Abstract
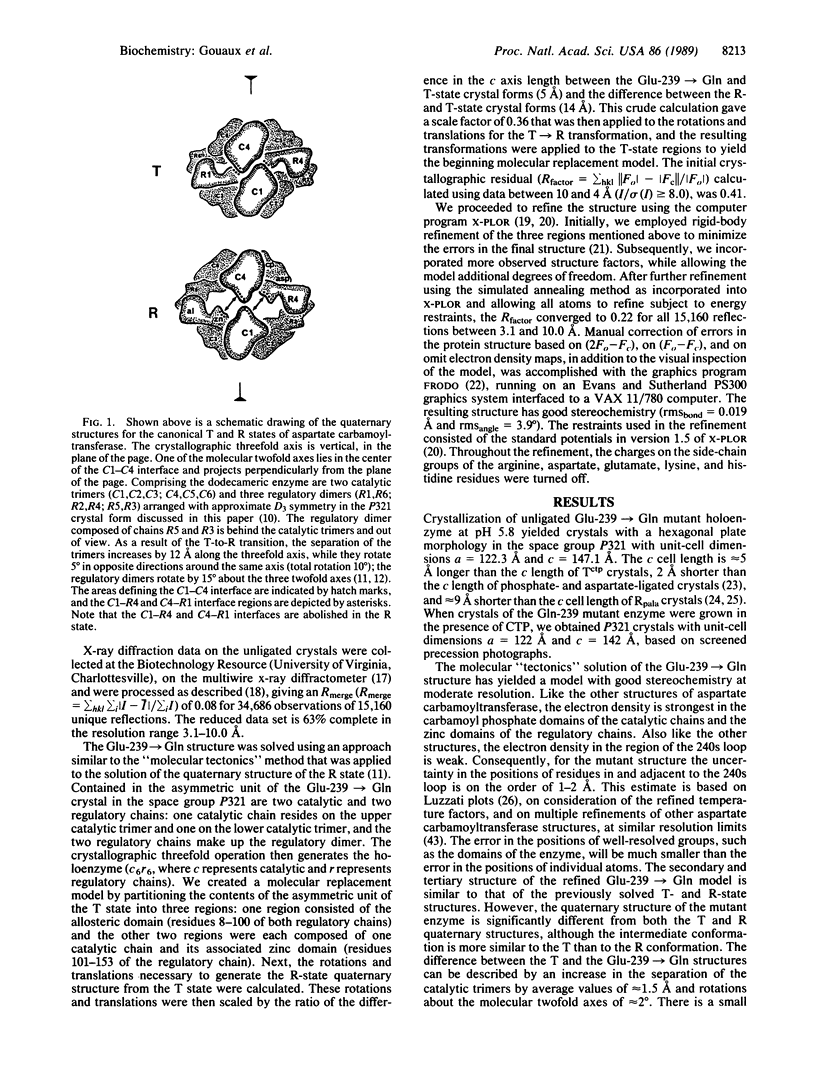
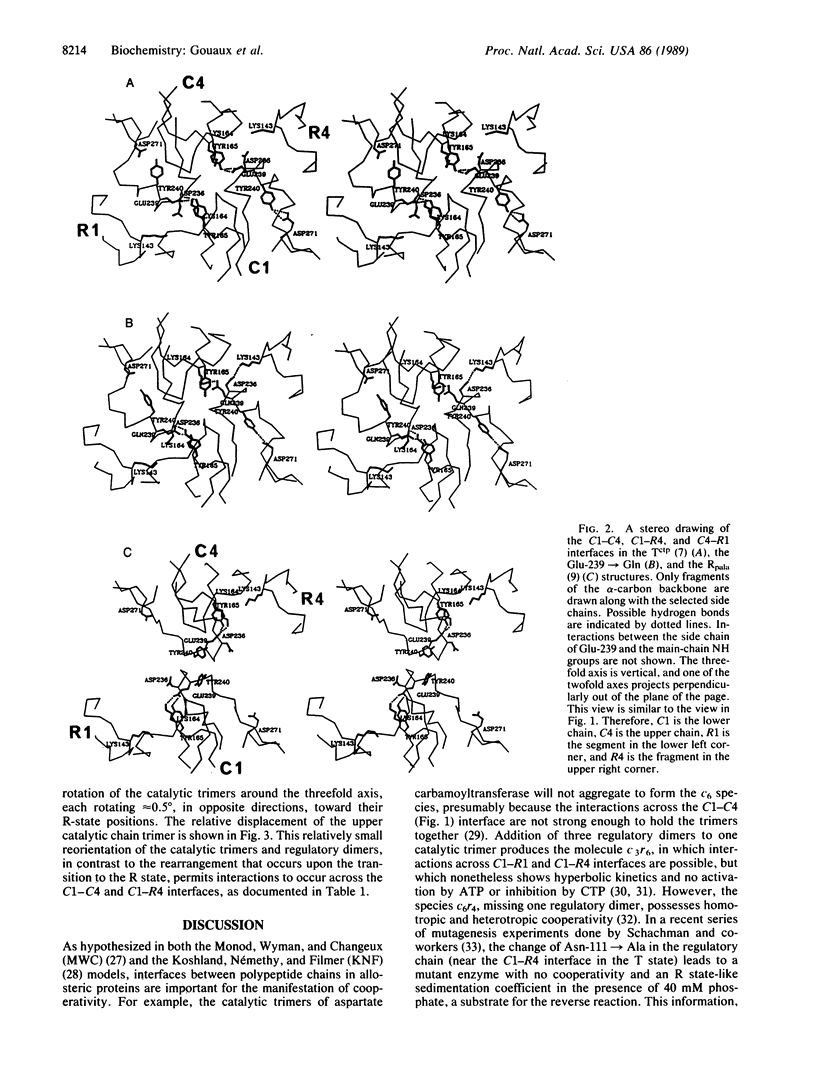
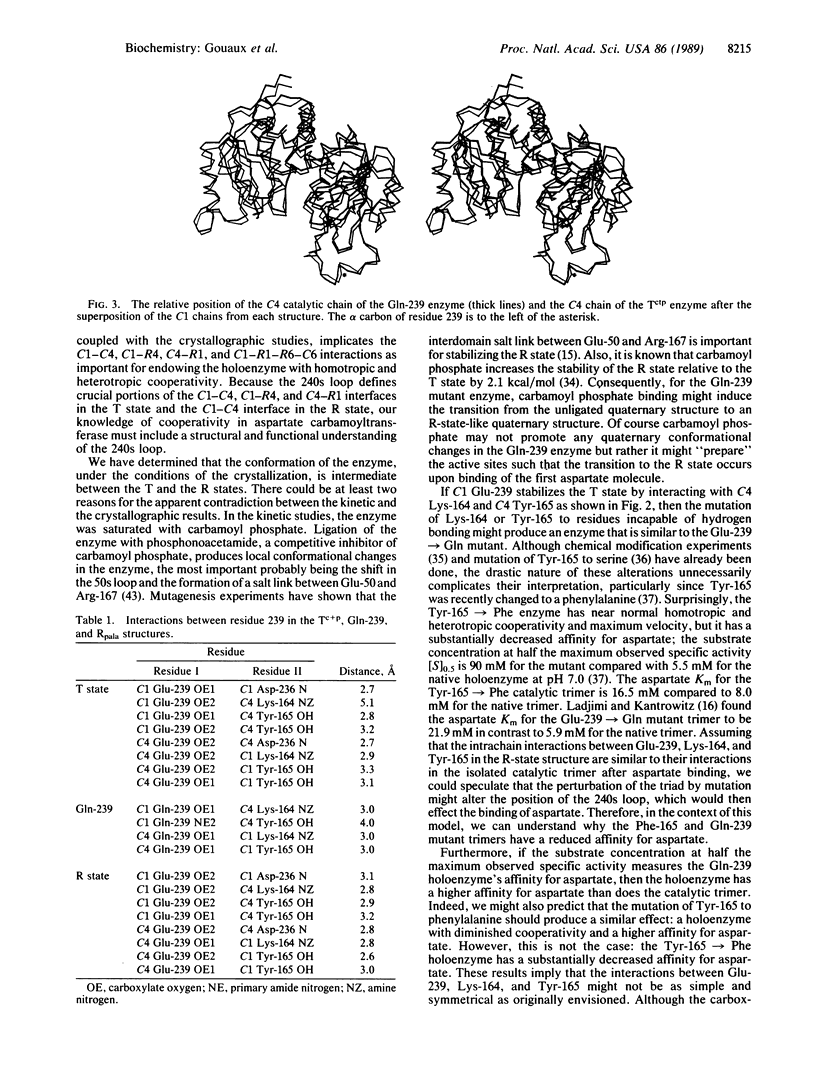
The structure of the unligated Glu 239----Gln mutant of Escherichia coli aspartate carbamoyltransferase (EC 2.1.3.2) has been determined to 3.1-A resolution and refined to a crystallographic residual of 0.22 in the space group P321. The unit-cell dimensions of the unligated enzyme are a = 122.3 A, c = 147.1 A. The c axis cell length is intermediate between the c axis lengths of the T (tense)(c = 142.2 A) and R (relaxed) (c = 156.2 A) state structures. Furthermore, the quaternary structure of the mutant enzyme is intermediate between the quaternary structures of the T form and the R form. The differences between the quaternary structures of the Glu-239----Gln and T-form enzymes can be described as follows: the separation between the catalytic trimers increases by approximately 1.5 A along the threefold axis, and they each rotate in opposite directions approximately 0.5 degree around the threefold axis, whereas the regulatory dimers rotate approximately 2 degrees around the twofold axes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman R. B., Ladner J. E., Lipscomb W. N. Quaternary structural changes in aspartate carbamoyltransferase of Escherichia coli at pH 8.3 and pH 5.8. Biochem Biophys Res Commun. 1982 Sep 30;108(2):592–595. doi: 10.1016/0006-291x(82)90869-5. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Chan W. W. Subunit interactions in aspartate transcarbamylase. The interaction between catalytic and regulatory subunits and the effect of ligands. J Biol Chem. 1975 Jan 25;250(2):661–667. [PubMed] [Google Scholar]
- Cherfils J., Vachette P., Tauc P., Janin J. The pAR5 mutation and the allosteric mechanism of Escherichia coli aspartate carbamoyltransferase. EMBO J. 1987 Sep;6(9):2843–2847. doi: 10.1002/j.1460-2075.1987.tb02581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunin R., Jacobs A., Charlier D., Crabeel M., Hervé G., Glansdorff N., Piérard A. Structure-function relationship in allosteric aspartate carbamoyltransferase from Escherichia coli. I. Primary structure of a pyrI gene encoding a modified regulatory subunit. J Mol Biol. 1985 Dec 20;186(4):707–713. doi: 10.1016/0022-2836(85)90390-0. [DOI] [PubMed] [Google Scholar]
