Abstract
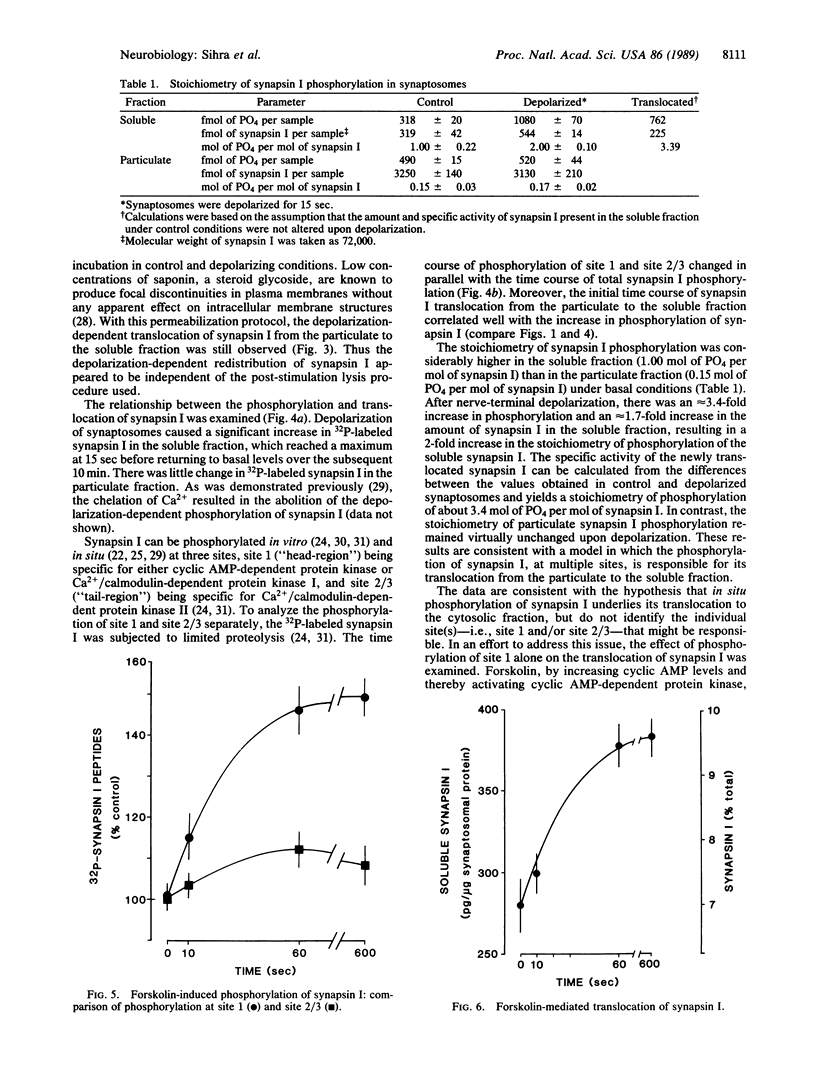
Depolarization of isolated nerve terminals (synaptosomes) has been shown to stimulate neurotransmitter release and to increase the phosphorylation state of a number of proteins, including synapsin I, in a Ca2+-dependent manner. Synapsin I, a prominent nerve terminal phosphoprotein, interacts with the cytoplasmic surface of small synaptic vesicles and with cytoskeletal elements in a phosphorylation-dependent manner. In the present study we have found that depolarization of synaptosomes resulted in a rapid (2-5 sec) translocation of synapsin I from the particulate to the cytosolic (soluble) fraction. This translocation of synapsin I correlated with its phosphorylation state and was dependent on the presence of Ca2+ in the incubation medium. The stoichiometry of phosphorylation of soluble synapsin I was considerably higher than that of synapsin I in the particulate fraction, under both basal and depolarizing conditions. These data are consistent with the hypothesis that, in situ, the phosphorylation of synapsin I promotes its translocation from synaptic vesicles/cytoskeleton to the cytosol. This phosphorylation/translocation may be instrumental in regulating the release of neurotransmitter.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines A. J., Bennett V. Synapsin I is a microtubule-bundling protein. Nature. 1986 Jan 9;319(6049):145–147. doi: 10.1038/319145a0. [DOI] [PubMed] [Google Scholar]
- Benfenati F., Bähler M., Jahn R., Greengard P. Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J Cell Biol. 1989 May;108(5):1863–1872. doi: 10.1083/jcb.108.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfenati F., Greengard P., Brunner J., Bähler M. Electrostatic and hydrophobic interactions of synapsin I and synapsin I fragments with phospholipid bilayers. J Cell Biol. 1989 May;108(5):1851–1862. doi: 10.1083/jcb.108.5.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks J. C., Treml S. Catecholamine secretion by chemically skinned cultured chromaffin cells. J Neurochem. 1983 Feb;40(2):468–473. doi: 10.1111/j.1471-4159.1983.tb11306.x. [DOI] [PubMed] [Google Scholar]
- Bähler M., Benfenati F., Valtorta F., Czernik A. J., Greengard P. Characterization of synapsin I fragments produced by cysteine-specific cleavage: a study of their interactions with F-actin. J Cell Biol. 1989 May;108(5):1841–1849. doi: 10.1083/jcb.108.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler M., Greengard P. Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature. 1987 Apr 16;326(6114):704–707. doi: 10.1038/326704a0. [DOI] [PubMed] [Google Scholar]
- Czernik A. J., Pang D. T., Greengard P. Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7518–7522. doi: 10.1073/pnas.84.21.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Cameron R., Greengard P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol. 1983 May;96(5):1337–1354. doi: 10.1083/jcb.96.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Greengard P. Synapsin I: a synaptic vesicle-associated neuronal phosphoprotein. Biochem Pharmacol. 1986 Dec 15;35(24):4349–4357. doi: 10.1016/0006-2952(86)90747-1. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Harris S. M., Jr, Huttner W. B., Greengard P. Synapsin I (Protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol. 1983 May;96(5):1355–1373. doi: 10.1083/jcb.96.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley P. R., Jarvie P. E., Heath J. W., Kidd G. J., Rostas J. A. A rapid method for isolation of synaptosomes on Percoll gradients. Brain Res. 1986 Apr 30;372(1):115–129. doi: 10.1016/0006-8993(86)91464-2. [DOI] [PubMed] [Google Scholar]
