Abstract
Inositol 1,4,5-trisphosphate receptors (IP3R) and their relatives, ryanodine receptors, are the channels that most often mediate Ca2+ release from intracellular stores. Their regulation by Ca2+ allows them also to propagate cytosolic Ca2+ signals regeneratively. This brief review addresses the structural basis of IP3R activation by IP3 and Ca2+. IP3 initiates IP3R activation by promoting Ca2+ binding to a stimulatory Ca2+-binding site, the identity of which is unresolved. We suggest that interactions of critical phosphate groups in IP3 with opposite sides of the clam-like IP3-binding core cause it to close and propagate a conformational change toward the pore via the adjacent N-terminal suppressor domain. The pore, assembled from the last pair of transmembrane domains and the intervening pore loop from each of the four IP3R subunits, forms a structure in which a luminal selectivity filter and a gate at the cytosolic end of the pore control cation fluxes through the IP3R.
IP3 binds to receptors that release calcium from intracellular stores. It causes a conformational change in the receptor, which is transmitted to a pore formed by the last two transmembrane domains from each of its four subunits.
A BRIEF HISTORY OF IP3 RECEPTORS
Sidney Ringer, in his famous correction to an earlier paper, showed that Ca2+ entry can evoke a physiological response by demonstrating that beating of the frog heart requires extracellular Ca2+ (Ringer 1883). Almost a century passed before it became clear that this Ca2+ entry, via voltage-gated Ca2+ channels, was not directly responsible for contraction, but instead provided the trigger for a much larger release of Ca2+ from stores within the sarcoplasmic reticulum (SR). The latter is mediated by type-2 ryanodine receptors (RyR) (Fabiato 1983; Cheng et al. 1993), which like many Ca2+ channels, are able both to transport Ca2+ through an open pore and respond to it. These observations highlight two general points. First, cells call upon two sources of Ca2+ to evoke increases in cytosolic Ca2+ concentration; second, interactions between these Ca2+ fluxes across the plasma membrane and the membranes of intracellular stores are important determinants of the physiological response. The same points apply to the Ca2+ signals evoked by receptors that stimulate phospholipase C (PLC) and, thereby, formation of inositol 1,4,5-trisphosphate (IP3).
The biochemical sequence linking these receptors to formation of IP3 emerged in the 1980s (Michell et al. 1989; Berridge 2005), but work in the decade before had established that many receptors regulate many different responses by increasing the cytosolic Ca2+ concentration (Rasmussen 1970; Berridge 1975). In his influential review, Bob Michell (Michell 1975), building on work showing that many of these receptors also stimulate phospholipid turnover (Hokin and Hokin 1953), had suggested a causal link between phosphoinositide hydrolysis and Ca2+ signals. Here, as in many studies, the emphasis was on Ca2+ entry, with a consensus only slowly emerging that Ca2+ fluxes across both the plasma membrane and the membranes of intracellular stores contribute to cytosolic Ca2+ signals (Rasmussen 1970; Berridge 1975; Williams 1980; Putney et al. 1981). In the years following Michell’s review, decisive evidence, much of it coming from Mike Berridge’s elegant studies of blowfly salivary gland, established that phosphoinositide hydrolysis is, as predicted by Michell, required for PLC-linked receptors to evoke Ca2+ signals (Berridge and Fain 1979). The same preparation was used to show that IP3 is the first water-soluble product of the signaling pathway (Berridge 1983). IP3, thus, emerged as a prime candidate for the cytosolic messenger linking events at the plasma membrane to release of Ca2+ from intracellular stores. Paradoxically, it was to be many years before the links between receptors that stimulate PLC and Ca2+ entry were resolved. These came with elaboration of the pathways linking empty Ca2+ stores to Ca2+ entry, the so-called store-operated Ca2+ entry pathway (Putney 1997; Park et al. 2009), and recognition that many trp channels are regulated by products of PLC activity (Nilius et al. 2007). IP3 receptors (IP3R) also contribute more directly to Ca2+ entry across the plasma membrane either because, at least in some cells, IP3R are functionally expressed in the plasma membrane (Dellis et al. 2006; Dellis et al. 2008), or perhaps through their direct interactions with other plasma membrane Ca2+ channels (Kiselyov et al. 1999). Here, we focus solely on Ca2+ release from the endoplasmic reticulum (ER) by IP3R. Some of the key steps in the evolution of our current understanding of IP3R are listed in Table 1.
Table 1.
Landmarks en route to a structural analysis of IP3 receptor behavior.
| RyR | IP3R | |
|---|---|---|
| 1883 | Ca2+ entry required for heart contraction.1 | |
| 1953 | Acetylcholine stimulates turnover of phospholipids.2 | |
| 1975 | Phosphoinositide hydrolysis proposed to cause Ca2+ signals.3 | |
| 1977 | Ca2+ waves occur at fertilization.4 | |
| 1977 | Ca2+-induced Ca2+ release in SR.5 | |
| 1979 | Phosphoinositide hydrolysis required for receptor-stimulated Ca2+ signals.6 | |
| 1980 | Introduction of Quin 27 and facile loading methods.8 | |
| 1983 | IP3 is first water-soluble product of PLC.9 | |
| 1983 | IP3 stimulates Ca2+ release from a non-mitochondrial store.10 | |
| 1985 | Ryanodine, selective RyR ligand.11 | |
| 1985 | Single channel records of RyR.12 | |
| 1986 | Frequency-coded Ca2+ spikes.13 | |
| 1987 | Ca2+ regulates IP3R.14,15 | |
| 1987 | RyR1 purified.16 | IP3R1 purified.17 |
| 1988 | Single channel records of IP3R.18 | |
| 1989 | Cloning of RyR1.19 | Cloning of IP3R1.20,21 |
| 1990 | Elementary Ca2+-release events.22 | |
| 1993 | Elementary Ca2+-release events.23 | |
| 2002 | Atomic structure of IBC.24 | |
| 2005 | Atomic structure of SD.25 | |
| 2009 | Atomic structure of N-terminal of RyR.26,27 |
14Iino (1987).
15Iino (1990).
The role of the SR as the intracellular source of Ca2+ signals in striated muscle was long-established (Endo et al. 1970), but there was no such agreement on the identity of the organelle from which Ca2+ was released in other cells. Competing claims suggested roles for mitochondria or the ER. Evidence that in resting hepatocytes only the ER contains appreciable amounts of Ca2+ (Burgess et al. 1983) was quickly followed by the demonstration that IP3 evoked Ca2+ release from a non-mitochondrial Ca2+ store in permeabilized pancreatic acinar cells (Streb et al. 1983). Countless groups quickly replicated these findings in many cells, and within months it was universally accepted that the ER is the major Ca2+ store from which IP3 stimulates Ca2+ release in most animal cells (Berridge and Irvine 1984; Berridge and Irvine 1989). Subsequent work has suggested that IP3 may also stimulate Ca2+ release from the Golgi apparatus (Pinton et al. 1998), from within the nucleus (Gerasimenko et al. 1995; Echevarria et al. 2003; Marchenko et al. 2005), and perhaps also from secretory vesicles (Gerasimenko et al. 1996), but ER remains the major IP3-sensitive Ca2+ store. Evidence that IP3 stimulates Ca2+ efflux from the ER (rather than inhibiting Ca2+ uptake) and the first single channel recordings (Ehrlich and Watras 1988) established that the IP3R is an IP3-gated, Ca2+-permeable channel. The first studies of 32P-IP3 binding (Spät et al. 1986) were followed by purification of IP3R from cerebellum (Maeda et al. 1988; Supattapone et al. 1988) and then cloning of the first IP3R subtype (IP3R1) (Furuichi et al. 1989; Mignery et al. 1989). Subsequent studies identified two additional genes encoding vertebrate IP3R (IP3R2 and IP3R3) and a single gene in invertebrates (Taylor et al. 1999). It remains far from clear whether plants express related IP3R (Krinke et al. 2007). These studies established that IP3R are unusually large proteins, comprising tetramers of closely-related subunits, each with about 2700 amino acid residues. RyR are even larger: they, too, are tetramers, but the subunits are almost twice the size of IP3R (∼5000 residues). This progress with identifying IP3R together with single channel recordings of IP3R, initially in artificial lipid bilayers and later in native membranes (Foskett et al. 2007; Rahman et al. 2009), provided the foundations from which to explore the structural determinants of IP3R behavior. The advances toward understanding the molecular mechanisms of IP3R behavior were accompanied by similar progress with RyR (Table 1). Recurrent themes, to which we return, are the similarities between RyR and IP3R, and the many instances where observations of one channel family have informed further analysis of the other. Very recently, a third family of intracellular Ca2+ channels, unrelated to RyR and IP3R, has been implicated in Ca2+ signaling. These are the two-pore channels (TPC) that are activated by NAADP and release Ca2+ from acidic Ca2+ stores, including lysosomes and endosomes (Patel et al. 2010; Zhu et al. 2010). Several trp (transient receptor protein) channels, in addition to their roles in the plasma membrane, may also mediate release of Ca2+ from intracellular stores (Gees et al. 2010).
Parallel to work addressing the workings of IP3R, there was growing interest in the spatiotemporal complexity of cytosolic Ca2+ signals. Ca2+ waves were first observed during fertilization. These waves were proposed to result from Ca2+-induced Ca2+ release (CICR) and were followed by smaller repetitive Ca2+ transients (Ridgway et al. 1977; Gilkey 1983). It was, however, the work of Peter Cobbold that focused most attention on the complexity of intracellular Ca2+ signals (Woods et al. 1986). Just as the activity of a nerve is conveyed by the frequency of its action potentials, Cobbold demonstrated that in hepatocytes the concentration of the extracellular stimulus determined the frequency of the cytosolic Ca2+ transients. As these ideas gathered momentum (Berridge 1995), evidence accumulated in support of cells using the information provided by frequency-encoded Ca2+ spikes as an efficient means of regulating cellular activity (Dolmetsch et al. 1997; Li et al. 1998; Berridge et al. 2000; Dupont et al. 2003). The single greatest contributor to progress in understanding the genesis of these intracellular Ca2+ signals was the introduction, by Roger Tsien in 1980, of simple, minimally disruptive methods for measuring the free cytosolic Ca2+ concentration in intact cells (Tsien 1980; Tsien 1981). These methods, in combination with improved optical microscopy, allowed Ian Parker to begin to resolve the subcellular organization of IP3-evoked Ca2+ signals (Parker and Ivorra 1990; Parker et al. 1996). He showed that as the IP3 concentration increases, it triggers a hierarchy of elementary Ca2+ release events, beginning with the openings of single IP3R (Ca2+ blips), progressing to the coordinated openings of a cluster of several IP3R (Ca2+ puffs) and finally, with sufficient IP3, culminating in a regenerative Ca2+ wave invading the entire cell (Bootman et al. 1997; Demuro and Parker 2007). The demonstration, in 1987 by Masamitsu Iino, that IP3R are stimulated by cytosolic Ca2+ (Iino 1987), and the later widespread recognition that all IP3R are biphasically regulated by cytosolic Ca2+ (Iino 1990; Taylor and Laude 2002), provided what has become the most widely accepted explanation for the recruitment of elementary Ca2+-release events. Namely, that CICR, already an established feature of RyR (Endo et al. 1970), allows an active IP3R to propagate its activity to neighboring IP3R.
