Abstract
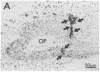
In situ hybridization histochemistry and immunocytochemistry were used to study the prenatal expression of luteinizing hormone-releasing hormone (LHRH) cells in the mouse. Cells expressing LHRH mRNA and peptide product were first detected on embryonic day 11.5 (E11.5) in the olfactory pit. On E12.5, the majority of LHRH cells were located on "tracks" extending from the olfactory pit to the base of the telencephalon. From E12.5 to E15.5, LHRH cells were detected in a rostral-to-caudal gradient in forebrain areas. Prior to E12.5, cells expressing LHRH mRNA were not detected in forebrain areas known to contain LHRH cells in postnatal animals. Quantitation of cells expressing LHRH mRNA showed that the number of labeled cells on E12.5 (approximately 800) equaled the number of LHRH cells in postnatal animals, but more than 90% of these cells were located in nasal regions. Between E12.5 and E15.5, the location of LHRH cells shifted. The number of LHRH cells in the forebrain increased, while the number of LHRH cells in nasal regions decreased over this same period. These findings establish that cells first found in the olfactory pit and thereafter in forebrain areas express the LHRH gene and correspond to the position of LHRH immunopositive cells found at these developmental times. To further examine the ontogeny of the LHRH system, immunocytochemistry in combination with [3H]thymidine autoradiography was used to determine when LHRH cells left the mitotic cycle. We show that LHRH neurons exhibit a discrete time of birth, suggesting that they arise as a single neuronal population between E10.0 and E11.0. Postnatal LHRH neurons were "birth-dated" shortly after differentiation of the olfactory placode and before LHRH mRNA was expressed in cells in the olfactory pit. Taken together, these studies support the hypothesis that all LHRH cells in the central nervous system arise from a discrete group of progenitor cells in the olfactory placode and that a subpopulation of these cells migrate into forebrain areas where they subsequently establish an adult-like distribution.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnefeld F. W., Dick W., Kilian J., Steuer W. Die Desinfektion von Beatmungsgeräten mit Formaldehyd im Aseptor. Offentl Gesundheitswes. 1980 Feb;42(2):79–83. [PubMed] [Google Scholar]
- Bojsen-Moller F. Demonstration of terminalis, olfactory, trigeminal and perivascular nerves in the rat nasal septum. J Comp Neurol. 1975 Jan 15;159(2):245–256. doi: 10.1002/cne.901590206. [DOI] [PubMed] [Google Scholar]
- Elkabes S., Loh Y. P., Nieburgs A., Wray S. Prenatal ontogenesis of pro-opiomelanocortin in the mouse central nervous system and pituitary gland: an in situ hybridization and immunocytochemical study. Brain Res Dev Brain Res. 1989 Mar 1;46(1):85–95. doi: 10.1016/0165-3806(89)90145-4. [DOI] [PubMed] [Google Scholar]
- Garcia M. S., Schwanzel-Fukuda M., Morrell J. I., Pfaff D. W. Immunochemical studies of the location of luteinizing hormone-releasing hormone neurons in the nervus terminalis of the mouse. Ann N Y Acad Sci. 1987;519:465–468. doi: 10.1111/j.1749-6632.1987.tb36317.x. [DOI] [PubMed] [Google Scholar]
- Hoffman G. E., Finch C. E. LHRH neurons in the female C57BL/6J mouse brain during reproductive aging: no loss up to middle age. Neurobiol Aging. 1986 Jan-Feb;7(1):45–48. doi: 10.1016/0197-4580(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Mason A. J., Hayflick J. S., Zoeller R. T., Young W. S., 3rd, Phillips H. S., Nikolics K., Seeburg P. H. A deletion truncating the gonadotropin-releasing hormone gene is responsible for hypogonadism in the hpg mouse. Science. 1986 Dec 12;234(4782):1366–1371. doi: 10.1126/science.3024317. [DOI] [PubMed] [Google Scholar]
- Oelschläger H. A., Buhl E. H., Dann J. F. Development of the nervus terminalis in mammals including toothed whales and humans. Ann N Y Acad Sci. 1987;519:447–464. doi: 10.1111/j.1749-6632.1987.tb36316.x. [DOI] [PubMed] [Google Scholar]
- Okamura H., Fukui K., Koyama E., Tsutou H. L., Tsutou T., Terubayashi H., Fujisawa H., Ibata Y. Time of vasopressin neuron origin in the mouse hypothalamus: examination by combined technique of immunocytochemistry and [3H]thymidine autoradiography. Brain Res. 1983 Aug;285(2):223–226. doi: 10.1016/0165-3806(83)90055-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Garcia M. S., Morrell J. I., Pfaff D. W. Distribution of luteinizing hormone-releasing hormone in the nervus terminalis and brain of the mouse detected by immunocytochemistry. J Comp Neurol. 1987 Jan 8;255(2):231–244. doi: 10.1002/cne.902550207. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M., Pfaff D. W. Origin of luteinizing hormone-releasing hormone neurons. Nature. 1989 Mar 9;338(6211):161–164. doi: 10.1038/338161a0. [DOI] [PubMed] [Google Scholar]
- Shimada M., Nakamura T. Time of neuron origin in mouse hypothalamic nuclei. Exp Neurol. 1973 Oct;41(1):163–173. doi: 10.1016/0014-4886(73)90187-8. [DOI] [PubMed] [Google Scholar]
- Whitnall M. H., Key S., Ben-Barak Y., Ozato K., Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. II. Immunocytochemical studies of the ontogeny of oxytocinergic and vasopressinergic neurons. J Neurosci. 1985 Jan;5(1):98–109. doi: 10.1523/JNEUROSCI.05-01-00098.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S., Gähwiler B. H., Gainer H. Slice cultures of LHRH neurons in the presence and absence of brainstem and pituitary. Peptides. 1988 Sep-Oct;9(5):1151–1175. doi: 10.1016/0196-9781(88)90103-9. [DOI] [PubMed] [Google Scholar]
- Wray S., Nieburgs A., Elkabes S. Spatiotemporal cell expression of luteinizing hormone-releasing hormone in the prenatal mouse: evidence for an embryonic origin in the olfactory placode. Brain Res Dev Brain Res. 1989 Apr 1;46(2):309–318. doi: 10.1016/0165-3806(89)90295-2. [DOI] [PubMed] [Google Scholar]
- Wray S., Zoeller R. T., Gainer H. Differential effects of estrogen on luteinizing hormone-releasing hormone gene expression in slice explant cultures prepared from specific rat forebrain regions. Mol Endocrinol. 1989 Aug;3(8):1197–1206. doi: 10.1210/mend-3-8-1197. [DOI] [PubMed] [Google Scholar]
- Zoeller R. T., Seeburg P. H., Young W. S., 3rd In situ hybridization histochemistry for messenger ribonucleic acid (mRNA) encoding gonadotropin-releasing hormone (GnRH): effect of estrogen on cellular levels of GnRH mRNA in female rat brain. Endocrinology. 1988 Jun;122(6):2570–2577. doi: 10.1210/endo-122-6-2570. [DOI] [PubMed] [Google Scholar]
- Zoeller R. T., Young W. S., 3rd Changes in cellular levels of messenger ribonucleic acid encoding gonadotropin-releasing hormone in the anterior hypothalamus of female rats during the estrous cycle. Endocrinology. 1988 Sep;123(3):1688–1689. doi: 10.1210/endo-123-3-1688. [DOI] [PubMed] [Google Scholar]