly in the cytoplasm. (C) Transcriptional activity of Rbf1 IE deletion and point mutant proteins assayed on PCNA-luciferase reporter. Mutant proteins lacking the IE, or with multiple lysine to alanine mutations, were compromised for transcriptional repression activity. Lysine to arginine mutant proteins exhibited wild-type repression activity. Error bars indicate SD, and asterisks indicate p < 0.05 (D) Rbf1 repression of Drosophila Polα-luciferase reporter. Deletion of the IE largely inactivates the protein for transcriptional repression (top panel). Data in 4A represent two biological replicates, each with three technical replicates, except for 1-845 and Δ 728-786, which represent 16 and 9 biological replicates. Other transfections include data from at least three biological replicates. Firefly luciferase activity is expressed relative to Renilla luciferase control.
Our previous data indicated that multiple lysine residues within the Rbf1 IE contributed to Rbf1 stability, thus we tested whether these same residues were involved in the transcriptional repression mediated by Rbf1. Indeed, as shown in Figure 4C, Rbf1 proteins bearing multiple lysine to alanine substitutions were less effective repressors, even though these proteins were more stable than the wild-type Rbf1. This effect was most notable for the Rbf1 4KA mutant whose repression capability was similar to that mediated by Rbf1 lacking the IE. Surprisingly, alanine substitution of two additional lysine resides (6KA) reproducibly improved the function of Rbf1 in repression. This observation raised the possibility that this region harbors elements that throttle Rbf1 repressor potency, as discussed further below. In contrast to alanine substitution, Rbf1 proteins harboring multiple lysine to arginine substitutions did not overaccumulate, and significantly, were just as potent as wild-type Rbf1 for transcriptional repression. Based on these data, we conclude that these residues contribute both Rbf1 instability and to repressor function. These data further indicate that modification of these residues is not essential to either process. To test whether the effects on transcriptional repression of these Rbf1 mutations were evident in other contexts, we compared transcriptional repression of wild-type and mutant Rbf1 proteins on the Polα promoter, which has somewhat different requirements for E2F and DP activation compared with the PCNA promoter (Figure 4D) (Dimova et al., 2003). Deletion of the IE or point mutations within this region similarly reduced the repression activity on this promoter as well, indicating that the relationship between protein activity and instability is independent of promoter context. Taken together, these data strongly indicate that the ability of the Rbf1 protein to act as a transcriptional repressor is tightly associated with its instability, and that the IE in the Rbf1 C terminus is multifunctional, linking these two features.
The Rbf1 IE Is Not Essential for E2F Interactions and Promoter Binding
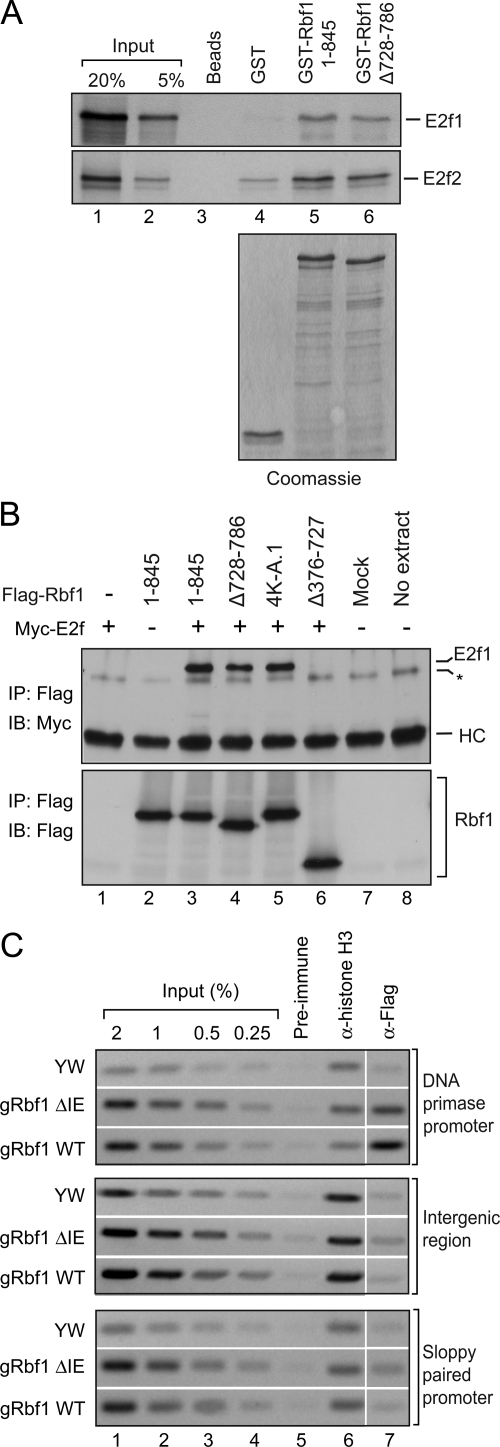