These observations and accumulating evidence that local Ca2+ signals can selectively regulate local events (Rizzuto et al. 1993; Berridge et al. 2000; Dyer et al. 2005; Willoughby and Cooper 2007) prompted a re-assessment of the ways in which Ca2+ signals convey information. It became untenable to think of responses to graded changes in the intensity of the extracellular stimulus as being simply encoded in graded changes in global cytosolic Ca2+ concentration. Ca2+ entering the cytosol via one channel can regulate different proteins to Ca2+ entering via another (Berridge et al. 2000; Dyer et al. 2005; Willoughby and Cooper 2007). Hence, the spatial organization of the changes in cytosolic Ca2+ concentration profoundly affects the physiological response, and that presents many opportunities for delivering different Ca2+ signals in response to different stimuli or different stimulus intensities. The duration of each Ca2+ increase, whether local or global, is also important in determining not only the amplitude of the response, but also its nature, because Ca2+-binding proteins differ in their responses to transient and sustained signals. Finally, the frequency with which Ca2+ signals are delivered can determine both the nature and amplitude of the cellular response. The key point is that the versatility of Ca2+ as an intracellular messenger capable of regulating diverse cellular events depends largely on the spatiotemporal complexity of cytosolic Ca2+ signals (Berridge et al. 2000). If we are to understand how Ca2+ functions as a ubiquitous intracellular messenger, we must explain how IP3-evoked Ca2+ signals grow from the opening of a single IP3R to much larger events. That explanation depends, ultimately, on putting IP3R into appropriate places within the cell, and on the interactions between IP3 and Ca2+ in regulating the opening of IP3R. In recent reviews (Taylor et al. 2009a; Taylor et al. 2009b) and original reports, we have described how IP3R are co-translationally targeted to the ER and then retained there by sequences within their transmembrane domains (TMD) (Parker et al. 2004; Pantazaka and Taylor 2010). We have also suggested that within the ER, IP3 causes IP3R to assemble into small clusters within which their regulation by both IP3 and Ca2+ is retuned to facilitate the Ca2+-mediated recruitment of IP3R activity by an active neighbor (Rahman and Taylor 2009; Rahman et al. 2009). Here, we focus entirely on the interactions between Ca2+ and IP3 in regulating IP3R activity, and the extent to which we can explain those interactions at the structural level.
REGULATION OF IP3 RECEPTORS BY Ca2+ AND IP3
Activation of IP3R requires both IP3 and its permeating ion, Ca2+ (Finch et al. 1991; Marchant and Taylor 1997; Adkins and Taylor 1999; Taylor and Laude 2002; Foskett et al. 2007). There are reports of IP3-independent activation of IP3R by CaBP1 (Yang et al. 2002), a member of the neuronal Ca2+-sensor family, and by Gβγ subunits (Zeng et al. 2003), but the physiological relevance is unclear (Haynes et al. 2004; Nadif Kasri et al. 2004). The current consensus is that binding of IP3 to the IP3R is essential for its activation, but whether all four IP3-binding sites of the tetrameric IP3R must be occupied is unresolved. Positively cooperative responses to IP3 in some (Dufour et al. 1997; Marchant and Taylor 1997; Tu et al. 2005a), though not all, studies (Finch et al. 1991; Watras et al. 1991; Laude et al. 2005), and delays before the first response to IP3 that decrease with increasing IP3 concentration (Marchant and Taylor 1997), indicate that channel opening requires occupancy of more than one IP3-binding site. However, gating by IP3 of heteromeric IP3R in which at least one subunit is mutated to prevent IP3 binding suggests that occupancy of fewer than four IP3-binding sites may be sufficient to cause some channel opening (Boehning and Joseph 2000a). IP3R subtypes differ in their affinities for IP3, with the general consensus being that IP3R2 is more sensitive than IP3R1, and both are considerably more sensitive than IP3R3 (Tu et al. 2005b; Iwai et al. 2007). In the cellular context, however, differences in expression level (Dellis et al. 2006; Tovey et al. 2010), subcellular distribution (Petersen et al. 1999), post-transcriptional and post-translational modifications, and association of IP3R with accessory proteins (Patterson et al. 2004) may be more important determinants of sensitivity.
Soon after the first report of IP3-evoked Ca2+ release, cytosolic Ca2+ was shown also to regulate IP3R (Suematsu et al. 1984; Jean and Klee 1986); thereafter, it emerged that the effects of Ca2+ were biphasic, with modest increases in cytosolic Ca2+ concentration enhancing responses to IP3, while higher concentrations were inhibitory (Iino 1987; Iino 1990; Finch et al. 1991; Parys et al. 1992; Marshall and Taylor 1993). This provided yet another parallel with RyR, which are also biphasically regulated by Ca2+ (Hamilton 2005). The coregulation of IP3R by IP3 and Ca2+ in permeabilized cells was confirmed by single-channel recordings of IP3R1 reconstituted into lipid bilayers (Bezprozvanny et al. 1991; Striggow and Ehrlich 1996; Kaftan et al. 1997; Ramos-Franco et al. 1998a; Ramos-Franco et al. 1998b; Tu et al. 2002; Tu et al. 2005b) and in native nuclear membranes (Stehno-Bittel et al. 1995; Mak et al. 1998; Boehning et al. 2001a; Marchenko et al. 2005). In each case, the single-channel open probability (Po) of IP3-activated channels displayed a bell-shaped dependence on cytosolic Ca2+ concentration. Evidence that purified IP3R1 could be stimulated, but not inhibited, by cytosolic Ca2+ (Thrower et al. 1998; Michikawa et al. 1999) raised the possibility that Ca2+ inhibition might be mediated by an accessory protein, although it has yet to be identified. The same explanation perhaps accounts for some reports, often derived from bilayer recordings, in which Ca2+ was suggested not to inhibit IP3R2 or IP3R3 (Horne and Meyer 1995; Hagar et al. 1998; Miyakawa et al. 1999; Ramos-Franco et al. 2000). The balance of opinion, supported by numerous studies of all three IP3R subtypes and using both single-channel and Ca2+-efflux studies, is that all three IP3R subtypes are biphasically regulated by cytosolic Ca2+ (Marshall and Taylor 1993; Oancea and Meyer 1996; Dufour et al. 1997; Missiaen et al. 1998; Miyakawa et al. 1999; Swatton et al. 1999; Boehning and Joseph 2000b; Mak et al. 2000; Mak et al. 2001; Tu et al. 2005a). Two independent Ca2+-binding sites, which differ in their interactions with different bivalent cations and in their affinities for Ca2+, mediate the stimulatory and inhibitory effects of cytosolic Ca2+ (Marshall and Taylor 1994; Striggow and Ehrlich 1996; Hajnóczky and Thomas 1997). Both sites are essential elements of many models proposed to explain regenerative Ca2+ signals (Lechleiter et al. 1991; Berridge 1997). This core biphasic pattern of regulation by cytosolic Ca2+ may be modulated by other intracellular signals (and these, too, may have contributed to some of the disparate findings) and by processing of IP3R. Ca2+-dependent inhibition of IP3R3, for example, is very sensitive to cytoplasmic ATP (Tu et al. 2005b), and the neuronal S2+ splice variant of IP3R1 has a broader Ca2+-dependence than the peripheral S2− form (Tu et al. 2002). However, IP3 is the major influence on what Ca2+ does to IP3R: The two ligands are essential co-agonists of IP3R (Finch et al. 1991). Activation of IP3R1 by Ca2+ is positively cooperative, enabling Po to reach its maximum value over a narrow range of Ca2+ concentrations, suggesting that IP3R1 may be well suited to mediating CICR and regenerative Ca2+ signals. Activation of IP3R3 is less cooperative, occurs over a broader range of Ca2+ concentrations, and requires lesser activation, making it well suited as a trigger for Ca2+ release as the level of IP3 increases (Mak et al. 2001; Foskett et al. 2007).
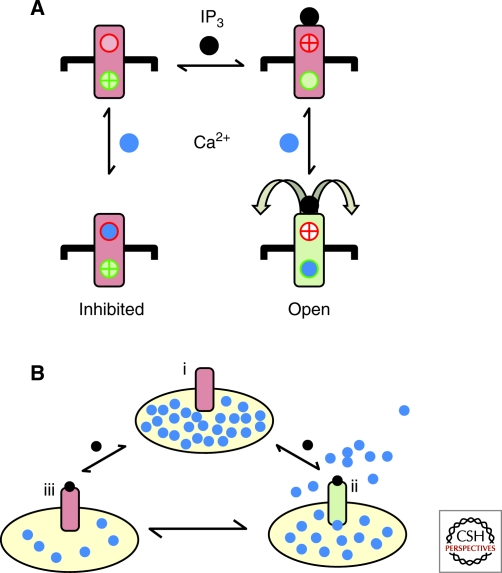
Foskett and colleagues have argued, from their analyses of patch-clamp recordings of nuclear IP3R, that IP3 decreases the sensitivity of the IP3R to inhibition by cytosolic Ca2+, and that this alone is the means whereby IP3 stimulates channel opening (Mak et al. 1998; Mak et al. 2001; Ionescu et al. 2006). This simple explanation, where IP3 serves only to relieve tonic inhibition by resting Ca2+ concentrations, is impossible to reconcile with their observation that pretreatment of cells with Ca2+-free media abolishes Ca2+ inhibition without preventing IP3 from activating IP3R (Mak et al. 2003). This simple model was later elaborated to include at least three different Ca2+ sensors (Mak et al. 2003), but at the core of this revised scheme is a single Ca2+-binding site that switches from being inhibitory in the absence of IP3 to stimulatory in its presence (Mak et al. 2003). The essential feature of this scheme is consistent with our initial model, derived from rapid superfusion analysis, which suggests that IP3 both relieves Ca2+ inhibition and promotes binding of Ca2+ to a stimulatory site (Marchant and Taylor 1997; Adkins and Taylor 1999). The latter is essential for the channel to open. We, however, argue that the stimulatory and inhibitory Ca2+-binding sites are distinct (Marshall and Taylor 1994). We suggest, therefore, that the essential role of IP3 is to promote Ca2+ binding to a stimulatory Ca2+-binding site. IP3, by priming this site, allows Ca2+ to provide instantaneous control over whether the channel opens (Fig. 1A).
Figure 1.
Regulation of IP3R by cytosolic and luminal Ca2+. (A) Binding of IP3 (black circle) to the IP3R determines whether a stimulatory (green) or inhibitory (red) Ca2+-binding site is available (Adkins and Taylor 1999). IP3 binding causes the stimulatory site to become accessible and the inhibitory site to be concealed; binding of Ca2+ (blue circle) to the former then triggers opening of the channel. (B) Luminal Ca2+ is proposed to tune the sensitivity of the IP3R to cytosolic IP3 and Ca2+ such that full stores (i) are most sensitive to IP3. As the IP3R opens (ii) and the stores lose Ca2+, they are proposed to lose sensitivity to IP3 until eventually the IP3R closes, despite the continued presence of the cytosolic stimuli, trapping Ca2+ within the ER (iii). Conversely, stores regain their sensitivity to IP3 as the stores refill, perhaps thereby determining the interval between Ca2+ spikes in stimulated cells (Berridge 2007).
The structural basis for Ca2+-regulation of IP3R is unresolved: it may be either direct, via Ca2+ binding to a site intrinsic to the IP3R or via an accessory Ca2+-binding protein (Taylor et al. 2004). Stimulation of IP3R by cytosolic Ca2+ is universally observed even with purified IP3R reconstituted into lipid bilayers (Ferris et al. 1989; Hirota et al. 1995; Michikawa et al. 1999), suggesting that this essential Ca2+-binding site probably resides within the primary sequence of the IP3R. At least seven cytosolic Ca2+-binding sites have been identified within IP3R1 (Sienaert et al. 1996; Sienaert et al. 1997), but the physiological relevance of these sites is unresolved. Two of the sites (residues 304-381 and 378-450) are within the IP3-binding core, for which there is a high-resolution structure (Bosanac et al. 2002). This structure shows two surface-exposed clusters of acidic residues that overlap with residues in the second N-terminal Ca2+-binding region. However, point mutations of several of these acidic residues had no effect on Ca2+-regulation of IP3R (Joseph et al. 2005). The remaining Ca2+-binding sites fall within the central region of the IP3R (Sienaert et al. 1996; Sienaert et al. 1997). The site between residues 1347–1426 is interesting because its proximity to a calmodulin (CaM)-binding region is reminiscent of RyR, which have two CaM-binding regions within ∼200 residues of high-affinity Ca2+-binding sites, and a third flanked by two high-affinity Ca2+-binding sites (Chen and MacLennan 1994). Interactions between these sites have been proposed to contribute to regulation of RyR by Ca2+ and CaM (Chen and MacLennan 1994). None of the Ca2+-binding sites within IP3R contain EF-hands or any other known Ca2+-binding motif, and none have obvious sequence similarity with similar regions in RyR. However, each site has clusters of negatively charged residues that may coordinate Ca2+ (Sienaert et al. 1997). There is presently no evidence to link any of these sites directly to Ca2+ regulation of IP3R. The only tangible link between specific residues and Ca2+ regulation comes from mutagenesis of a glutamate residue that is conserved in all IP3R and RyR. Mutation of this residue in RyR massively reduced the Ca2+ sensitivity of the channel (Chen et al. 1998; Li and Chen 2001). Mutation of the same residue (Glu-2100) to another acidic residue (Asp) caused a ∼5- to 10-fold decrease in the Ca2+-sensitivity of the IP3R to both stimulation and inhibition, abolished oscillatory Ca2+ transients in response to agonist stimulation, and reduced the Ca2+-binding affinity of a large fragment that includes the residue (Miyakawa et al. 2001; Tu et al. 2003). A rather puzzling aspect of these results is the observation that mutation of a single residue similarly attenuates both stimulation and inhibition by Ca2+, when other evidence suggests that the two effects are mediated by distinct sites. This, together with the lack of direct evidence that Ca2+ is coordinated by the conserved glutamate, leaves open the possibility that rather than itself contributing to an essential Ca2+-binding site, this residue may be allosterically coupled to the site.
