Abstract
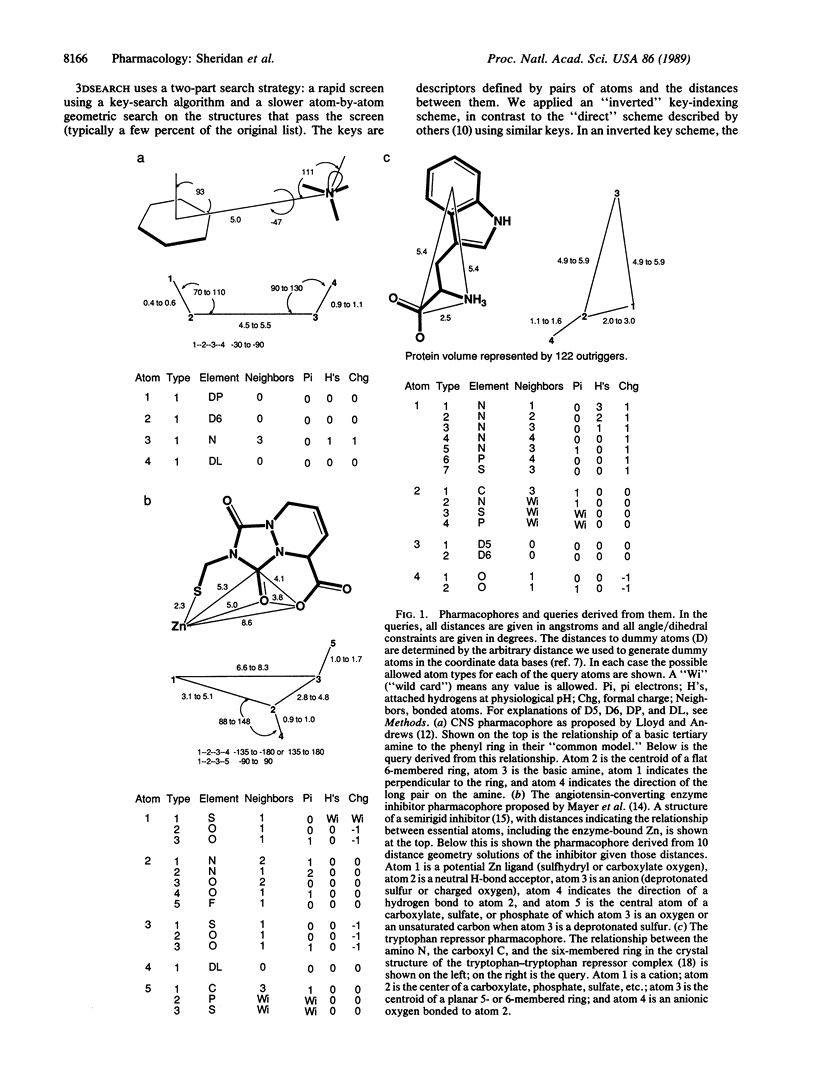
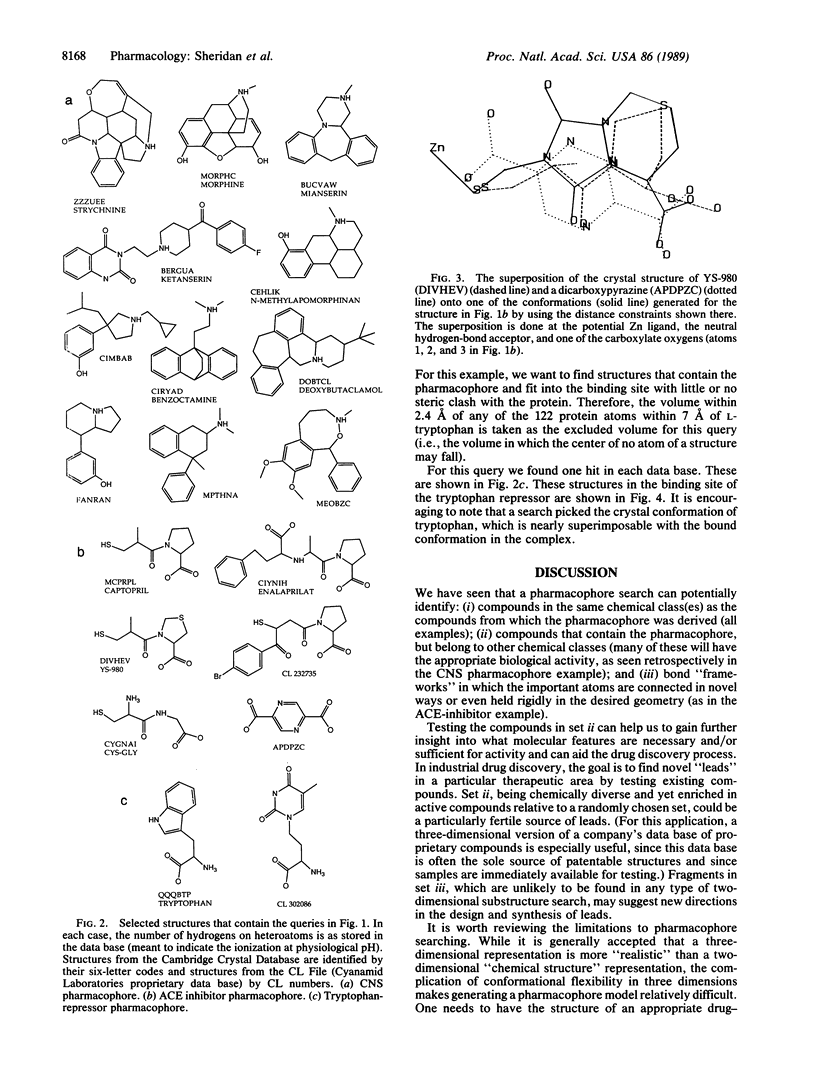
Pharmacophores, three-dimensional arrangements of chemical groups essential for biological activity, are being proposed in increasing numbers. We have developed a system to search data bases of three-dimensional coordinates for compounds that contain a particular pharmacophore. The coordinates can be derived from experiment (e.g., Cambridge Crystal Database) or be generated from data bases of connection tables (e.g., Cyanamid Laboratories proprietary compounds) via the program CONCORD. We discuss the results of searches for three sample pharmacophores. Two have been proposed by others based on the conformational analysis of active compounds, and one is inferred from the crystal structure of a protein-ligand complex. These examples show that such searches can identify classes of compounds that are structurally different from the compounds from which the pharmacophore was derived but are known to have the appropriate biological activity. Occasionally, the searches find bond "frameworks" in which the important groups are rigidly held in the proper geometry. These may suggest new structural classes for synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Cheung H. S., Wang F. L., Ondetti M. A., Sabo E. F., Cushman D. W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. Importance of the COOH-terminal dipeptide sequence. J Biol Chem. 1980 Jan 25;255(2):401–407. [PubMed] [Google Scholar]
- Crippen G. M. Distance geometry approach to rationalizing binding data. J Med Chem. 1979 Aug;22(8):988–997. doi: 10.1021/jm00194a020. [DOI] [PubMed] [Google Scholar]