Previous studies have shown that both the pocket domain as well as the carboxy terminus of the human RB protein can make molecular contacts with E2F1 (Lee et al., 2002; Xiao et al., 2003; Rubin et al., 2005). We reasoned that the reduced activity of the Rbf1 instability element mutants might be a direct result of their inability to physically associate with the E2F transcription factors. Therefore, we performed GST pull-down and coimmunoprecipitation (CoIP) assays to test for interactions between Rbf1 and E2f proteins. In the GST pull-down assays, both GST-Rbf1 1-845 and the IE mutant (Δ728-786) displayed similar binding ability to in vitro translated E2f1 and E2f2 proteins (Figure 5A, lanes 5 and 6). No interaction was observed with beads alone or GST protein (Figure 5A, lanes 3 and 4). Similarly in CoIP assays from Drosophila S2 cells, Myc-tagged E2f1 coprecipitated with Rbf1 1-845 and two IE mutants (Δ728-786 and 4K-A.1) but not with the pocket domain deletion mutant (Δ376-727) (Figure 5B; top panel, lanes 3–6). These results show that the IE mutants retain a capacity to interact with both E2f1 and E2f2 proteins.
Figure 5.
Rbf1 IE is not essential for E2F interactions and promoter binding. (A and B) Physical association between Rbf1 IE mutants and E2F proteins. (A) GST-Rbf1 and E2f interaction assay. Indicated GST fusion proteins were bound to radio-labeled E2f proteins and bound proteins were analyzed by SDS-PAGE and autoradiography. GST-Rbf1 1-845 and ΔIE mutant displayed similar binding ability to both in vitro translated E2f1 and E2f2 proteins (compare lanes 5 and 6). No interaction was observed with beads alone and GST protein (lanes 3 and 4). Coomassie stained gel showing equal amounts of GST fusion proteins used in binding assays (bottom panel). The data shown are representative of three biological replicates. (B) Coimmunoprecipitation assay. Rbf1/E2f1 interactions in cotransfected S2 cells. Cells were cotransfected with Myc-tagged E2f1 and Flag-tagged Rbf1 expression constructs. Whole cell lysates were used for Flag immunoprecipitations (IP) and the samples were assayed using Western blots with anti-Myc antibody (top panel). Myc-tagged E2f1 coprecipitated with Rbf1 1-845 and two IE mutants (Δ728-786 and 4K-A.1) but not with the pocket domain deletion mutant (Δ376-727) (top panel, lanes 3–6). Mock is IP performed using cell lysate from untransfected cells (lane 7). The asterisk indicates a nonspecific band that is contributed by the Flag M2 beads since it appeared in the no extract control where IP was performed in the absence of any cell lysate (lane 8). Equivalent levels of the heavy chain IgG (marked as HC) were seen in all samples indicating the use of equal amount of antibody for each IP reaction. The IP samples were also blotted with the anti-Flag antibody (bottom panel) to verify the amount of Flag-tagged protein that was captured in each assay. The data shown are representative of two biological replicates. (C) Promoter occupancy by Flag-tagged Rbf1 wild-type and Rbf1 IE mutant proteins measured by chromatin immunoprecipitation. Formaldehyde cross-linked chromatin was prepared from 0 to 20 h embryos expressing the wild-type or mutant Rbf1 protein and immunoprecipitated using the indicated antibodies. Enrichment of the Rbf-regulated promoter (DNA primase) was observed by anti-Flag antibody immunoprecipitation reactions with both wild-type and IE mutant fly embryos but not in reactions using pre-immune IgG (top panel) or at an intergenic locus (middle panel) and a nontarget gene promoter (sloppy paired 1) (bottom panel).