Ca2+-mediated inhibition of IP3R is widely assumed to contribute to termination of local cytosolic Ca2+ signals, but it remains far from clear whether such inhibition is mediated by Ca2+ binding directly to IP3R or to an associated protein (Taylor and Laude 2002). The effects of Ca2+ on IP3 binding differ between subtypes: It inhibits binding to IP3R1 (Worley et al. 1987; Supattapone et al. 1988; Joseph et al. 1989; Varney et al. 1990; Richardson and Taylor 1993; Benevolensky et al. 1994; Cardy et al. 1997; Yoneshima et al. 1997), but the effects of Ca2+ on IP3 binding to IP3R from cells expressing predominantly IP3R2 or IP3R3 are confused (Pietri et al. 1990; Mohr et al. 1993; Marshall and Taylor 1994; Cardy et al. 1997; Yoneshima et al. 1997; Lin et al. 2000; Swatton and Taylor 2002). These conflicting results, and evidence that purified IP3R1 is not inhibited by Ca2+ (Danoff et al. 1988; Richardson and Taylor 1993; Benevolensky et al. 1994; Lin et al. 2000), lend some support to the idea that Ca2+ inhibition may be mediated by an accessory protein. It is, however, noteworthy that deletion of the suppressor domain (SD, residues 1-223) of IP3R1, which appears not to include a Ca2+-binding site, abolishes inhibition of IP3 binding by Ca2+ (Sienaert et al. 2002). This suggests that effective regulation by an accessory protein might require the SD.
Calmodulin (CaM) is one candidate for the accessory protein through which Ca2+ inhibition is exercised (Nadif Kasri et al. 2002; Taylor and Laude 2002). CaM is a ubiquitously expressed, EF-hand containing, Ca2+-binding protein that serves as the Ca2+-sensor for many cellular events (Gnegy 1993). All IP3R subtypes are inhibited by Ca2+-CaM (Hirota et al. 1999; Michikawa et al. 1999; Missiaen et al. 1999; Adkins et al. 2000; Missiaen et al. 2000), and CaM has been shown to restore Ca2+ inhibition to purified IP3R (Hirota et al. 1999; Michikawa et al. 1999; Nosyreva et al. 2002). Yet, it has proven difficult to relate these functional effects of CaM to either its effects on IP3 binding or to identified CaM-binding sites within IP3R. CaM inhibits IP3 binding to IP3R1 in a Ca2+-independent manner (Patel et al. 1997; Cardy and Taylor 1998), through a site that probably lies within the SD (Adkins et al. 2000; Sienaert et al. 2002). Its properties are clearly inconsistent with the ability of CaM to inhibit IP3R function only in the presence of Ca2+. There is a high-affinity Ca2+-CaM-binding site within the central region of IP3R1 and IP3R2, but not IP3R3 (Yamada et al. 1995; Lin et al. 2000). However, mutations that prevented Ca2+-CaM binding to this site had no affect on Ca2+-dependent inhibition of IP3R (Zhang and Joseph 2001; Nosyreva et al. 2002). This evidence and the absence of the site from IP3R3 suggest that the central Ca2+-CaM-binding site cannot be responsible for Ca2+ inhibition of IP3R. An additional high-affinity Ca2+-CaM-binding site is created in IP3R1 after removal of the S2 splice region: While this may increase the Ca2+-CaM sensitivity of peripheral S2− IP3R1, it is not a universal candidate for mediating Ca2+ inhibition of IP3R (Islam et al. 1996; Lin et al. 2000). Recently, it was suggested that bound CaM is essential for IP3R function because a peptide antagonist of CaM inhibited IP3-evoked Ca2+ release (Nadif Kasri et al. 2006). It is now clear that this peptide acts directly on IP3R, with no requirement for CaM (Sun and Taylor 2008). While this eliminates an essential role for tethered CaM, it raises the intriguing possibility that an endogenous CaM-like structure might be essential for IP3R activation (Sun and Taylor 2008). In summary, all IP3R subtypes are inhibited by Ca2+-CaM, but the molecular basis of this inhibition has not been established. It seems, on balance, that CaM is unlikely to be the accessory protein through which Ca2+ universally inhibits IP3R. That need not preclude a role for CaM in modulating IP3R function (Taylor and Laude 2002), just as it does for RyR (Chen et al. 1997; Fruen et al. 2000; Rodney et al. 2001), but we must look elsewhere for the site through which Ca2+ inhibits IP3R.
We turn now to the luminal surface of the IP3R, where, and again drawing parallels with RyR, we consider regulation by luminal Ca2+. Persuasive evidence suggests that Ca2+ release by RyR may be terminated before Ca2+ stores are entirely depleted because luminal Ca2+ is required to maintain RyR activity (Györke and Györke 1998; Launikonis et al. 2006; Jiang et al. 2008), possibly via its interaction with calsequestrin, a luminal high-capacity Ca2+-binding protein (Launikonis et al. 2006; Terentyev et al. 2006). A similar scheme has been proposed to account for two features of IP3-evoked Ca2+ release: the initiation of Ca2+ release after the quiescent interspike interval during repetitive Ca2+ spikes (Berridge 2007) and quantal Ca2+ release via IP3R. The latter describes the situation wherein unidirectional Ca2+ efflux from intracellular stores terminates before the stores have fully emptied after stimulation with submaximally effective concentrations of IP3 without loss of their ability to respond to a further increase in IP3 concentration (Muallem et al. 1989; Meyer and Stryer 1990; Taylor and Potter 1990; Oldershaw et al. 1991; Bootman et al. 1992; Brown et al. 1992; Combettes et al. 1992; Ferris et al. 1992; Hirota et al. 1995). The proposal is that luminal Ca2+ sets the gain on the regulation by cytosolic IP3 and Ca2+, so that as the luminal free Ca2+ concentration falls, it causes the sensitivity of the IP3R to IP3 to fall until, as Ca2+ leaks from the ER, the IP3R closes despite the continued presence of cytosolic IP3 and residual Ca2+ within the ER (Irvine 1990). Conversely, as stores refill between Ca2+ spikes in an intact cell, the model predicts that the sensitivity of the IP3R increases until it exceeds the threshold at which prevailing cytosolic IP3 and Ca2+ concentrations become sufficient to trigger opening (Fig. 1B). Despite the enduring appeal of the model, evidence that luminal Ca2+ directly regulates IP3R is not yet entirely convincing.
Stores loaded with Ca2+ have been shown to become more sensitive to IP3 in some studies (Missiaen et al. 1992; Nunn and Taylor 1992; Oldershaw and Taylor 1993; Parys et al. 1993; Missiaen et al. 1994; Horne and Meyer 1995; Combettes et al. 1996; Tanimura and Turner 1996), but not in others (Combettes et al. 1992; Shuttleworth 1992; Combettes et al. 1993; van de Put et al. 1994). However, even the supportive results do not eliminate the possibility that the increased sensitivity to IP3 arises from having Ca2+ pass through active IP3R and increase their sensitivity from the cytosolic surface. Similar difficulties have plagued analyses of the effects of luminal Ca2+ on RyR (Tripathy and Meissner 1996; Laver 2007; Laver 2009). In bilayer recordings of IP3R1, where essential accessory proteins may be lost, luminal Ca2+ failed to potentiate the Ca2+ release evoked by IP3 (Bezprozvanny and Ehrlich 1994). Despite the caveats, regulation of IP3R by luminal Ca2+ deserves serious consideration. A high-affinity Ca2+-binding site within the luminal loop linking TMD 5 and 6 (Sienaert et al. 1996) contains conserved acidic residues that could mediate luminal Ca2+ regulation, although the sub-µM affinity of this site for Ca2+ would be ill-suited to detecting likely changes in luminal Ca2+ concentration. Luminal accessory proteins, akin to those that regulate RyR, are another possibility, with ERp44 being one candidate. ERp44 belongs to the thioredoxin protein family and regulates IP3R in a pH- and luminal Ca2+-dependent manner (Higo et al. 2005). Binding of ERp44 to the TMD5-6 loop of IP3R inhibits channel activity, and the interaction is disrupted by high concentrations of Ca2+ consistent with the suggestion that luminal Ca2+ might enhance IP3R activity.
To summarize, IP3 works by tuning the Ca2+ sensitivity of the IP3R: It stimulates Ca2+ binding to a stimulatory site and inhibits Ca2+ binding to an inhibitory site (Fig. 1A). Binding to the stimulatory site is the trigger for opening of the pore. The identity of neither Ca2+-binding site is known: The stimulatory site probably resides within the IP3R itself, but the inhibitory site may require an accessory protein, though this is unlikely to be CaM. Luminal Ca2+ may further tune the sensitivity of the IP3R to regulation by its cytosolic ligands, but this remains unproven.
STRUCTURAL DETERMINANTS OF IP3R ACTIVATION
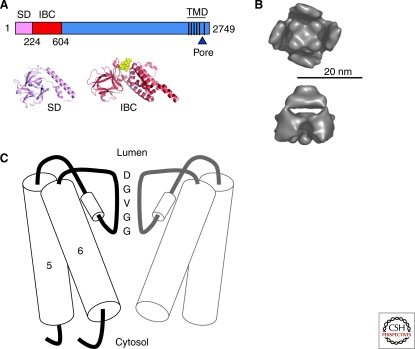
Judged by their primary amino acid sequences, all known IP3R subunits are assumed to have a similar architecture. Each subunit, of about 2700 residues, comprises three major regions: the N-terminal to which IP3 binds, the C-terminal region with its six transmembrane regions (TMD) (Galvan et al. 1999), and a large intervening sequence (Fig. 2A). Functional IP3Rs are tetrameric, assembled either from identical subunits or from mixtures of the three subtypes and their many splice variants (Taylor et al. 1999; Foskett et al. 2007). Several structures of the entire IP3R1 have been published, each derived from single particle analysis of images from electron microscopy (Hamada and Mikoshiba 2002; Jiang et al. 2002a; da Fonseca et al. 2003; Hamada et al. 2003; Serysheva et al. 2003; Sato et al. 2004). These studies confirm the tetrameric state of IP3R, but variability between the structures and their relatively low resolution (∼30 Å) have, so far, limited any realistic interpretation of the structural basis of IP3R activation (Taylor et al. 2004) (Fig. 2B). Whether structures of recombinant IP3R will contribute to resolving this impasse remains to be seen (Wolfram et al. 2010).
Figure 2.
Major structural domains of IP3R. (A) The three key regions defined by the primary sequence of a single IP3R subunit are highlighted: the N-terminal with its SD and IBC, the C-terminal region with its six TMD and pore, and the large central region. Atomic structures of the SD (Bosanac et al. 2005) and IBC with IP3 bound (Bosanac et al. 2002) are also shown. (B) Two views of the IP3R derived from single particle analysis (da Fonseca et al. 2003) (top, from the cytosol; bottom, across the ER membrane with the ER lumen at the top). (C) A possible structure of the IP3R pore, with a luminal selectivity filter and a constriction formed by the tepee-like structure of TMD6. Only two of the four IP3R subunits are shown.
