Abstract
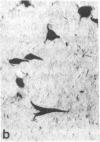
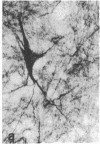
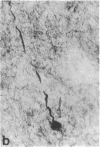
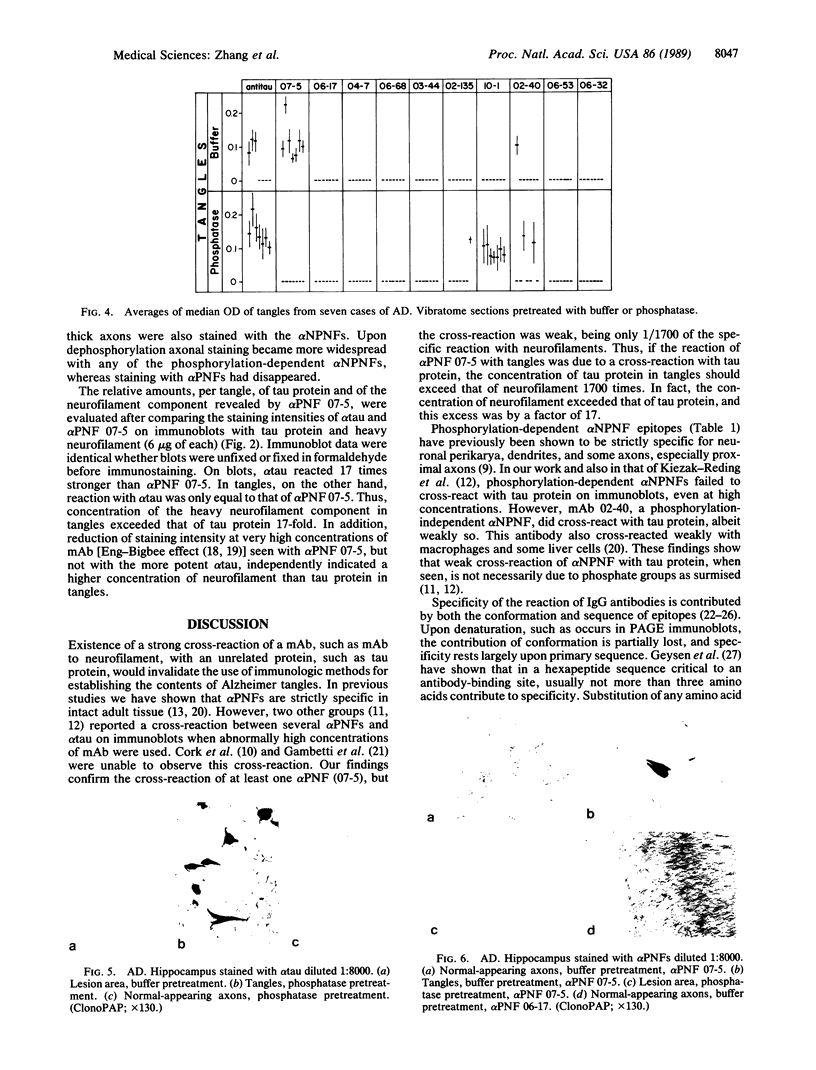
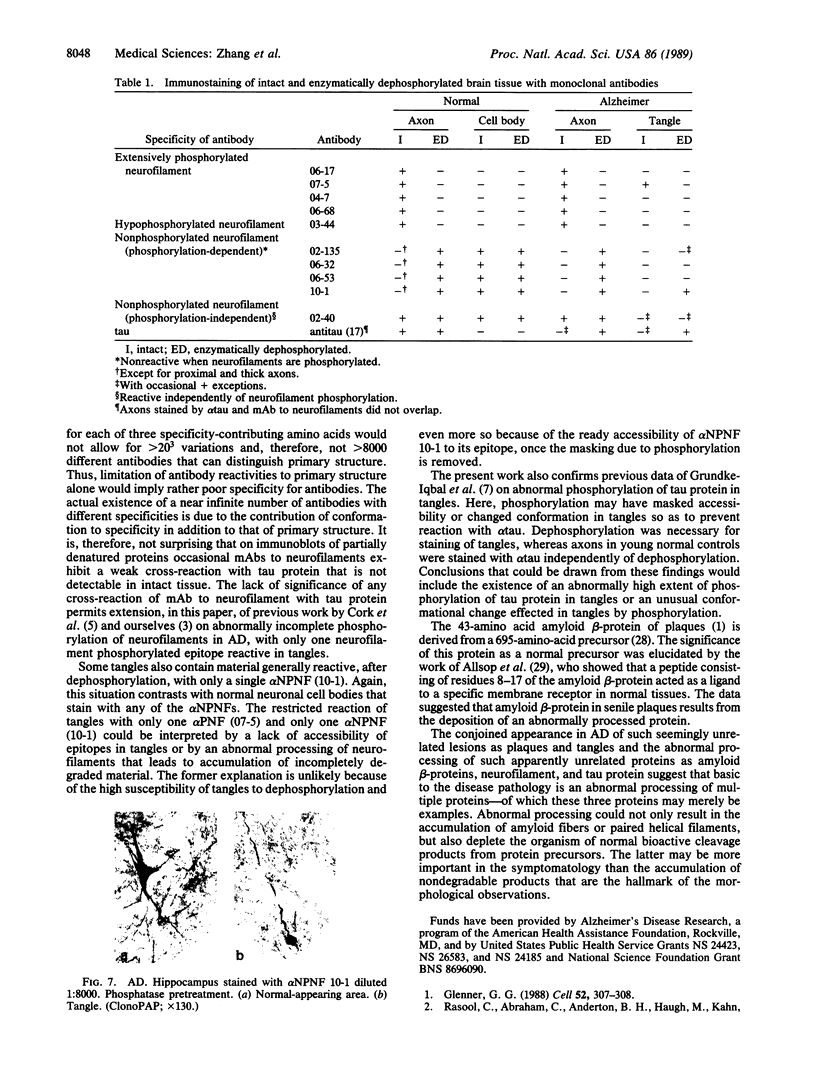
Cerebrovascular amyloid is the main constituent of the perivascular and neuritic plaques typical of Alzheimer disease, whereas neurofilaments and microtubule-associated tau protein have been considered primary contributors to the formation of the characteristic Alzheimer tangles. Plaques and tangles and their constituents have at times been ascribed a role in pathogenesis of the disease. Normally, neurofilaments become phosphorylated only upon axonal entry. In many neurologic disorders, neurofilament phosphorylation, as detected by any of the available monoclonal antibodies (mAbs) to neurofilament phosphorylated epitopes is shifted from an axonal to a cell-body location. An exception is provided by Alzheimer disease, where tangles (which are neuronal cell-body-derived structures) exhibit only one phosphorylated epitope. However, the very presence of neurofilaments in tangles and plaques has been questioned because of a reported cross-reaction of mAbs to phosphorylated neurofilaments with tau protein. On reinvestigating this cross-reactivity we found that four of five mAbs to phosphorylated neurofilaments and four of five mAbs to nonphosphorylated neurofilaments failed to react with tau protein. A fifth mAb (07-5) to phosphorylated neurofilament cross-reacted with partially denatured tau protein at an affinity 1/1700th of that for denatured neurofilaments; nondenatured tau protein in tissue sections did not cross-react. A fifth mAb (02-40) to nonphosphorylated neurofilament also cross-reacted weakly. In Alzheimer disease normal-appearing axons were revealed with all the mAbs to phosphorylated neurofilaments, but tangles were revealed with only one of them (mAb 07-5). mAb to tau protein did not stain or did so indistinctly. Four of five mAbs to nonphosphorylated neurofilaments failed to reveal axons. Upon dephosphorylation of tissue, staining by mAbs to phosphorylated neurofilaments disappeared, and axons were revealed with the mAb to tau protein and all mAbs to the nonphosphorylated neurofilaments. Tangles became stained with tau mAb and one mAb to the nonphosphorylated neurofilaments (mAb 10-1). Quantitative evaluation of immunocytochemical staining intensities and immunoblot cross-reactivity showed that neurofilaments are, indeed, constituents of tangles--apparently exceeding the concentration of tau protein 17-fold. Contribution of both conformation and primary structure to IgG specificity