- Eisenstein E., Markby D. W., Schachman H. K. Changes in stability and allosteric properties of aspartate transcarbamoylase resulting from amino acid substitutions in the zinc-binding domain of the regulatory chains. Proc Natl Acad Sci U S A. 1989 May;86(9):3094–3098. doi: 10.1073/pnas.86.9.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. R., Pastra-Landis S. C., Lipscomb W. N. An intermediate complex in the dissociation of aspartate transcarbamylase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1351–1355. doi: 10.1073/pnas.71.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gao J., Kuczera K., Tidor B., Karplus M. Hidden thermodynamics of mutant proteins: a molecular dynamics analysis. Science. 1989 Jun 2;244(4908):1069–1072. doi: 10.1126/science.2727695. [DOI] [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Distinct subunits for the regulation and catalytic activity of aspartate transcarbamylase. Biochemistry. 1965 Jun;4(6):1054–1062. doi: 10.1021/bi00882a012. [DOI] [PubMed] [Google Scholar]
- Gouaux J. E., Lipscomb W. N., Middleton S. A., Kantrowitz E. R. Structure of a single amino acid mutant of aspartate carbamoyltransferase at 2.5-A resolution: implications for the cooperative mechanism. Biochemistry. 1989 Feb 21;28(4):1798–1803. doi: 10.1021/bi00430a056. [DOI] [PubMed] [Google Scholar]
- Gouaux J. E., Lipscomb W. N. Structural transitions in crystals of native aspartate carbamoyltransferase. Proc Natl Acad Sci U S A. 1989 Feb;86(3):845–848. doi: 10.1073/pnas.86.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Kantrowitz E. R., Lipscomb W. N. Escherichia coli aspartate transcarbamylase: the relation between structure and function. Science. 1988 Aug 5;241(4866):669–674. doi: 10.1126/science.3041592. [DOI] [PubMed] [Google Scholar]