There has been more progress with RyR, although only recently has the resolution of these structures (∼30 Å) improved on that obtained for IP3R. These structures of native RyR, and all three subtypes of recombinant RyR reveal a shape like a square mushroom with a very large, open cytoplasmic structure tethered to a much smaller TMD region (the stalk). At ∼30 Å resolution, the structures of the three RyR subtypes are almost indistinguishable, and because they, like the three subtypes of IP3R, share about 65% sequence identity, it seems reasonable to suppose that the 3D structures of all IP3R are also likely to be similar to each other. These studies of RyR have identified positions of critical residues within the 3D structure, the sites to which accessory proteins bind, and conformational changes associated with opening of the pore (Orlova et al. 1996; Serysheva et al. 2005; Wang et al. 2007; Jones et al. 2008). Activation of RyR is associated with considerable changes in both the pore and cytoplasmic regions: The four corners of the latter dip down toward the SR, while the central region lifts away from it (Samso et al. 2009). It is noteworthy, in the context of schemes for activation of IP3R (see below), that large movements of some cytoplasmic domains of RyR1 appear to occur around hinges that link them to relatively immobile domains.
The highest resolution maps (∼10Å), although still insufficient to map 3D structure to primary sequence, have come close to defining the likely secondary structure of the pore of RyR1 (Ludtke et al. 2005; Samso et al. 2005; Samso et al. 2009). This region appears to have six α-helices (Samso et al. 2009), consistent with models of RyR that suggest six TMD (Meur et al. 2007). Along the central axis, it has a luminal constriction (probably the selectivity filter, see below) and a tepee-like assembly of four inner helices (likely to be TMD6), with the apex pointing into the cytoplasmic structure. By analogy with MthK channels, this constriction may form the gate of the RyR. Kinking of the inner helix around a central Gly residue causes splitting of the tepee and thereby opening of the channel for MthK (Jiang et al. 2002b). One structure (Samso et al. 2009) is consistent with a similar mechanism operating for RyR1, but another structure (Ludtke et al. 2005) and mutagenesis of the critical Gly (G4863 in RyR1) (Wang et al. 2003) contradict it. These insights into the possible workings of the RyR pore are significant for IP3R, because it is within the pore region (TMD5-6) that RyR and IP3R share the greatest sequence similarity. We turn, therefore, to the pore of the IP3R to explore its properties and structure.
All IP3R (like all RyR) are cation channels with extremely large conductance, but only modest selectivity for Ca2+ over monovalent cations (permeability ratio, PCa/PK ∼ 6) (Williams et al. 2001; Foskett et al. 2007). The voltage-gated and store-operated Ca2+ channels that mediate Ca2+ entry across the plasma membrane are vastly more selective (PCa/PK > 1000). In the ER, where most IP3Rs are located, this lack of selectivity is unlikely to be a problem because Ca2+ is probably the only cation with an appreciable electrochemical gradient across the ER membrane. In effect, the ER Ca2+ pump (SERCA), by creating a steep Ca2+ concentration gradient across the ER membrane, assumes responsibility for determining which cations flow through an open IP3R. Indeed, the K+ permeability of IP3R and RyR may facilitate rapid Ca2+ release by allowing K+ to move into the ER to electrically compensate the efflux of Ca2+ (Gillespie and Fill 2008). The pore of the IP3R, like that of RyR, is formed by the final pair of TMD (TMD5-6) and the luminal loop that links them from each of the four subunits (Ramos-Franco et al. 1999; Williams et al. 2001) (Fig. 2C). The loop includes a sequence (GGVGD in IP3R) similar to that of the selectivity filter of K+ channels (Balshaw et al. 1999), consistent with the idea that the overall architecture of the pore region may be broadly similar to that of K+ channels (MacKinnon 2004). For both IP3R and RyR, however, the pore must be larger and less-selective than for K+ channels, and probably able to accommodate only one cation at a time (Williams et al. 2001). This model for the IP3R pore, where TMD5 (the outer helix) and TMD6 (inner helix) cradle a short pore helix and selectivity filter (Fig. 2C), is consistent with mutagenesis of residues within this region affecting ion permeation (Boehning et al. 2001b; Dellis et al. 2006; Dellis et al. 2008; Schug et al. 2008), with biophysical evidence that the narrowest region of the pore lies close to the luminal entrance of the RyR (Williams et al. 2001) and with the intermediate resolution structures of the pore region of RyR1 (Samso et al. 2009). A conserved acidic residue (D2550 in IP3R1) at the luminal end of the selectivity filter (Fig. 2C) contributes to the modest Ca2+ selectivity of IP3R (Boehning et al. 2001b; Dellis et al. 2008) and RyR (Gao et al. 2000; Wang et al. 2005; Gillespie 2008), but the structural determinants of ion selectivity and permeation by IP3R are otherwise poorly understood. The changes in pore structure that allow it to open are also minimally understood. Indeed, mutation of the conserved Gly within TMD6 of IP3R (G2586 in IP3R1), which might have been thought to provide the gating hinge (Samso et al. 2009), appears not to prevent IP3 from opening IP3R (Schug et al. 2008). In short, aside from knowing the regions of primary sequence that form the IP3R pore (TMD5-6) and a rather vague notion that its structure perhaps resembles that of K+ channels, we have only the most rudimentary knowledge of the structural determinants of how the IP3R pore opens and selects between ions.
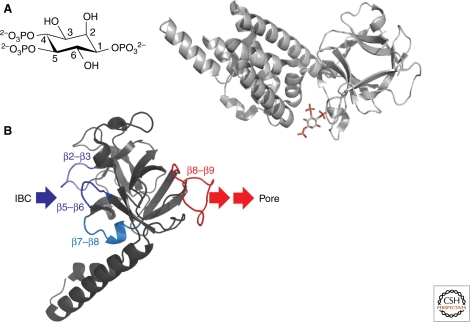
The conformational changes in the IP3R that lead to opening of its pore are initiated by IP3 binding to the IP3-binding core (IBC, residues 224–604 in IP3R1) (Fig. 3A). Although IP3 is the only endogenous ligand of the IBC, there are many synthetic agonists, all of which have structures equivalent to the equatorial 6-hydroxyl and the 4- and 5-phosphate groups of IP3 (Fig. 3A) (Rossi et al. 2010). It is noteworthy that neither of the immediate products of IP3 metabolism, IP2 and IP4, binds to the IBC; both metabolic pathways are therefore effective means of terminating activation of IP3R by IP3. An atomic structure of the IBC with IP3 bound (Bosanac et al. 2002) shows IP3 held within a clam-like structure in which the phosphate groups of IP3 are coordinated by basic residues (Fig. 3A). The two sides of the clam, the α- and β-domains, form a network of interactions with the essential groups of IP3. The 4-phosphate is hydrogen-bonded with residues in the β-domain, the 5-phosphate forms hydrogen bonds with residues predominantly in the α-domain, and the 6-hydroxyl interacts with the backbone of a residue within the α-domain. It is easy to imagine how these interactions with IP3 might pull the α- and β-domains together, causing the clam to close in a manner similar to glutamate binding to ionotropic glutamate receptors (Mayer 2006). Structures of the IBC without IP3 bound are urgently needed to assess this proposal, but two lines of evidence lend circumstantial support. First, the IBC adopts a more constrained structure when it binds IP3 (Chan et al. 2007). Second, adenophostins, which are high-affinity agonists of IP3R (Rossi et al. 2010), retain some activity after loss of the 3-phosphate (analogous to the 5-phosphate of IP3), probably because their adenine moiety interacts strongly with a residue in the α-domain and thereby partially mimics the clam-closure that would otherwise require the 5-phosphate to bind to the α-domain (Sureshan et al. 2009). We envisage, therefore, that when IP3 binds to the IBC, the essential vicinal phosphate groups through their contacts with the α- and β-domains effectively cross-bridge the two sides of the clam-like structure, causing it to close, and thereby initiate the processes that will culminate in opening of the pore.
Figure 3.
Initiation of IP3R activation by IP3. (A) The structure of IP3, with its critical vicinal 4,5-bisphosphate and 6-hydroxyl groups, is shown alongside the structure of the IBC with IP3 bound. The latter shows the 4- and 5-phosphates contacting the β- and α-domains, respectively (Bosanac et al. 2002), and thereby pulling the clam into a more closed state. (B) Structure of the SD (Bosanac et al. 2005) showing possible sites of interaction with the IBC and downstream domains through which it signals to the pore. See text for further details.
It is worth commenting briefly on available antagonists of IP3R because of their obvious value as experimental tools. There are no specific antagonists of IP3R, although with appropriate caution some antagonists can yield useful insight (Michelangeli et al. 1995). Heparin is a competitive antagonist of IP3 (Worley et al. 1987), although it is not membrane-permeant and, among many additional effects, it uncouples G-protein-coupled receptors from their G proteins (Dasso and Taylor 1991) and activates RyR (Ehrlich et al. 1994). 2-aminoethyl diphenylboronate (2-APB) is membrane-permeant and inhibits IP3-evoked Ca2+ release without affecting IP3 binding (Maruyama et al. 1997); its mechanism of action is unresolved. However, 2-APB also inhibits Ca2+ uptake and many other Ca2+ channels. It has recently aroused interest as a modulator of STIM and, therefore, store-operated Ca2+ entry (Goto et al. 2010). A screen of 2-APB analogues with selectivity for store-operated Ca2+ entry may yet also provide IP3R-selective antagonists (Goto et al. 2010). Xestospongins, isolated from an Australian sponge, are high-affinity membrane-permeant inhibitors of IP3-evoked Ca2+ release that do not affect IP3 binding (Gafni et al. 1997), but they, too, have side effects (Solovyova et al. 2002). High concentrations of caffeine inhibit IP3-evoked Ca2+ release (Parker and Ivorra 1991) without affecting IP3 binding (Worley et al. 1987), but caffeine also stimulates RyR, inhibits cyclic nucleotide phosphodiesterases, and interferes with many Ca2+ indicators. Membrane-permeant peptide antagonists of IP3R may provide another potential source of selective antagonists (Sun and Taylor 2008).
How IP3 binding to the IBC leads to binding of Ca2+ to the IP3R, and thereby opening of the pore, remains largely unknown, but it is clear that the suppressor domain (SD, residues 1-223 of IP3R1), which is connected to the IBC by a flexible linkage (Chan et al. 2007), plays an essential role. The clearest evidence is that IP3 binds to IP3R without an SD, but it fails to open the pore (Uchida et al. 2003; Szlufcik et al. 2006). The name of this region derives from the observation that, although the SD itself is unlikely to make any direct contacts with IP3, its presence decreases the affinity of IP3R for IP3 (Uchida et al. 2003). We have interpreted this effect to reflect the use of binding energy from the binding of IP3 to the IBC to cause a conformational change within the SD. This interpretation gains considerable support from our analysis of partial agonists of the IP3R (Rossi et al. 2009). The crux of our argument is that the energy provided by agonist binding drives both the conformational changes that lead to receptor activation and tighter binding of the ligand to its receptor. There is, therefore, a playoff between these two claims on the binding energy. Partial agonists, because they less effectively activate the receptor, divert more binding energy into stabilizing the binding, while full agonists evoke more substantial conformational changes; therefore, less binding energy remains to stabilize binding. Our results show that although full and partial agonists bind with similar affinities to the IBC, the SD causes the affinity of full agonists to decrease more than for partial agonists (Rossi et al. 2009). Quantitative analyses of these results lead to the conclusion that the most energetically costly conformational change in the IP3R evoked by IP3 occurs within its N-terminal (residues 1-604), and that these conformational changes pass entirely via the SD to the pore region (Rossi et al. 2009). We suggest, therefore, that the SD is the essential link between IP3 binding to the IBC and the subsequent conformational changes that lead to opening of the pore. Without a structure of the entire N-terminal of the IP3R, we can only speculate on the physical relationship between the IBC and SD, but our results with partial agonists and mutagenesis are consistent with three exposed loops of the SD (β2–β3, β5–β6, and β7–β8, blue in Fig. 3B) being the most likely sites of interaction with the IBC (Rossi et al. 2009).