To assess whether the IE plays a role in Rbf1 promoter occupancy we performed chromatin immunoprecipitation (ChIP) assays using embryos expressing the Flag-tagged Rbf1 wild-type or ΔIE mutant to test for promoter binding of these proteins at the DNA primase promoter (Figure 5C). Binding at an intergenic locus and a nontarget gene (sloppy paired 1) promoter was assessed as negative controls. Interestingly, the DNA primase promoter was found to be enriched in immunoprecipitates from chromatin derived from embryos expressing both the wild-type Rbf1 as well as the Rbf1 IE mutant proteins indicating that the Rbf1 IE mutant can still occupy promoters (Figure 5C; top panel). Binding of the IE mutant at this locus was slightly reduced compared with the wild-type Rbf1 although the association was significantly above background as no enrichment was observed at an intergenic locus (middle panel) or the nontarget sloppy paired 1 promoter (bottom panel). It appears that, unlike the Rbf1 pocket deletion mutant, the reduced activity of the Rbf1 IE mutants cannot be attributed simply to their inability to interact with E2F proteins or target gene promoters.
The Rbf1 IE Is a Dual-Function Regulator of Repressor Potency
Our data indicates that the Rbf1 IE region influences Rbf1 instability and contributes to Rbf1 repression potency, providing a link between these two activities. However, during these analyses we additionally observed that Rbf1 (6KA), harboring substitutions of all lysine residues within the IE was reproducibly a more potent repressor than Rbf1 (4KA), harboring substitutions of only the four most N-terminal lysine residues within the IE. This observation raised the possibility that while most of the lysines play a positive role in Rbf1 repression, one or both of the C-terminal-most lysine residues (K774, K782) play a negative role, restricting Rbf1 activity. Therefore, to determine whether the lysine residues within the IE contribute to both positive and negative regulation of Rbf1 function, we tested the repression activities of Rbf1 proteins with individual alanine substitutions of each lysine residue within the IE. A subset of these results is shown in Figure 6A, revealing three outcomes. In one case (K732), alanine substitution did not affect repressor potency and was indistinguishable from wild-type Rbf1. The second class of mutants were hypomorphic (K739, K740, K754), exhibiting modest but reproducible inhibitory effects on repression, consistent with these residues contributing a positive influence on repressor potency (Figure 6, A and B). In contrast, three mutants, K774A, K774R, and K782A exhibited hypermorphic phenotypes with modest but reproducibly higher repression activity than the wild-type Rbf1 protein, suggesting that these residues are involved in a negative control of repressor activity (Figure 6, A and B). In cases where lysine to arginine substitution did not moderate activity to wild-type levels, such as with K754 and K774, it is possible that the lysine in question is a target of modification, as a positive charge is not the sole important feature. However, for mutants with only single point mutations, we did not observe the robust stabilization of mutant proteins compared with the wild-type protein (not shown). Together, these data also indicate that the IE exerts both positive and negative influences on transcriptional activity. Those mutant forms of Rbf1 lacking all lysines exhibited intermediate repression phenotypes because of two distinct and opposite effects, with decreased activity caused by mutations in K739, 740, and 754 partially offset by increased activity mediated by the mutation of K774 and K782.
Figure 6.
Rbf1 IE harbors positive and negative regulatory elements. (A) Transcriptional repression activity of Rbf1 lysine point mutant proteins. Examples of mutant proteins that show either enhanced or reduced repression activity. Mutation of K754 to alanine or arginine attenuates repression activity while K774 to alanine mutant exhibited enhanced repression activity with respect to the wild-type protein (top panel). Under these transfection conditions, proteins were expressed at similar levels (lower panel). Error bars indicate standard deviations, and asterisks indicate p < 0.05 compared with wild-type Rbf1. (B) The lysine point mutants were classified as neutral, hypo-, or hypermorphic based on the indicated t test results. (C) Schematic representation of the Rbf1 IE indicating the location of lysine residues that play a positive or negative role in Rbf1-mediated repression.