- Ke H. M., Lipscomb W. N., Cho Y. J., Honzatko R. B. Complex of N-phosphonacetyl-L-aspartate with aspartate carbamoyltransferase. X-ray refinement, analysis of conformational changes and catalytic and allosteric mechanisms. J Mol Biol. 1988 Dec 5;204(3):725–747. doi: 10.1016/0022-2836(88)90365-8. [DOI] [PubMed] [Google Scholar]
- Kim K. H., Pan Z. X., Honzatko R. B., Ke H. M., Lipscomb W. N. Structural asymmetry in the CTP-liganded form of aspartate carbamoyltransferase from Escherichia coli. J Mol Biol. 1987 Aug 20;196(4):853–875. doi: 10.1016/0022-2836(87)90410-4. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. 2.5 A structure of aspartate carbamoyltransferase complexed with the bisubstrate analog N-(phosphonacetyl)-L-aspartate. J Mol Biol. 1987 Feb 5;193(3):527–553. doi: 10.1016/0022-2836(87)90265-8. [DOI] [PubMed] [Google Scholar]
- Krause K. L., Volz K. W., Lipscomb W. N. Structure at 2.9-A resolution of aspartate carbamoyltransferase complexed with the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1643–1647. doi: 10.1073/pnas.82.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladjimi M. M., Ghellis C., Feller A., Cunin R., Glansdorff N., Piérard A., Hervé G. Structure-function relationship in allosteric aspartate carbamoyltransferase from Escherichia coli. II. Involvement of the C-terminal region of the regulatory chain in homotropic and heterotropic interactions. J Mol Biol. 1985 Dec 20;186(4):715–724. doi: 10.1016/0022-2836(85)90391-2. [DOI] [PubMed] [Google Scholar]
- Ladjimi M. M., Kantrowitz E. R. A possible model for the concerted allosteric transition in Escherichia coli aspartate transcarbamylase as deduced from site-directed mutagenesis studies. Biochemistry. 1988 Jan 12;27(1):276–283. doi: 10.1021/bi00401a042. [DOI] [PubMed] [Google Scholar]
- Ladjimi M. M., Middleton S. A., Kelleher K. S., Kantrowitz E. R. Relationship between domain closure and binding, catalysis, and regulation in Escherichia coli aspartate transcarbamylase. Biochemistry. 1988 Jan 12;27(1):268–276. doi: 10.1021/bi00401a041. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Kitchell J. P., Honzatko R. B., Ke H. M., Volz K. W., Kalb A. J., Ladner R. C., Lipscomb W. N. Gross quaternary changes in aspartate carbamoyltransferase are induced by the binding of N-(phosphonacetyl)-L-aspartate: A 3.5-A resolution study. Proc Natl Acad Sci U S A. 1982 May;79(10):3125–3128. doi: 10.1073/pnas.79.10.