Remarkably, and despite their rather low sequence identities (∼30%), the crystal structures of the SD from IP3R1 (Bosanac et al. 2005) and of the analogous N-terminal regions from RyR1 and RyR2 (Amador et al. 2009; Lobo and Van Petegem 2009) are extremely similar. Several mutations associated with malignant hyperthermia and central core disease (RyR1) or catecholaminergic polymorphic ventricular tachycardia (RyR2), all of which impair the normal regulation of gating, are clustered in an exposed loop (β8–β9) of the N-terminal of RyR (Amador et al. 2009). Furthermore, and consistent with the N-terminal of the RyR mediating essential interdomain interactions, a peptide derived from this region causes RyR2 to open spontaneously, apparently by uncoupling an interaction between the endogenous loop and a central region of the RyR that includes residues 2460–2495 (Oda et al. 2005; Tateishi et al. 2009). In light of the conservation of structure between IP3R and RyR, it is tempting to speculate that the same loop in the SD of the IP3R (β8–β9, red in Fig. 3B) may mediate transfer of conformational changes onward toward the pore. Co-immunoprecipitation studies have suggested an interaction between the N-terminal of IP3R1 (most likely the SD) and the pore region of an adjacent subunit (Boehning and Joseph 2000a), perhaps mediated by the cytosolic loop linking TMD4 to TMD5 (Schug and Joseph 2006). An attractive possibility, therefore, is that the SD (perhaps its β8-β9 loop) interacts directly with the short cytosolic helix linking TMD4 and TMD5, and thereby gates the pore (Schug and Joseph 2006; Rossi et al. 2009). Such an interaction would require that the SD comes very close to the pore, but the exact location of the SD within the 3D structure of either the IP3R or RyR is unknown. The N-terminal of the RyR probably lies within the clamp region at the periphery of the large square cytoplasmic structure (Wang et al. 2007), and it does change shape during RyR activation (Samso et al. 2009). Yet, in this location the N-terminal is too far from the pore to interact directly with the TMD4-5 loop, consistent perhaps with evidence that in RyR the N-terminal may interact directly with a neighboring domain that includes residues from the central part of the primary sequence (Wang et al. 2007). These observations and the evidence that the uncoupling peptide derived from the N-terminal of RyR2 appears to interact with residues remote from the pore (Oda et al. 2005; Tateishi et al. 2009), suggest that the links between the SD and pore may, at least for RyR, be indirect.
In summary, we suggest that IP3R activation is initiated when IP3 binds to the IBC, and perhaps thereby causes closure of its clam-like structure. That conformational change, which must also initiate the events that allow Ca2+ to bind to a stimulatory site, is passed to the rest of the IP3R entirely via the SD. The location of that Ca2+-binding site and, therefore, the structural links between it and the SD, are unresolved. We speculate that one face of the SD interacts directly with the IBC, and the opposite face interacts with the structure through which conformational changes pass to the pore. The pore is a relatively nonselective, large-conductance cation channel formed by the tetrameric assembly of the TMD5-6 regions of each subunit. Its structure is unresolved but likely to be broadly similar to K+ channels with a selectivity filter and gate at opposite ends of its membrane-spanning structure.
ACKNOWLEDGMENTS
Work from the authors’ laboratory is supported by the Wellcome Trust.
Footnotes
Editors: Martin D. Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Adkins CE, Morris SA, De Smedt H, Török K, Taylor CW 2000. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem J 345: 357–363 [PMC free article] [PubMed] [Google Scholar]
- Adkins CE, Taylor CW 1999. Lateral inhibition of inositol 1,4,5-trisphosphate receptors by cytosolic Ca2+. Curr Biol 9: 1115–1118 [DOI] [PubMed] [Google Scholar]
- Amador FJ, Liu S, Ishiyama N, Plevin MJ, Wilson A, Maclennan DH, Ikura M 2009. Crystal structure of type I ryanodine receptor amino-terminal β-trefoil domain reveals a disease-associated mutation “hot spot” loop. Proc Natl Acad Sci USA 106: 11040–11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshaw D, Gao L, Meissner G 1999. Luminal loop of the ryanodine receptor: A pore-forming segment? Proc Natl Acad Sci USA 96: 3345–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benevolensky D, Moraru II, Watras J 1994. Micromolar calcium decreases the affinity of inositol trisphosphate receptor in vascular smooth muscle. Biochem J 299: 631–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ 1975. The interaction of cyclic nucleotides and calcium in the control of cellular activity. In Advances in Cyclic Nucleotide and Protein Phosphorylation Research, (ed. Greengard P, Robison GA), pp. 1–98 Raven Press, New York: [PubMed] [Google Scholar]
- Berridge MJ 1983. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem J 212: 849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, (ed.) 1995. CIBA Foundation Symposium Calcium waves, gradients and oscillations. John Wiley and Sons, Chichester [Google Scholar]
- Berridge MJ 1997. Elementary and global aspects of calcium signalling. J Physiol 499: 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ 2005. Unlocking the secrets of cell signaling. Annu Rev Physiol 67: 1–21 [DOI] [PubMed] [Google Scholar]
- Berridge MJ 2007. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp 74: 1–7 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Fain JN 1979. Inhibition of phosphatidylinositol synthesis and the inactivation of calcium entry after prolonged exposure of the blowfly salivary gland to 5-hydroxytryptamine. Biochem J 178: 59–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF 1984. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature 312: 315–321 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF 1989. Inositol phosphates and cell signalling. Nature 341: 197–205 [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD 2000. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 1: 11–21 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Ehrlich BE 1994. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol 104: 821–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE 1991. Bell-shaped calcium-response curves for Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754 [DOI] [PubMed] [Google Scholar]
- Boehning D, Joseph SK 2000a. Direct association of ligand-binding and pore domains in homo- and heterotetrameric inositol 1,4,5-trisphosphate receptors. EMBO J 19: 5450–5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Joseph SK 2000b. Functional properties of recombinant type I and type III inositol 1,4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J Biol Chem 275: 21492–21499 [DOI] [PubMed] [Google Scholar]
- Boehning D, Joseph SK, Mak D-OD, Foskett JK 2001a. Single-channel recordings of recombinant inositol trisphosphate receptors in mammalian nuclear envelope. Biophys J 81: 117–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehning D, Mak D-OD, Foskett JK, Joseph SK 2001b. Molecular determinants of ion permeation and selectivity in inositol 1,4,5-trisphosphate receptor Ca2+ channels. J Biol Chem 276: 13509–13512 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Lipp P 1997. Cooking with calcium: the recipes for composing global signals from elementary events. Cell 91: 367–373 [DOI] [PubMed] [Google Scholar]
- Bootman MD, Berridge MJ, Taylor CW 1992. All-or-nothing Ca2+ mobilization from the intracellular stores of single histamine-stimulated HeLa cells. J Physiol 450: 163–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosanac I, Alattia J-R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, et al. 2002. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 420: 696–700 [DOI] [PubMed] [Google Scholar]
- Bosanac I, Yamazaki H, Matsu-ura T, Michikawa M, Mikoshiba K, Ikura M 2005. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol Cell 17: 193–203 [DOI] [PubMed] [Google Scholar]
- Brown GR, Sayers LG, Kirk CJ, Michell RH, Michelangeli F 1992. The opening of the inositol 1,4,5-trisphosphate-sensitive Ca2+ channel in rat cerebellum is inhibited by caffeine. Biochem J 282: 309–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess GM, McKinney JS, Fabiato A, Leslie BA, Putney JW Jr 1983. Calcium pools in saponin-permeabilized guinea pig hepatocytes. J Biol Chem 258: 15336–15345 [PubMed] [Google Scholar]
- Cardy TJA, Taylor CW 1998. A novel role for calmodulin: Ca2+-independent inhibition of type-1 inositol trisphosphate receptors. Biochem J 334: 447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardy TJA, Traynor D, Taylor CW 1997. Differential regulation of types 1 and 3 inositol trisphosphate receptors by cytosolic Ca2+. Biochem J 328: 785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J, Whitten AE, Jeffries CM, Bosanac I, Mal TK, Ito J, Porumb H, Michikawa T, Mikoshiba K, Trewhella J, et al. 2007. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J Mol Biol 373: 1269–1280 [DOI] [PubMed] [Google Scholar]
- Chen SRW, Ebisawa K, Li X, Zhang L 1998. Molecular identification of the ryanodine receptor Ca2+ sensor. J Biol Chem 273: 14675–14678 [DOI] [PubMed] [Google Scholar]
- Chen SRW, Li X, Ebisawa K, Zhang L 1997. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J Biol Chem 272: 24234–24236 [DOI] [PubMed] [Google Scholar]
- Chen SRW, MacLennan DH 1994. Identification of calmodulin-, Ca2+- and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem 269: 22698–22704 [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB 1993. Calcium sparks. Elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744 [DOI] [PubMed] [Google Scholar]
- Combettes L, Cheek TR, Taylor CW 1996. Regulation of inositol trisphosphate receptors by luminal Ca2+ contributes to quantal Ca2+ mobilization. EMBO J 15: 2086–2093 [PMC free article] [PubMed] [Google Scholar]
- Combettes L, Claret M, Champeil P 1992. Do submaximal InsP3 concentrations only induce partial release discharge of permeabilized hepatocyte calcium pools because of the concomitant reduction of intraluminal Ca2+ concentration? FEBS Lett 301: 287–290 [DOI] [PubMed] [Google Scholar]
- Combettes L, Claret M, Champeil P 1993. Calcium control of InsP3-induced discharge of calcium from permeabilised hepatocyte pools. Cell Calcium 14: 279–292 [DOI] [PubMed] [Google Scholar]
- da Fonseca PCA, Morris SA, Nerou EP, Taylor CW, Morris EP 2003. Domain organisation of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis. Proc Natl Acad Sci USA 100: 3936–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danoff SK, Supattapone S, Snyder SH 1988. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J 254: 701–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso LLT, Taylor CW 1991. Heparin and other polyanions uncouple a1-adrenoceptors from G-proteins. Biochem J 280: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellis O, Dedos S, Tovey SC, Rahman T-U-, Dubel SJ, Taylor CW 2006. Ca2+ entry through plasma membrane IP3 receptors. Science 313: 229–233 [DOI] [PubMed] [Google Scholar]
- Dellis O, Rossi AM, Dedos SG, Taylor CW 2008. Counting functional IP3 receptors into the plasma membrane. J Biol Chem 283: 751–755 [DOI] [PubMed] [Google Scholar]
- Demuro A, Parker I 2007. Multi-dimensional resolution of elementary Ca2+ signals by simultaneous multi-focal imaging. Cell Calcium 43: 367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI 1997. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386: 855–858 [DOI] [PubMed] [Google Scholar]
- Dufour J-F, Arias IM, Turner TJ 1997. Inositol 1,4,5-trisphosphate and calcium regulate the calcium channel function of the hepatic inositol 1,4,5-trisphosphate receptor. J Biol Chem 272: 2675–2681 [DOI] [PubMed] [Google Scholar]
- Dupont G, Houart G, De Koninck P 2003. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations: a simple model. Cell Calcium 34: 485–497 [DOI] [PubMed] [Google Scholar]
- Dyer JL, Liu Y, Pino de la Huerga I, Taylor CW 2005. Long-lasting inhibition of adenylyl cyclase selectively mediated by inositol 1,4,5-trisphosphate-evoked calcium release. J Biol Chem 280: 8936–8944 [DOI] [PubMed] [Google Scholar]
- Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH 2003. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol 5: 440–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich BE, Kaftan E, Bezprozvannaya S, Bezprozvanny I 1994. The pharmacology of intracellular Ca2+-release channels. Trends Pharmacol Sci 15: 145–149 [DOI] [PubMed] [Google Scholar]
- Ehrlich BE, Watras J 1988. Inositol 1,4,5-trisphosphate activates a channel from smooth muscle sarcoplasmic reticulum. Nature 336: 583–586 [DOI] [PubMed] [Google Scholar]
- Endo M 1977. Calcium release from the sarcoplasmic reticulum. Physiol Rev 57: 71–108 [DOI] [PubMed] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y 1970. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 228: 34–36 [DOI] [PubMed] [Google Scholar]
- Fabiato A 1983. Calcium-induced release of calcium from cardiac sarcoplasmic reticulum. Am J Physiol 245: C1–C14 [DOI] [PubMed] [Google Scholar]
- Ferris CD, Cameron AM, Huganir RL, Snyder SH 1992. Quantal calcium release by purified reconstituted inositol 1,4,5-trisphosphate receptors. Nature 356: 350–352 [DOI] [PubMed] [Google Scholar]
- Ferris CD, Huganir RL, Supattapone S, Snyder SH 1989. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature 342: 87–89 [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252: 443–446 [DOI] [PubMed] [Google Scholar]
- Foskett JK, White C, Cheung KH, Mak DO 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruen BR, Bardy JM, Byrem TM, Strasburg GM, Louis CF 2000. Differential Ca2+ sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am J Physiol 279: C724–C733 [DOI] [PubMed] [Google Scholar]
- Furuichi T, Yoshikawa S, Miyawaki A, Wada K, Maeda M, Mikoshiba K 1989. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 342: 32–38 [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN 1997. Xestospongins: Potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733 [DOI] [PubMed] [Google Scholar]
- Galvan DL, Borrego-Diaz E, Perez PJ, Mignery GA 1999. Subunit oligomerization, and topology of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 274: 29483–29492 [DOI] [PubMed] [Google Scholar]
- Gao L, Balshaw D, Xu L, Tripathy A, Xin C, Meissner G 2000. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca2+ release channel (ryanodine receptor) activity and conductance. Biophys J 79: 828–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gees M, Colsoul B, Nilius B 2010. The Role of Transient Receptor Potential Cation Channels in Ca2+ Signaling. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a003962 [DOI] [PMC free article] [PubMed]
- Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH 1996. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell 84: 473–480 [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH 1995. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell 80: 439–444 [DOI] [PubMed] [Google Scholar]
- Gilkey JC 1983. Roles of calcium and pH in activation of eggs of the medaka fish, Oryzias latipes. J Cell Biol 97: 669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D 2008. Energetics of divalent selectivity in a calcium channel: the ryanodine receptor case study. Biophys J 94: 1169–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D, Fill M 2008. Intracellular calcium release channels mediate their own countercurrent: the ryanodine receptor case study. Biophys J 95: 3706–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnegy ME 1993. Calmodulin in neurotransmitter and hormone action. Annu Rev Pharmacol Toxicol 33: 45–70 [DOI] [PubMed] [Google Scholar]
- Goto J, Suzuki AZ, Ozaki S, Matsumoto N, Nakamura T, Ebisui E, Fleig A, Penner R, Mikoshiba K 2010. Two novel 2-aminoethyl diphenylborinate (2-APB) analogues differentially activate and inhibit store-operated Ca2+ entry via STIM proteins. Cell Calcium 47: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I, Györke S 1998. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75: 2801–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE 1998. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature 296: 81–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnóczky G, Thomas AP 1997. Minimal requirements for calcium oscillations driven by the IP3 receptor. EMBO J 16: 3533–3543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K, Mikoshiba K 2002. Two-state conformational changes in inositol 1,4,5-trisphosphate receptor regulated by calcium. J Biol Chem 277: 21115–21118 [DOI] [PubMed] [Google Scholar]
- Hamada K, Terauchi A, Mikoshiba K 2003. Three-dimensional rearrangements with inositol 1,4,5-trisphosphate receptor by calcium. J Biol Chem 278: 52881–52889 [DOI] [PubMed] [Google Scholar]
- Hamilton SL 2005. Ryanodine receptors. Cell Calcium 38: 253–260 [DOI] [PubMed] [Google Scholar]
- Haynes LP, Tepikin AV, Burgoyne RD 2004. Calcium-binding protein 1 is an inhibitor of agonist-evoked, inositol 1,4,5-trisphosphate-mediated calcium signaling. J Biol Chem 279: 547–555 [DOI] [PubMed] [Google Scholar]
- Higo T, Hattori M, Nakamura T, Natsume T, Michikawa T, Mikoshiba K 2005. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120: 85–98 [DOI] [PubMed] [Google Scholar]
- Hirota J, Michikawa T, Miyawaki A, Furuichi T, Okura I, Mikoshiba K 1995. Kinetics of calcium release by immunoaffinity-purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles. J Biol Chem 270: 19046–19051 [DOI] [PubMed] [Google Scholar]
- Hirota J, Michikawa T, Natsume T, Furuichi T, Mikoshiba K 1999. Calmodulin inhibits inositol 1,4,5-trisphosphate-induced calcium release through the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett 456: 322–326 [DOI] [PubMed] [Google Scholar]
- Hokin MR, Hokin LE 1953. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem 203: 967–977 [PubMed] [Google Scholar]
- Horne JH, Meyer T 1995. Luminal calcium regulates the inositol trisphosphate receptor of rat basophilic leukemia cells at the cytosolic side. Biochemistry 34: 12738–12746 [DOI] [PubMed] [Google Scholar]
- Iino M 1987. Calcium dependent inositol trisphosphate-induced calcium release in the guinea-pig taenia caeci. Biochem Biophys Res Commun 142: 47–52 [DOI] [PubMed] [Google Scholar]
- Iino M 1990. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol 95: 1103–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa T, Smith JS, Coronado R, Campbell KP 1987. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem 262: 16636–16643 [PubMed] [Google Scholar]
- Ionescu L, Cheung KH, Vais H, Mak DO, White C, Foskett JK 2006. Graded recruitment and inactivation of single InsP3 receptor Ca2+-release channels: implications for quantal Ca2+ release. J Physiol 573: 645–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine RF 1990. “Quantal” Ca2+ release and the control of Ca2+ entry by inositol phosphates - a possible mechanism. FEBS Lett 262: 5–9 [DOI] [PubMed] [Google Scholar]
- Islam MO, Yoshida Y, Koga T, Kojima M, Kanagawa K, Imai S 1996. Isolation and characterization of vascular smooth muscle inositol 1,4,5-trisphosphate receptor. Biochem J 316: 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M, Michikawa T, Bosanac I, Ikura M, Mikoshiba K 2007. Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J Biol Chem 282: 12755–12764 [DOI] [PubMed] [Google Scholar]
- Jean T, Klee CB 1986. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem 261: 16414–16420 [PubMed] [Google Scholar]
- Jiang D, Chen W, Xiao J, Wang R, Kong H, Jones PP, Zhang L, Fruen B, Chen SR 2008. Reduced threshold for luminal Ca2+ activation of RyR1 underlies a causal mechanism of porcine malignant hyperthermia. J Biol Chem 283: 20813–20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q-X, Thrower EC, Chester DW, Ehrlich BE, Sigworth FJ 2002a. Three-dimensional structure of the type 1 inositol 1,4,5-trisphosphate receptor at 24 Å resolution. EMBO J 21: 3575–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R 2002b. The open pore conformation of potassium channels. Nature 417: 523–526 [DOI] [PubMed] [Google Scholar]
- Jones PP, Meng X, Xiao B, Cai S, Bolstad J, Wagenknecht T, Liu Z, Chen SR 2008. Localization of PKA phosphorylation site, Ser2030, in the three-dimensional structure of cardiac ryanodine receptor. Biochem J 410: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SK, Brownell S, Khan MT 2005. Calcium regulation of inositol 1,4,5-trisphosphate receptors. Cell Calcium 38: 539–546 [DOI] [PubMed] [Google Scholar]
- Joseph SK, Rice HL, Williamson JR 1989. The effect of external calcium and pH on inositol trisphosphate-mediated calcium release from cerebellum microsomal fractions. Biochem J 258: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaftan EJ, Ehrlich BE, Watras J 1997. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J Gen Physiol 110: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Mignery GA, Zhu MX, Muallem S 1999. The N-terminal domain of the IP3 receptor gates store-operated hTrp3 channels. Mol Cell 4: 423–429 [DOI] [PubMed] [Google Scholar]
- Krinke O, Novotna Z, Valentova O, Martinec J 2007. Inositol trisphosphate receptor in higher plants: is it real? J Exp Bot 58: 361–376 [DOI] [PubMed] [Google Scholar]
- Laude AJ, Tovey SC, Dedos S, Potter BVL, Lummis SCR, Taylor CW 2005. Rapid functional assays of recombinant IP3 receptors. Cell Calcium 38: 45–51 [DOI] [PubMed] [Google Scholar]
- Launikonis BS, Zhou J, Royer L, Shannon TR, Brum G, Rios E 2006. Depletion “skraps” and dynamic buffering inside the cellular calcium store. Proc Natl Acad Sci USA 103: 2982–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR 2007. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J 92: 3541–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR 2009. Luminal Ca2+ activation of cardiac ryanodine receptors by luminal and cytoplasmic domains. Eur J Biophys 39: 19–26 [DOI] [PubMed] [Google Scholar]
- Lechleiter J, Girard S, Peralta E, Clapham D 1991. Spiral calcium wave propagation and annihilation in Xenopus laevis occytes. Science 252: 123–126 [DOI] [PubMed] [Google Scholar]
- Li P, Chen SR 2001. Molecular basis of Ca2+ activation of the mouse cardiac Ca2+ release channel (ryanodine receptor). J Gen Physiol 118: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W-H, Llopis J, Whitney M, Zlokarnik G, Tsien RY 1998. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature 392: 936–941 [DOI] [PubMed] [Google Scholar]
- Lin C, Widjaja J, Joseph SK 2000. The interaction of calmodulin with alternatively spliced isoforms of the type-I inositol trisphosphate receptor. J Biol Chem 275: 2305–2311 [DOI] [PubMed] [Google Scholar]
- Lobo PA, Van Petegem F 2009. Crystal structures of the N-terminal domains of cardiac and skeletal muscle ryanodine receptors: insights into disease mutations. Structure 17: 1505–1514 [DOI] [PubMed] [Google Scholar]
- Ludtke SJ, Serysheva II, Hamilton SL, Chiu W 2005. The pore structure of the closed RYR1 channel. Structure 13: 1203–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R 2004. Potassium channels and the atomic basis of selective ion conduction (Nobel Lecture). Angew Chem Int Edn Engl 43: 4265–4277 [DOI] [PubMed] [Google Scholar]
- Maeda N, Niinobe M, Nakahira K, Mikoshiba K 1988. Purification and characterization of P400 protein, a glycoprotein characteristic of purkinje cell from mouse cerebellum. J Neurochem 51: 1724–1730 [DOI] [PubMed] [Google Scholar]
- Mak D-OD, McBride S, Foskett JK 1998. Inositol 1,4,5-tris-phosphate activation of inositol tris-phosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc Natl Acad Sci USA 95: 15821–15825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D-O, McBride S, Foskett JK 2001. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channels: Ca2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J Gen Physiol 117: 435–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D-O, McBride SMJ, Petrenko NB, Foskett JK 2003. Novel regulation of calcium inhibition of the inositol 1,4,5-trisphosphate receptor calcium-release channel. J Gen Physiol 122: 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak DO, McBride S, Raghuram V, Yue Y, Joseph SK, Foskett JK 2000. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J Gen Physiol 115: 241–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant JS, Taylor CW 1997. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr Biol 7: 510–518 [DOI] [PubMed] [Google Scholar]