may explain the lack of any cross-reaction of mAbs to neurofilaments with tau protein in intact tissue and the appearance of cross-reaction in immunoblots where conformation specificity may be largely lost. The present data extend earlier findings of abnormal processing of neurofilaments and tau protein in Alzheimer disease and, together with reported abnormal processing of cerebrovascular amyloid beta-protein, suggest that inhibition of the processing of multiple proteins is basic to the pathogenesis of Alzheimer disease, whereas formation of plaques and tangles could be merely the most striking histologic result.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsop D., Wong C. W., Ikeda S., Landon M., Kidd M., Glenner G. G. Immunohistochemical evidence for the derivation of a peptide ligand from the amyloid beta-protein precursor of Alzheimer disease. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2790–2794. doi: 10.1073/pnas.85.8.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigbee J. W., Kosek J. C., Eng L. F. Effects of primary antiserum dilution on staining of "antigenrich" tissues with the peroxidase antiperoxidase technique. J Histochem Cytochem. 1977 Jun;25(6):443–447. doi: 10.1177/25.6.69655. [DOI] [PubMed] [Google Scholar]
- Binder L. I., Frankfurter A., Rebhun L. I. The distribution of tau in the mammalian central nervous system. J Cell Biol. 1985 Oct;101(4):1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Shi C. H., Kilbourne E. D. Hemagglutinin of swine influenza virus: a single amino acid change pleiotropically affects viral antigenicity and replication. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6996–7000. doi: 10.1073/pnas.80.22.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cork L. C., Sternberger N. H., Sternberger L. A., Casanova M. F., Struble R. G., Price D. L. Phosphorylated neurofilament antigens in neurofibrillary tangles in Alzheimer's disease. J Neuropathol Exp Neurol. 1986 Jan;45(1):56–64. doi: 10.1097/00005072-198601000-00005. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Troncoso J. C., Klavano G. G., Johnson E. S., Sternberger L. A., Sternberger N. H., Price D. L. Neurofilamentous abnormalities in motor neurons in spontaneously occurring animal disorders. J Neuropathol Exp Neurol. 1988 Jul;47(4):420–431. doi: 10.1097/00005072-198807000-00003. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Alzheimer's disease: its proteins and genes. Cell. 1988 Feb 12;52(3):307–308. doi: 10.1016/s0092-8674(88)80021-7. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Ksiezak-Reding H., Dickson D. W., Davies P., Yen S. H. Recognition of tau epitopes by anti-neurofilament antibodies that bind to Alzheimer neurofibrillary tangles. Proc Natl Acad Sci U S A. 1987 May;84(10):3410–3414. doi: 10.1073/pnas.84.10.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. The purification of tau protein and the occurrence of two phosphorylation states of tau in brain. J Biol Chem. 1984 Oct 10;259(19):12241–12245. [PubMed] [Google Scholar]