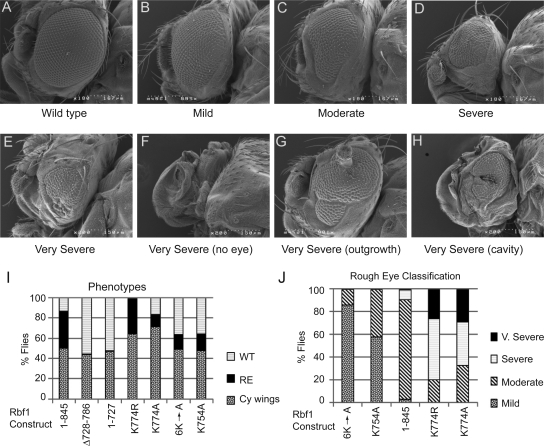
To test the physiological importance of these positively and negatively-acting residues for repressor regulation in Drosophila, we expressed Rbf1 isoforms in the developing eye imaginal disc using an eyeless-Gal4 driver system (Figure 7, A–H). As noted in previous studies, misexpression of the wild-type Rbf1 protein induced rough eyes in a large percentage of offspring. The mutant form of Rbf1 (Δ728-786) lacking the IE was completely inert, despite robust expression of the protein in the fly (not shown), consistent with a role for the IE in repression. Individual point mutations that had modest effects on repression in cell culture assays similarly showed modest effects on eye development, exhibiting milder phenotypes, and lower penetrance than the wild-type Rbf1. In contrast, the hypermorphic K774A mutant, which exhibited elevated repression activity in cell culture assays, induced dramatic phenotypes (Figure 7, E–H). A large percentage of offspring expressing this protein exhibited very severe eye defects, including complete loss of the eye or developmental abnormalities including antennal outgrowths and fewer transgenic individuals were recovered relative to nonexpressing controls, suggesting lethality (Figure 7, I and J). Thus, the effects of the mutant forms of Rbf1 on eye development mirror exactly the relative potencies of these proteins as measured in cell-based repression assays indicating that Rbf1 is subjected to both positive and negative regulation of repressor potency via the C-terminal IE in vivo. This result additionally demonstrates the importance of limiting Rbf1 repression activity during development.
Figure 7.
Severe developmental consequences of expression of hyperactive Rbf1. cDNAs of rbf1 wild-type and IE hypermorphic and hypomorphic mutants were misexpressed in the eye imaginal disc using the eye-Gal4 driver. (A–H) representative eyes exhibiting wild-type, mild, moderate, severe, and four very severe phenotypes. (I) Bar graphs representing frequency with which flies carrying the eye-Gal4 driver and UAS-rbf1 gene were recovered, as well as frequency with which these latter flies exhibited a phenotype (“WT” normal eye, “RE” rough eye of any degree of severity, “Cy wings” indicates flies that lacked the Gal4 driver, did not express the rbf1 transgene, and had wild-type eyes). Note that Δ728-786 and 1-727, which lack the IE and were inactive in cell culture, never showed a phenotype, and that the hyperactive K774 mutants exhibited a partially lethal phenotype, as judged by lower recovery of flies containing the eye-Gal4 driver. (J) Severity of eye phenotype in flies exhibiting rough eyes. Mutants are shown in order of increasing severity; point mutations in the IE that decreased function in cell culture assays also exhibited weaker eye phenotypes, and hypermorphic K774 alleles exhibited much stronger phenotypes.
Conserved Instability Domain of Mammalian p107
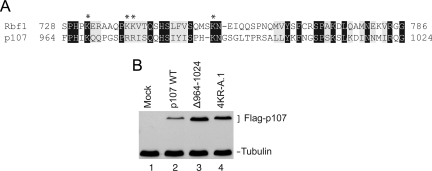
The correlation between Rbf1 activity and instability in Drosophila prompted us to examine whether similar regulation affects mammalian RB proteins. The overall level of amino acid conservation is highest between the “pocket” domains of RB family members, but there are clearly conserved blocks of residues in the C-terminal region. The primary structure of the C terminus of Rbf1 most closely resembles that of p107, including the amino acids residues located in the instability element of Rbf1 (Figure 8A). To directly compare Rbf1 and p107, we transfected S2 cells with wild-type p107 and mutant forms in which conserved lysine and arginine residues were replaced with alanine, as well as a deletion of the region most similar to the Rbf1 IE (amino acids 964-1024). Similar to the stabilization effects noted with Rbf1, mutant p107 exhibited increased accumulation compared with the wild-type protein (Figure 8B), suggesting that the C-terminal region of p107 harbors an instability element that funnels p107 into similar turnover pathways even in this heterologous system.
Figure 8.