- Marchenko SM, Yarotskyy VV, Kovalenko TN, Kostyuk PG, Thomas RC 2005. Spontaneously active and InsP3-activated ion channels in cell nuclei from rat cerebellar Purkinje and granule neurones. J Physiol 565: 897–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall ICB, Taylor CW 1993. Biphasic effects of cytosolic calcium on Ins(1,4,5)P3-stimulated Ca2+ mobilization in hepatocytes. J Biol Chem 268: 13214–13220 [PubMed] [Google Scholar]
- Marshall ICB, Taylor CW 1994. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J 301: 591–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem 122: 498–505 [DOI] [PubMed] [Google Scholar]
- Mayer ML 2006. Glutamate receptors at atomic resolution. Nature 440: 456–462 [DOI] [PubMed] [Google Scholar]
- Meur G, Parker AKT, Gergely FV, Taylor CW 2007. Targeting and retention of type 1 ryanodine receptors to the endoplasmic reticulum. J Biol Chem 282: 23096–23103 [DOI] [PubMed] [Google Scholar]
- Meyer T, Stryer L 1990. Transient calcium release induced by successive increments of inositol 1,4,5-trisphosphate. Proc Natl Acad Sci USA 87: 3841–3845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelangeli F, Mezna M, Tovey S, Sayers LG 1995. Pharmacological modulators of the inositol 1,4,5-trisphosphate receptor. Neuropharmacol 34: 1111–1122 [DOI] [PubMed] [Google Scholar]
- Michell RH 1975. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta 415: 81–147 [DOI] [PubMed] [Google Scholar]
- Michell RH, Drummond AH, Downes CP 1989. Inositol Lipids in Cell Signalling. Academic Press Ltd, London [Google Scholar]
- Michikawa T, Hirota J, Kawano S, Hiraoka M, Yamada M, Furuichi T, Mikoshiba K 1999. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron 23: 799–808 [DOI] [PubMed] [Google Scholar]
- Mignery GA, Südhof TC, Takei K, De Camilli P 1989. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature 342: 192–195 [DOI] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Bultynck G, Vanlingen S, De Smet P, Callewaert G, Parys J 2000. Calmodulin increases the sensitivity of type 3 inositol 1,4,5-trisphosphate receptors to Ca2+ inhibition in human bronchial mucosal cells. Mol Pharmacol 57: 564–567 [DOI] [PubMed] [Google Scholar]
- Missiaen L, De Smedt H, Parys JB, Casteels R 1994. Co-activation of inositol trisphosphate-induced Ca2+ release by cytosolic Ca2+ is loading-dependent. J Biol Chem 269: 7238–7242 [PubMed] [Google Scholar]
- Missiaen L, Parys JB, Sienaert I, Maes K, Kunzelmann K, Takahashi M, Tanzawa K, De Smedt H 1998. Functional properties of the type-3 InsP3 receptor in 16HBE14o- bronchial mucosal cells. J Biol Chem 273: 8983–8986 [DOI] [PubMed] [Google Scholar]
- Missiaen L, Parys JB, Weidema AF, Sipma H, Vanlingen S, De Smet P, Callewaert G, De Smedt H 1999. The bell-shaped Ca2+-dependence of the inositol 1,4,5-trisphosphate induced Ca2+ release is modulated by Ca2+/calmodulin. J Biol Chem 274: 13748–13751 [DOI] [PubMed] [Google Scholar]
- Missiaen L, Taylor CW, Berridge MJ 1992. Luminal Ca2+ promoting spontaneous Ca2+ release from inositol trisphosphate-sensitive stores of rat hepatocytes. J Physiol 455: 623–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M 1999. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J 18: 1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Mizushima A, Hirose K, Yamazawa T, Bezprozvanny I, Kurosaki T, Iino M 2001. Ca2+-sensor region of IP3 receptor controls intracellular Ca2+ signaling. EMBO J 20: 1674–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr FC, Hershey PEC, Zimányi I, Pessah IN 1993. Regulation of inositol 1,4,5-trisphosphate receptors in rat basophilic leukemia cells. I. Multiple conformational states of the receptor in a microsomal preparation. Biochim Biophys Acta 1147: 105–114 [DOI] [PubMed] [Google Scholar]
- Muallem S, Pandol SJ, Beeker TG 1989. Hormone-evoked calcium release from intracellular stores is a quantal process. J Biol Chem 264: 205–212 [PubMed] [Google Scholar]
- Nadif Kasri N, Bultynck G, Sienaert I, Callewaert G, Erneux C, Missiaen L, Parys JB, De Smedt H 2002. The role of calmodulin for inositol 1,4,5-trisphosphate receptor function. Biochim Biophys Acta 1600: 19–31 [DOI] [PubMed] [Google Scholar]
- Nadif Kasri N, Holmes AM, Bultynck G, Parys JB, Bootman MD, Rietdorf K, Missiaen L, McDonald F, De Smedt H, Conway SJ, et al. 2004. Regulation of InsP3 receptor activity by neuronal Ca2+-binding proteins. EMBO J 23: 312–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadif Kasri N, Torok K, Galione A, Garnham C, Callewaert G, Missiaen L, Parys JB, De Smedt H 2006. Endogenously bound calmodulin is essential for the function of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 281: 8332–8338 [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA 2007. Transient receptor potential cation channels in disease. Physiol Rev 87: 165–217 [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Miyakawa T, Wang Z, Glouchankova L, Iino M, Bezprozvanny I 2002. The high-affinity calcium-calmodulin-binding site does not play a role in the modulation of type 1 inositol 1,4,5-trisphosphate receptor function by calcium and calmodulin. Biochem J 365: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunn DL, Taylor CW 1992. Luminal Ca2+ increases the sensitivity of Ca2+ stores to inositol 1,4,5-trisphosphate. Mol Pharmacol 41: 115–119 [PubMed] [Google Scholar]
- Oancea E, Meyer T 1996. Reversible desensitization of inositol trisphosphate-induced calcium release provides a mechanism for repetitive calcium spikes. J Biol Chem 271: 17253–17260 [DOI] [PubMed] [Google Scholar]
- Oda T, Yano M, Yamamoto T, Tokuhisa T, Okuda S, Doi M, Ohkusa T, Ikeda Y, Kobayashi S, Ikemoto N, et al. 2005. Defective regulation of interdomain interactions within the ryanodine receptor plays a key role in the pathogenesis of heart failure. Circulation 111: 3400–3410 [DOI] [PubMed] [Google Scholar]
- Oldershaw KA, Nunn DL, Taylor CW 1991. Quantal Ca2+ mobilization stimulated by inositol 1,4,5-trisphosphate in permeabilized hepatocytes. Biochem J 278: 705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldershaw KA, Taylor CW 1993. Luminal Ca2+ increases the affinity of inositol 1,4,5-trisphosphate for its receptor. Biochem J 292: 631–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlova EV, Serysheva II, van Heel M, Hamilton SL, Chiu W 1996. Two structural configurations of the skeletal muscle calcium release channel. Nat Struct Biol 3: 547–552 [DOI] [PubMed] [Google Scholar]
- Pantazaka E, Taylor CW 2010. Targeting of inositol 1,4,5-trisphosphate receptor to the endoplasmic reticulum by its first transmembrane domain. Biochem J 425: 61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, Raunser S, Walz T, Garcia KC, Dolmetsch RE, Lewis RS 2009. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker AKT, Gergely FV, Taylor CW 2004. Targeting of inositol 1,4,5-trisphosphate receptors to the endoplasmic reticulum by multiple signals within their transmembrane domains. J Biol Chem 279: 23797–23805 [DOI] [PubMed] [Google Scholar]
- Parker I, Choi J, Yao Y 1996. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: hot spots, puffs and blips. Cell Calcium 20: 105–121 [DOI] [PubMed] [Google Scholar]
- Parker I, Ivorra I 1990. Localized all-or-nothing calcium liberation by inositol trisphosphate. Science 250: 977–979 [DOI] [PubMed] [Google Scholar]
- Parker I, Ivorra I 1991. Caffeine inhibits inositol trisphosphate-mediated liberation of intracellular calcium in Xenopus oocytes. J Physiol 433: 229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parys JB, Missiaen L, De Smedt H, Casteels R 1993. Loading dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in the clonal cell line A7r5. J Biol Chem 268: 25206–25212 [PubMed] [Google Scholar]
- Parys JB, Sernett SW, DeLisle S, Snyder PM, Welsh MJ, Campbell KP 1992. Isolation, characterization, and localization of the inositol 1,4,5-trisphosphate receptor protein in Xenopus laevis oocytes. J Biol Chem 267: 18776–18782 [PubMed] [Google Scholar]
- Patel S, Marchant JS, Brailoiu E 2010. Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47: 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Morris SA, Adkins CE, O’Beirne G, Taylor CW 1997. Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulin: Redistribution of calmodulin as a possible means of regulating Ca2+ mobilization. Proc Natl Acad Sci USA 94: 11627–11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, Boehning D, Snyder SH 2004. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem 73: 437–465 [DOI] [PubMed] [Google Scholar]
- Petersen OH, Burdakov D, Tepikin AV 1999. Polarity in intracellular calcium signalling. Bioessays 21: 851–860 [DOI] [PubMed] [Google Scholar]
- Pietri F, Hilly M, Mauger J-P 1990. Calcium mediates the interconversion between two states of the liver inositol 1,4,5-trisphosphate receptor. J Biol Chem 265: 17478–17485 [PubMed] [Google Scholar]
- Pinton P, Pozzan T, Rizzuto R 1998. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J 17: 5298–5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW Jr 1997. Capacitative calcium entry. RGLandes Company, Austin, Texas, USA [Google Scholar]
- Putney JW, Poggioli J, Weiss SJ 1981. Receptor regulation of calcium release and calcium permeability in parotid gland cells. Philos Trans R Soc London [Biol] 296: 37–45 [DOI] [PubMed] [Google Scholar]
- Rahman T-U, Skupin A, Falcke M, Taylor CW 2009. Clustering of IP3 receptors by IP3 retunes their regulation by IP3 and Ca2+. Nature 458: 655–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Taylor CW 2009. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels 3: 336–332 [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J, Bare D, Caenepeel S, Nani A, Fill M, Mignery G 2000. Single-channel function of recombinant type 2 inositol 1,4,5-trisphosphate receptor. Biophys J 79: 1388–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco J, Caenepeel S, Fill M, Mignery G 1998a. Single channel function of recombinant type-1 inositol 1,4,5-trisphosphate receptor ligand binding domain splice variants. Biophys J 75: 2783–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco J, Fill M, Mignery GA 1998b. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys J 75: 834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Franco J, Galvan D, Mignery GA, Fill M 1999. Location of the permeation pathway in the recombinant type-1 inositol 1,4,5-trisphosphate receptor. J Gen Physiol 114: 243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H 1970. Cell communciation, calcium ion, and cyclic adenosine monophosphate. Science 170: 404–412 [DOI] [PubMed] [Google Scholar]
- Richardson A, Taylor CW 1993. Effects of Ca2+ chelators on purified inositol 1,4,5-trisphosphate (InsP3) receptors and InsP3-stimulated Ca2+ mobilization. J Biol Chem 268: 11528–11533 [PubMed] [Google Scholar]
- Ridgway EB, Gilkey JC, Jaffe LF 1977. Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci USA 74: 623–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringer S 1883. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol 4: 29–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, Brini M, Murgia M, Pozzan T 1993. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighbouring mitochondria. Science 262: 744–747 [DOI] [PubMed] [Google Scholar]
- Rodney GG, Moore CP, Williams BY, Zhang J-Z, Krol J, Pedersen SE, Hamilton SL 2001. Calcium binding to calmodulin leads to an N-terminal shift in its binding site on the ryanodine receptor. J Biol Chem 276: 2069–2074 [DOI] [PubMed] [Google Scholar]
- Rossi AM, Riley AM, Potter BVL, Taylor CW 2010. Adenophostins: high-affinity agonists of IP3 receptors. Curr Topics Membr 66: 209–233 [DOI] [PubMed] [Google Scholar]
- Rossi AM, Riley AM, Tovey SC, Rahman T, Dellis O, Taylor EJA, Veresov VG, Potter BVL, Taylor CW 2009. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat Chem Biol 5: 631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samso M, Feng W, Pessah IN, Allen PD 2009. Coordinated movement of cytoplasmic and transmembrane domains of RyR1 upon gating. PLoS Biol 7: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samso M, Wagenknecht T, Allen PD 2005. Internal structure and visualization of transmembrane domains of the RyR1calcium release channel by cryo-EM. Nat Struct Mol Biol 6: 539–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato C, Hamada K, Ogura T, Miyazawa A, Iwasaki K, Hiroaki Y, Tani K, Terauchi A, Fujiyoshi Y, Mikoshiba K 2004. Inositol 1,4,5-trisphosphate receptor contains multiple cavities and L-shaped ligand-binding domains. J Mol Biol 336: 155–164 [DOI] [PubMed] [Google Scholar]