- Nukina N., Kosik K. S., Selkoe D. J. Recognition of Alzheimer paired helical filaments by monoclonal neurofilament antibodies is due to crossreaction with tau protein. Proc Natl Acad Sci U S A. 1987 May;84(10):3415–3419. doi: 10.1073/pnas.84.10.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann E., Sternberger N. H., Sternberger L. A. Immunocytochemistry of brain-reactive monoclonal antibodies in peripheral tissues. Cell Tissue Res. 1983;228(3):459–473. doi: 10.1007/BF00211468. [DOI] [PubMed] [Google Scholar]
- Perry G., Friedman R., Shaw G., Chau V. Ubiquitin is detected in neurofibrillary tangles and senile plaque neurites of Alzheimer disease brains. Proc Natl Acad Sci U S A. 1987 May;84(9):3033–3036. doi: 10.1073/pnas.84.9.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry G., Rizzuto N., Autilio-Gambetti L., Gambetti P. Paired helical filaments from Alzheimer disease patients contain cytoskeletal components. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3916–3920. doi: 10.1073/pnas.82.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasool C. G., Abraham C., Anderton B. H., Haugh M., Kahn J., Selkoe D. J. Alzheimer's disease: immunoreactivity of neurofibrillary tangles with anti-neurofilament and anti-paired helical filament antibodies. Brain Res. 1984 Sep 24;310(2):249–260. doi: 10.1016/0006-8993(84)90148-3. [DOI] [PubMed] [Google Scholar]
- Reichlin M. Amino acid substitution and the antigenicity of globular proteins. Adv Immunol. 1975;20:71–123. doi: 10.1016/s0065-2776(08)60207-2. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. The unlabeled antibody method: comparison of peroxidase-antiperoxidase with avidin-biotin complex by a new method of quantification. J Histochem Cytochem. 1986 May;34(5):599–605. doi: 10.1177/34.5.3517144. [DOI] [PubMed] [Google Scholar]
- Sternberger N. H., Sternberger L. A., Ulrich J. Aberrant neurofilament phosphorylation in Alzheimer disease. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4274–4276. doi: 10.1073/pnas.82.12.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesande F. A critical review of immunocytochemical methods for light microscopy. J Neurosci Methods. 1979 Mar;1(1):3–23. doi: 10.1016/0165-0270(79)90003-7. [DOI] [PubMed] [Google Scholar]