Mutations in the conserved IE of p107 enhance expression. (A) Similarities between Rbf1 IE and homologous region of p107, which is most similar to Rbf1. Asterisks mark basic residues mutated in each protein to stabilize expression. (B) Genes for Flag-tagged wild-type p107 or IE mutants were transfected into S2 cells and expression quantitated by Western blot. The 60-aa region deleted from p107 in Δ964-1024 is similar to the Rbf1 IE. Endogenous tubulin levels are shown as controls.
DISCUSSION
During Drosophila development, cell-cycle regulation deviates considerably from the classical four-stage G1/S/G2/M pattern, exhibiting rapid direct S-M cycling early in development, stepwise acquisition of G2 and G1 phases, and endoreplication. These alternative cycles involve a variety of regulatory features, including constitutive inactivation of Rbf proteins by phosphorylation, transcriptional regulation of the rbf1 and rbf2 genes, and regulated degradation of the E2F1 protein. Here we provide evidence that this regulatory richness also includes a novel developmentally-triggered degradation of Rbf1 that paradoxically appears to be required for repression activity. Our study indicates that Rbf1 lability is tightly linked to repression activity, both in a cellular as well as a whole organismal context. The IE identified in the C terminus of this protein appears to be a complex domain with dual functions, so that even a few lysine to alanine mutations can dramatically enhance protein stability while inhibiting transcriptional activity, while other lesions enhance the protein's activity (Figures 1, 3, and 4).
Not only is the turnover of Rbf1 required for effective gene regulation, but it appears that this turnover can be developmentally cued, presumably to be coordinated with the engagement of Rbf1 with regulation of the cell cycle (Figure 3). Highly proliferative imaginal disc tissue appears to provide one such context, where levels of wild-type, but not an instability element mutant, Rbf1 protein decrease sharply, presumably in response to the engagement of this protein during cell cycling. In the eye imaginal disc, the Rbf1 protein levels drop sharply in the posterior, where cells are becoming terminally differentiated. Presumably, Rbf1 is activated and consumed in the coordinated cell divisions that occur in the two stripes flanking the morphogenetic furrow; the absence of any further transcription leads to global depletion of Rbf1. The Rbf1 protein lacking the IE accumulates inappropriately in differentiating cells.
How might the repression activity of Rbf1 be linked to protein turnover? Protein lability has previously been found to underlie the action of some eukaryotic transcriptional activators (Salghetti et al., 2001; Kim et al., 2003). The activation domain of the VP16 protein was found to be subject to modification by ubiquitylation, enhancing the transcriptional potency of this factor as well as destabilizing it. This process is thought to affect other transcriptional activators as well (Salghetti et al., 2000). The exact mechanism by which ubiquitylation enhances transcriptional activation is poorly understood. The ubiquitin tag may serve a dual purpose of facilitating interactions with the transcriptional machinery as well as attracting the 26S proteasome. Alternatively, the proteasome itself, or portions of this multi-protein complex, may directly enhance transcription; chromatin immunoprecipitation experiments have placed the “lid” of the proteasome on specific genomic locations (Gonzalez et al., 2002; Ferdous et al., 2007).
Until now, there have been no examples of a connection between transcriptional repression and turnover. If it is the modification of the protein with ubiquitin that potentiates Rbf1's repressor activity, this moiety may allow efficient interaction with the transcriptional machinery, similar to the manner in which SUMOylation of PPAR-γ enhances interaction with NCoR corepressors to silence inflammatory genes (Pascual et al., 2005). Ubiquitylation would in this case attract the 26S proteasome in a competing, parallel reaction that enables Rbf1 turnover. Alternatively, Rbf1 recruitment of the proteasome may allow this complex to directly mediate repression, in a way opposite to that produced by activation domains.
The C terminus of Rbf1 appears to represent a regulatory nexus for this protein; in addition to the instability/repression activity described here, key residues appear to provide a damper to modulate its overall activity (Figure 6), and phosphorylation within this region by cyclin kinases can inactivate the protein (Xin et al., 2002). The deep conservation of residues within the Rbf1 IE argues strongly for similar activities in mammalian pocket proteins; indeed, mutations of key residues in p107, the closest homolog to Rbf1, strongly stabilize the levels of this protein (Figure 8). In addition, the spectrum of mutations associated with the human retinoblastoma gene indicates that the C-terminal region correlating to the Rbf1 IE may similarly contain critical functions for the mammalian RB protein. One common class of genetic lesion associated with retinoblastomas are nonsense mutations that cause a truncation of the C terminus of the RB protein, and several cancer-associated missense mutations have similarly been mapped to the region corresponding to the Rbf1 IE (Lohmann, 1999).