- Schug ZT, da Fonseca PC, Bhanumathy CD, Wagner L 2nd, Zhang X, Bailey B, Morris EP, Yule DI, Joseph SK 2008. Molecular characterization of the inositol 1,4,5-trisphosphate receptor pore-forming segment. J Biol Chem 283: 2939–2948 [DOI] [PubMed] [Google Scholar]
- Schug ZT, Joseph SK 2006. The role of the S4-S5 linker and C-terminal tail in inositol 1,4,5-trisphosphate receptor function. J Biol Chem 281: 24431–24440 [DOI] [PubMed] [Google Scholar]
- Serysheva II, Bare DJ, Ludtke SJ, Kettlun CS, Chiu W, Mignery GA 2003. Structure of the type 1 inositol 1,4,5-trisphosphate receptor revealed by cryomicroscopy. J Biol Chem 278: 21319–21322 [DOI] [PubMed] [Google Scholar]
- Serysheva II, Hamilton SL, Chiu W, Ludtke SJ 2005. Structure of a Ca2+ release channel at 14Å resolution. J Struct Biol 345: 427–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth TJ 1992. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+]. J Biol Chem 267: 3573–3576 [PubMed] [Google Scholar]
- Sienaert I, De Smedt H, Parys JB, Missiaen L, Vanlingen S, Sipma H, Casteels R 1996. Characterization of a cytosolic and a luminal Ca2+ binding site in the type I inositol 1,4,5-trisphosphate receptor. J Biol Chem 271: 27005–27012 [DOI] [PubMed] [Google Scholar]
- Sienaert I, Kasri NN, Vanlingen S, Parys J, Callewaert G, Missiaen L, De Smedt H 2002. Localization and function of a calmodulin/apocalmodulin binding domain in the N-terminal part of the type 1 inositol 1,4,5-trisphosphate receptor. Biochem J 365: 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienaert I, Missiaen L, De Smedt H, Parys JB, Sipma H, Casteels R 1997. Molecular and functional evidence for multiple Ca2+-binding domains on the type 1 inositol 1,4,5-trisphosphate receptor J Biol Chem 272: 25899–25906 [DOI] [PubMed] [Google Scholar]
- Smith JS, Coronado R, Meissner G 1985. Sarcoplasmic reticulum contains adenine nucleotide-activated calcium channels. Nature 316: 446–449 [DOI] [PubMed] [Google Scholar]
- Solovyova N, Fernyhough P, Glazner G, Verkhratsky A 2002. Xestospongin C empties the ER calcium store but does not inhibit InsP3-induced Ca2+ release in cultured dorsal root ganglia neurones. Cell Calcium 32: 49–52 [DOI] [PubMed] [Google Scholar]
- Spät A, Bradford PG, McKinney JS, Rubin RP, Putney JW Jr 1986. A saturable receptor for 32P-inositol-1,4,5-trisphosphate in hepatocytes and neutrophils. Nature 319: 514–516 [DOI] [PubMed] [Google Scholar]
- Stehno-Bittel L, Lückhoff A, Clapham DE 1995. Calcium release from the nucleus by InsP3 receptor channels. Neuron 14: 163–167 [DOI] [PubMed] [Google Scholar]
- Streb H, Irvine RF, Berridge MJ, Schulz I 1983. Release of Ca2+ from a nonmitochondrial store of pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306: 67–69 [DOI] [PubMed] [Google Scholar]
- Striggow F, Ehrlich BE 1996. The inositol 1,4,5-trisphosphate receptor of cerebellum. Mn2+ permeability and regulation by cytosolic Mn2+. J Gen Physiol 108: 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu E, Hirata M, Hashimoto T, Kuriyama H 1984. Inositol 1,4,5-trisphosphate releases Ca2+ from intracellular store sites in skinned single cells of porcine coronary artery. Biochem Biophys Res Commun 120: 481–485 [DOI] [PubMed] [Google Scholar]
- Sun Y, Taylor CW 2008. A calmodulin antagonist reveals a calmodulin-independent interdomain interaction essential for activation of inositol 1,4,5-trisphosphate receptors. Biochem J 416: 243–253 [DOI] [PubMed] [Google Scholar]
- Supattapone S, Worley PF, Baraban JM, Snyder SH 1988. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem 263: 1530–1534 [PubMed] [Google Scholar]
- Sureshan KM, Riley AM, Rossi AM, Tovey SC, Dedos SG, Taylor CW, Potter BVL 2009. Activation of IP3 receptors by synthetic bisphosphate ligands. Chem Commun 14: 1204–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko JL, Ito K, Kenyon JL 1985. Ryanodine: a modifier of sarcoplasmic reticulum calcium release in striated muscle. FASEB J 44: 2984–2988 [PubMed] [Google Scholar]
- Swatton JE, Morris SA, Cardy TJA, Taylor CW 1999. Type 3 inositol trisphosphate receptors in RINm5F cells are biphasically regulated by cytosolic Ca2+ and mediate quantal Ca2+ mobilization. Biochem J 344: 55–60 [PMC free article] [PubMed] [Google Scholar]
- Swatton JE, Taylor CW 2002. Fast biphasic regulation of type 3 inositol trisphosphate receptors by cytosolic calcium. J Biol Chem 277: 17571–17579 [DOI] [PubMed] [Google Scholar]
- Szlufcik K, Bultynck G, Callewaert G, Missiaen L, Parys JB, De Smedt H 2006. The suppressor domain of inositol 1,4,5-trisphosphate receptor plays an essential role in the protection against apoptosis. Cell Calcium 39: 325–336 [DOI] [PubMed] [Google Scholar]
- Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. 1989. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339: 439–445 [DOI] [PubMed] [Google Scholar]
- Tanimura A, Turner RJ 1996. Calcium release in HSY cells conforms to a steady-state mechanism involving regulation of the inositol 1,4,5-trisphosphate receptor Ca2+ channel by luminal [Ca2+]. J Cell Biol 132: 607–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi H, Yano M, Mochizuki M, Suetomi T, Ono M, Xu X, Uchinoumi H, Okuda S, Oda T, Kobayashi S, et al. 2009. Defective domain-domain interactions within the ryanodine receptor as a critical cause of diastolic Ca2+ leak in failing hearts. Cardiovasc Res 81: 536–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, da Fonseca PCA, Morris EP 2004. IP3 receptors: the search for structure. Trends Biochem Sci 29: 210–219 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Genazzani AA, Morris SA 1999. Expression of inositol trisphosphate receptors. Cell Calcium 26: 237–251 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Laude AJ 2002. IP3 receptors and their regulation by calmodulin and cytosolic Ca2+. Cell Calcium 32: 321–334 [DOI] [PubMed] [Google Scholar]
- Taylor CW, Potter BVL 1990. The size of inositol 1,4,5-trisphosphate-sensitive Ca2+ stores depends on inositol 1,4,5-trisphosphate concentration. Biochem J 266: 189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Prole DL, Rahman T 2009a. Ca2+ channels on the move. Biochemistry 48: 12062–12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, Ur-Rahman T, Pantazaka E 2009b. Targeting and clustering of IP3 receptors: key determinants of spatially organized Ca2+ signals. Chaos 19: 037102-037101–037102-037110 [DOI] [PubMed] [Google Scholar]
- Terentyev D, Nori A, Santoro M, Viatchenko-Karpinski S, Kubalova Z, Gyorke I, Terentyeva R, Vedamoorthyrao S, Blom NA, Valle G, et al. 2006. Abnormal interactions of calsequestrin with the ryanodine receptor calcium release channel complex linked to exercise-induced sudden cardiac death. Circ Res 98: 1151–1158 [DOI] [PubMed] [Google Scholar]
- Thrower EC, Lea EJA, Dawson AP 1998. The effects of free [Ca2+] on the cytosolic face of inositol (1,4,5)-trisphosphate receptor at the single channel level. Biochem J 330: 559–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey SC, Dedos SG, Rahman T, Taylor EJA, Pantazaka E, Taylor CW 2010. Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J Biol Chem 285: 12979–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A, Meissner G 1996. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J 70: 2600–2615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RY 1980. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry 19: 2396–2404 [DOI] [PubMed] [Google Scholar]
- Tsien RY 1981. A non-disruptive technique for loading calcium buffers and indicators into cells. Nature 290: 527–528 [DOI] [PubMed] [Google Scholar]
- Tu H, Miyakawa T, Wang Z, Glouchankova L, Iino M, Bezprozvanny I 2002. Functional characterization of the type 1 inositol 1,4,5-trisphosphate receptor coupling doamain SII(±) splice variants and the Opisthotonos mutant form. Biophys J 82: 1995–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Nosyreva E, Miyakawa T, Wang Z, Mizushima A, Iino M, Bezprozvanny I 2003. Functional and biochemical analysis of the type 1 inositol (1,4,5)-trisphosphate receptor calcium sensor. Biophys J 85: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Wang Z, Bezprozvanny I 2005a. Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys J 88: 1056–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I 2005b. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J 88: 1046–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K, Miyauchi H, Furuichi T, Michikawa T, Mikoshiba K 2003. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J Biol Chem 278: 16551–16560 [DOI] [PubMed] [Google Scholar]
- van de Put FHMM, De Pont JJHHM, Willems PHGM 1994. Heterogeneity between intracellular Ca2+ stores as the underlying principle of quantal Ca2+ release by inositol 1,4,5-trisphosphate in permeabilized pancreatic acinar cells. J Biol Chem 269: 12438–12443 [PubMed] [Google Scholar]
- Varney MA, Rivera J, Lopez Bernal A, Watson SP 1990. Are there subtypes of the inositol 1,4,5-trisphosphate receptor? Biochem J 269: 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Chen W, Cai S, Zhang J, Bolstad J, Wagenknecht T, Liu Z, Chen SR 2007. Localization of an NH2-terminal disease-causing mutation hot spot to the “clamp” region in the three-dimensional structure of the cardiac ryanodine receptor. J Biol Chem 282: 17785–17793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang L, Bolstad J, Diao N, Brown C, Ruest L, Welch W, Williams AJ, Chen SR 2003. Residue Gln4863 within a predicted transmembrane sequence of the Ca2+ release channel (ryanodine receptor) is critical for ryanodine interaction. J Biol Chem 278: 51557–51565 [DOI] [PubMed] [Google Scholar]
- Wang Y, Xu L, Pasek DA, Gillespie D, Meissner G 2005. Probing the role of negatively charged amino acid residues in ion permeation of skeletal muscle ryanodine receptor. Biophys J 89: 256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watras J, Bezprozvanny I, Ehrlich BE 1991. Inositol 1,4,5-trisphosphate-gated channels in cerebellum: presence of multiple conductance states. J Neurosci 11: 3239–3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AJ, West DJ, Sitsapesan R 2001. Light at the end of the Ca2+-release channel tunnel: structures and mechanisms involved in ion translocation in ryanodine receptor channels. Quart Rev Biophys 34: 61–104 [DOI] [PubMed] [Google Scholar]
- Williams JA 1980. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol 238: G269–G279 [DOI] [PubMed] [Google Scholar]
- Willoughby D, Cooper DM 2007. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010 [DOI] [PubMed] [Google Scholar]
- Wolfram F, Morris E, Taylor CW 2010. Three-dimensional structure of recombinant type 1 inositol 1,4,5-trisphosphate receptor. Biochem J 428: 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods NM, Cuthbertson KSR, Cobbold PH 1986. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature 319: 600–602 [DOI] [PubMed] [Google Scholar]
- Worley PF, Baraban JM, Supattapone S, Wilson VS, Snyder SH 1987. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem 262: 12132–12136 [PubMed] [Google Scholar]
- Yamada M, Miyawaki A, Saito K, Yamamoto-Hino M, Ryo Y, Furuichi T, Mikoshiba K 1995. The calmodulin-binding domain in the mouse type 1 inositol 1,4,5-trisphosphate receptor. Biochem J 308: 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, McBride S, Mak D-OD, Vardi N, Palczewski K, Haeseleer F, Foskett JK 2002. Identification of a family of calcium sensors as protein ligands of the inositol trisphosphate receptor Ca2+ release channels. Proc Natl Acad Sci USA 99: 7711–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshima H, Miyawaki A, Michikawa T, Furuichi T, Mikoshiba K 1997. Ca2+ differentially regulates ligand-affinity states of type 1 and 3 inositol 1,4,5-trisphosphate receptors. Biochem J 322: 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Mak DD, Li Q, Shin DM, Foskett JK, Muallem S 2003. A new mode of Ca2+ signaling by G protein-coupled receptors: gating of IP3 receptor Ca2+ release channels by Gβγ. Curr Biol 13: 872–876 [DOI] [PubMed] [Google Scholar]
- Zhang X, Joseph SK 2001. Effect of mutation of a calmodulin-binding sites on Ca2+ regulation of inositol trisphosphate receptors. Biochem J 360: 395–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM 2010. Calcium signaling via two-pore channels: local or global, that is the question. Am J Physiol 298: C430–C441 [DOI] [PMC free article] [PubMed] [Google Scholar]