- Hassall C. H., Kröhn A., Moody C. J., Thomas W. A. The design of a new group of angiotensin-converting enzyme inhibitors. FEBS Lett. 1982 Oct 18;147(2):175–179. doi: 10.1016/0014-5793(82)81036-3. [DOI] [PubMed] [Google Scholar]
- Lloyd E. J., Andrews P. R. A common structural model for central nervous system drugs and their receptors. J Med Chem. 1986 Apr;29(4):453–462. doi: 10.1021/jm00154a005. [DOI] [PubMed] [Google Scholar]
- Martin Y. C., Danaher E. B., May C. S., Weininger D. MENTHOR, a database system for the storage and retrieval of three-dimensional molecular structures and associated data searchable by substructural, biologic, physical, or geometric properties. J Comput Aided Mol Des. 1988 Apr;2(1):15–29. doi: 10.1007/BF01532050. [DOI] [PubMed] [Google Scholar]
- Mayer D., Naylor C. B., Motoc I., Marshall G. R. A unique geometry of the active site of angiotensin-converting enzyme consistent with structure-activity studies. J Comput Aided Mol Des. 1987 Apr;1(1):3–16. doi: 10.1007/BF01680553. [DOI] [PubMed] [Google Scholar]
- McEvoy F. J., Lai F. M., Albright J. D. Antihypertensive agents: angiotensin converting enzyme inhibitors. 1-[3-(Acylthio)-3-aroylpropionyl]-L-prolines. J Med Chem. 1983 Mar;26(3):381–393. doi: 10.1021/jm00357a013. [DOI] [PubMed] [Google Scholar]
- Schevitz R. W., Otwinowski Z., Joachimiak A., Lawson C. L., Sigler P. B. The three-dimensional structure of trp repressor. 1985 Oct 31-Nov 6Nature. 317(6040):782–786. doi: 10.1038/317782a0. [DOI] [PubMed] [Google Scholar]
- Sheridan R. P., Nilakantan R., Dixon J. S., Venkataraghavan R. The ensemble approach to distance geometry: application to the nicotinic pharmacophore. J Med Chem. 1986 Jun;29(6):899–906. doi: 10.1021/jm00156a005. [DOI] [PubMed] [Google Scholar]