Previous studies have shown that the RB C terminus interacts with the E3 ligase Skp2 and the anaphase promoting complex (APC/C) to regulate turnover of the p27 cyclin kinase inhibitor (Ji et al., 2004; Binne et al., 2007). This pathway has been suggested to represent a transcription-independent mechanism by which RB controls the cell cycle, and indeed RB was shown not to be subject to APC/C degradation (Binne et al., 2007). Our results indicate that a clean separation of transcription and proteolytic control in the context of RB proteins may be oversimplified; here we see evidence for a separate route of proteolytic regulation that modulates transcriptional regulatory potential and protein stability of Rbf1, and possibly related mammalian pocket proteins. Interestingly, the regulation of this pathway may involve the evolutionarily conserved COP9 signalosome. Our previous biochemical studies indicated that the COP9 signalosome regulatory complex is physically associated with Rbf proteins and limits turnover of these repressors (Ullah et al., 2007). From the results of the current study, we postulate that COP9 antagonizes the function of the Rbf1 IE, perhaps by blocking the access of ubiquitin-modifying E3 ligases that would otherwise potentiate Rbf1 activity and turnover. Alternatively, inhibition of E3 ligases may involve the enzymatic activity of COP9, whereby this complex downregulates E3 ligases by deneddylation of their cullin subunits (Wei et al., 2008). How the instability of pocket proteins potentiates their activities, and how these processes relate to developmental control of retinoblastoma family proteins and cancer, will be an area of active investigation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Nick Dyson for sharing the PCNA and Polα luciferase reporter genes, Maxim Frolov for providing the Myc-E2f1 construct, Stefan Gaubatz for providing the p107 expression construct, Min-Hao Kuo and Jianjun Luo for aid with fluorescence microscopy, Jaclyn Peraino for generating Rbf1 single lysine point mutants, and John Wang for reagents and friendly advice on protein localization assays. We also appreciate the assistance from Satyaki Sengupta for his helpful discussions. This work was supported by an undergraduate summer training grant from the Michigan State University Genetics Program (to S.D.), the Michigan State University Gene Expression in Development and Disease Group, and a grant from the National Institutes of Health (to D.N.A. and R.W.H.).
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0520) on September 22, 2010.
REFERENCES
- Binne U. K., Classon M. K., Dick F. A., Wei W., Rape M., Kaelin W. G., Jr, Naar A. M., Dyson N. J. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat. Cell. Biol. 2007;9:225–232. doi: 10.1038/ncb1532. [DOI] [PubMed] [Google Scholar]
- Boyer S. N., Wazer D. E., Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitin-proteasome pathway. Cancer Res. 1996;56:4620–4624. [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Schwechheimer C. ZOMES III: the interface between signalling and proteolysis. Meeting on The COP9 Signalosome, Proteasome and eIF3. EMBO Rep. 2004;5:1041–1045. doi: 10.1038/sj.embor.7400275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classon M., Harlow E. The retinoblastoma tumour suppressor in development and cancer. Nat. Rev. Cancer. 2002;2:910–917. doi: 10.1038/nrc950. [DOI] [PubMed] [Google Scholar]
- Collins G. A., Tansey W. P. The proteasome: a utility tool for transcription? Curr. Opin. Genet. Dev. 2006;16:197–202. doi: 10.1016/j.gde.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Daulny A., Geng F., Muratani M., Geisinger J. M., Salghetti S. E., Tansey W. P. Modulation of RNA polymerase II subunit composition by ubiquitylation. Proc. Natl. Acad. Sci. USA. 2008;105:19649–19654. doi: 10.1073/pnas.0809372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova D. K., Stevaux O., Frolov M. V., Dyson N. J. Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev. 2003;17:2308–2320. doi: 10.1101/gad.1116703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- Ferdous A., Sikder D., Gillette T., Nalley K., Kodadek T., Johnston S. A. The role of the proteasomal ATPases and activator monoubiquitylation in regulating Gal4 binding to promoters. Genes Dev. 2007;21:112–123. doi: 10.1101/gad.1493207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolov M. V., Moon N. S., Dyson N. J. dDP is needed for normal cell proliferation. Mol. Cell. Biol. 2005;25:3027–3039. doi: 10.1128/MCB.25.8.3027-3039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M. K., Tan Y. Y., Hart C. M. The Drosophila boundary element-associated factors BEAF-32A and BEAF-32B affect chromatin structure. Genetics. 2006;173:1365–1375. doi: 10.1534/genetics.106.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F., Delahodde A., Kodadek T., Johnston S. A. Recruitment of a 19S proteasome subcomplex to an activated promoter. Science. 2002;296:548–550. doi: 10.1126/science.1069490. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992;6:177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Ji P., Jiang H., Rekhtman K., Bloom J., Ichetovkin M., Pagano M., Zhu L. An Rb-Skp2–p27 pathway mediates acute cell cycle inhibition by Rb and is retained in a partial-penetrance Rb mutant. Mol. Cell. 2004;16:47–58. doi: 10.1016/j.molcel.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Keller S. A., Ullah Z., Buckley M. S., Henry R. W., Arnosti D. N. Distinct developmental expression of Drosophila retinoblastoma factors. Gene. Expr. Patterns. 2005;5:411–421. doi: 10.1016/j.modgep.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Herbst A., Tworkowski K. A., Salghetti S. E., Tansey W. P. Skp2 regulates Myc protein stability and activity. Mol. Cell. 2003;11:1177–1188. doi: 10.1016/s1097-2765(03)00173-4. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Retinoblastoma: a prototypic hereditary neoplasm. Semin. Oncol. 1978;5:57–60. [PubMed] [Google Scholar]
- Lee C., Chang J. H., Lee H. S., Cho Y. Structural basis for the recognition of the E2F transactivation domain by the retinoblastoma tumor suppressor. Genes Dev. 2002;16:3199–3212. doi: 10.1101/gad.1046102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D., Ezhkova E., Li B., Pattenden S. G., Tansey W. P., Workman J. L. The proteasome regulatory particle alters the SAGA coactivator to enhance its interactions with transcriptional activators. Cell. 2005;123:423–436. doi: 10.1016/j.cell.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Lohmann D. R. RB1 gene mutations in retinoblastoma. Hum. Mutat. 1999;14:283–288. doi: 10.1002/(SICI)1098-1004(199910)14:4<283::AID-HUMU2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Martinez C. A., Arnosti D. N. Spreading of a corepressor linked to action of long-range repressor hairy. Mol. Cell. Biol. 2008;28:2792–2802. doi: 10.1128/MCB.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratani M., Tansey W. P. How the ubiquitin-proteasome system controls transcription. Nat. Rev. Mol. Cell. Biol. 2003;4:192–201. doi: 10.1038/nrm1049. [DOI] [PubMed] [Google Scholar]
- Pascual G., Fong A. L., Ogawa S., Gamliel A., Li A. C., Perissi V., Rose D. W., Willson T. M., Rosenfeld M. G., Glass C. K. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., May J. S., Burton G. R., Lyle R. E., McGehee R. E., Jr Proteasomal degradation of retinoblastoma-related p130 during adipocyte differentiation. Biochem. Biophys. Res. Commun. 2002;290:1066–1071. doi: 10.1006/bbrc.2001.6291. [DOI] [PubMed] [Google Scholar]
- Rubin S. M., Gall A. L., Zheng N., Pavletich N. P. Structure of the Rb C-terminal domain bound to E2F1-DP 1, a mechanism for phosphorylation-induced E2F release. Cell. 2005;123:1093–1106. doi: 10.1016/j.cell.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Ryu J. R., Arnosti D. N. Functional similarity of Knirps CtBP-dependent and CtBP-independent transcriptional repressor activities. Nucleic Acids Res. 2003;31:4654–4662. doi: 10.1093/nar/gkg491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti S. E., Caudy A. A., Chenoweth J. G., Tansey W. P. Regulation of transcriptional activation domain function by ubiquitin. Science. 2001;293:1651–1653. doi: 10.1126/science.1062079. [DOI] [PubMed] [Google Scholar]