- Gandy S., Czernik A. J., Greengard P. Phosphorylation of Alzheimer disease amyloid precursor peptide by protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6218–6221. doi: 10.1073/pnas.85.16.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S. E., Nestler E. J., Chehrazi B., Greengard P. Distribution of protein I in mammalian brain as determined by a detergent-based radioimmunoassay. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2130–2134. doi: 10.1073/pnas.78.4.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenring J. R., Lasher R. S., Vallano M. L., Ueda T., Naito S., Sternberger N. H., Sternberger L. A., DeLorenzo R. J. Association of synapsin I with neuronal cytoskeleton. Identification in cytoskeletal preparations in vitro and immunocytochemical localization in brain of synapsin I. J Biol Chem. 1986 Jun 25;261(18):8495–8504. [PubMed] [Google Scholar]
- Greengard P. Neuronal phosphoproteins. Mediators of signal transduction. Mol Neurobiol. 1987 Spring-Summer;1(1-2):81–119. doi: 10.1007/BF02935265. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Sobue K., Kanda K., Harada A., Yorifuji H. The cytoskeletal architecture of the presynaptic terminal and molecular structure of synapsin 1. J Cell Biol. 1989 Jan;108(1):111–126. doi: 10.1083/jcb.108.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., DeGennaro L. J., Greengard P. Differential phosphorylation of multiple sites in purified protein I by cyclic AMP-dependent and calcium-dependent protein kinases. J Biol Chem. 1981 Feb 10;256(3):1482–1488. [PubMed] [Google Scholar]
- Huttner W. B., Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B., Greengard P. Two calcium/calmodulin-dependent protein kinases, which are highly concentrated in brain, phosphorylate protein I at distinct sites. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1293–1297. doi: 10.1073/pnas.78.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger B. K., Forn J., Greengard P. Depolarization-induced phosphorylation of specific proteins, mediated by calcium ion influx, in rat brain synaptosomes. J Biol Chem. 1977 Apr 25;252(8):2764–2773. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., McGuinness T. L., Leonard C. S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci U S A. 1985 May;82(9):3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navone F., Greengard P., De Camilli P. Synapsin I in nerve terminals: selective association with small synaptic vesicles. Science. 1984 Dec 7;226(4679):1209–1211. doi: 10.1126/science.6438799. [DOI] [PubMed] [Google Scholar]
- Schiebler W., Jahn R., Doucet J. P., Rothlein J., Greengard P. Characterization of synapsin I binding to small synaptic vesicles. J Biol Chem. 1986 Jun 25;261(18):8383–8390. [PubMed] [Google Scholar]
- Schweinsberg P. D., Loo T. L. Simultaneous analysis of ATP, ADP, AMP, and other purines in human erythrocytes by high-performance liquid chromatography. J Chromatogr. 1980 Jan 11;181(1):103–107. doi: 10.1016/s0378-4347(00)81276-1. [DOI] [PubMed] [Google Scholar]
- Steiner J. P., Ling E., Bennett V. Nearest neighbor analysis for brain synapsin I. Evidence from in vitro reassociation assays for association with membrane protein(s) and the Mr = 68,000 neurofilament subunit. J Biol Chem. 1987 Jan 15;262(2):905–914. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T., Greengard P. Adenosine 3':5'-monophosphate-regulated phosphoprotein system of neuronal membranes. I. Solubilization, purification, and some properties of an endogenous phosphoprotein. J Biol Chem. 1977 Jul 25;252(14):5155–5163. [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Greengard P. Protein phosphorylation in nerve terminals: comparison of calcium/calmodulin-dependent and calcium/diacylglycerol-dependent systems. J Neurosci. 1988 Jan;8(1):281–288. doi: 10.1523/JNEUROSCI.08-01-00281.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. K., Walaas S. I., Sihra T. S., Aderem A., Greengard P. Phosphorylation and associated translocation of the 87-kDa protein, a major protein kinase C substrate, in isolated nerve terminals. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2253–2256. doi: 10.1073/pnas.86.7.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]