- Salghetti S. E., Kim S. Y., Tansey W. P. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 1999;18:717–726. doi: 10.1093/emboj/18.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salghetti S. E., Muratani M., Wijnen H., Futcher B., Tansey W. P. Functional overlap of sequences that activate transcription and signal ubiquitin-mediated proteolysis. Proc. Natl. Acad. Sci. USA. 2000;97:3118–3123. doi: 10.1073/pnas.050007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter E. D., Shilo B. Z. The Drosophila EGF receptor homolog (DER) gene is allelic to faint little ball, a locus essential for embryonic development. Cell. 1989;56:1093–1104. doi: 10.1016/0092-8674(89)90642-9. [DOI] [PubMed] [Google Scholar]
- Sdek P., Ying H., Chang D. L., Qiu W., Zheng H., Touitou R., Allday M. J., Xiao Z. X. MDM2 promotes proteasome-dependent ubiquitin-independent degradation of retinoblastoma protein. Mol. Cell. 2005;20:699–708. doi: 10.1016/j.molcel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Stevaux O., Dimova D., Frolov M. V., Taylor-Harding B., Morris E., Dyson N. Distinct mechanisms of E2F regulation by Drosophila RBF1 and RBF2. EMBO J. 2002;21:4927–4937. doi: 10.1093/emboj/cdf501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevaux O., Dimova D. K., Ji J. Y., Moon N. S., Frolov M. V., Dyson N. J. Retinoblastoma family 2 is required in vivo for the tissue-specific repression of dE2F2 target genes. Cell. Cycle. 2005;4:1272–1280. doi: 10.4161/cc.4.9.1982. [DOI] [PubMed] [Google Scholar]
- Stubdal H., Zalvide J., Campbell K. S., Schweitzer C., Roberts T. M., DeCaprio J. A. Inactivation of pRB-related proteins p130 and p107 mediated by the J domain of simian virus 40 large T antigen. Mol. Cell. Biol. 1997;17:4979–4990. doi: 10.1128/mcb.17.9.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H. W., Qu L. J., Chen Z. L., Gu H. Y. Evolution of COP9 signalosome and proteasome lid complex. Acta. Botanica Sinica. 2003;45:523–529. [Google Scholar]
- Sutcliffe J. E., Korenjak M., Brehm A. Tumour suppressors–a fly's perspective. Eur. J. Cancer. 2003;39:1355–1362. doi: 10.1016/s0959-8049(03)00263-6. [DOI] [PubMed] [Google Scholar]
- Swanhart L. M., Sanders A. N., Duronio R. J. Normal regulation of Rbf1/E2f1 target genes in Drosophila type 1 protein phosphatase mutants. Dev. Dyn. 2007;236:2567–2577. doi: 10.1002/dvdy.21265. [DOI] [PubMed] [Google Scholar]
- Uchida C., Miwa S., Kitagawa K., Hattori T., Isobe T., Otani S., Oda T., Sugimura H., Kamijo T., Ookawa K., Yasuda H., Kitagawa M. Enhanced Mdm2 activity inhibits pRB function via ubiquitin-dependent degradation. EMBO J. 2005;24:160–169. doi: 10.1038/sj.emboj.7600486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah Z., Buckley M. S., Arnosti D. N., Henry R. W. Retinoblastoma protein regulation by the COP9 signalosome. Mol. Biol. Cell. 2007;18:1179–1186. doi: 10.1091/mbc.E06-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel S., Dyson N. J. Conserved functions of the pRB and E2F families. Nat. Rev. Mol. Cell. Biol. 2008;9:713–724. doi: 10.1038/nrm2469. [DOI] [PubMed] [Google Scholar]
- Wei N., Serino G., Deng X. W. The COP9 signalosome: more than a protease. Trends Biochem. Sci. 2008;33:592–600. doi: 10.1016/j.tibs.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Xiao B., Spencer J., Clements A., Ali-Khan N., Mittnacht S., Broceno C., Burghammer M., Perrakis A., Marmorstein R., Gamblin S. J. Crystal structure of the retinoblastoma tumor suppressor protein bound to E2F and the molecular basis of its regulation. Proc. Natl. Acad. Sci. USA. 2003;100:2363–2368. doi: 10.1073/pnas.0436813100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin S., Weng L., Xu J., Du W. The role of RBF in developmentally regulated cell proliferation in the eye disc and in Cyclin D/Cdk4 induced cellular growth. Development. 2002;129:1345–1356. doi: 10.1242/dev.129.6.1345. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.