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen A. M., Landfear S. M., Lipscomb W. N. Inactivation of the catalytic subunit of aspartate transcarbamylase by nitration with tetranitromethane. J Biol Chem. 1980 Jan 25;255(2):602–607. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Middleton S. A., Kantrowitz E. R. Function of arginine-234 and aspartic acid-271 in domain closure, cooperativity, and catalysis in Escherichia coli aspartate transcarbamylase. Biochemistry. 1988 Nov 15;27(23):8653–8660. doi: 10.1021/bi00423a022. [DOI] [PubMed] [Google Scholar]
- Middleton S. A., Stebbins J. W., Kantrowitz E. R. A loop involving catalytic chain residues 230-245 is essential for the stabilization of both allosteric forms of Escherichia coli aspartate transcarbamylase. Biochemistry. 1989 Feb 21;28(4):1617–1626. doi: 10.1021/bi00430a029. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort J. S., Chan W. W. Subunit interactions in aspartate transcarbamylase. Characterization of a complex between the catalytic and the regulatory subunits. J Biol Chem. 1975 Jan 25;250(2):653–660. [PubMed] [Google Scholar]
- PARDEE A. B., YATES R. A. Control of pyrimidine biosynthesis in Escherichia coli by a feed-back mechanism. J Biol Chem. 1956 Aug;221(2):757–770. [PubMed] [Google Scholar]
- Robey E. A., Schachman H. K. Site-specific mutagenesis of aspartate transcarbamoylase. Replacement of tyrosine 165 in the catalytic chain by serine reduces enzymatic activity. J Biol Chem. 1984 Sep 25;259(18):11180–11183. [PubMed] [Google Scholar]
- Wales M. E., Hoover T. A., Wild J. R. Site-specific substitutions of the Tyr-165 residue in the catalytic chain of aspartate transcarbamoylase promotes a T-state preference in the holoenzyme. J Biol Chem. 1988 May 5;263(13):6109–6114. [PubMed] [Google Scholar]
- Wild J. R., Loughrey-Chen S. J., Corder T. S. In the presence of CTP, UTP becomes an allosteric inhibitor of aspartate transcarbamoylase. Proc Natl Acad Sci U S A. 1989 Jan;86(1):46–50. doi: 10.1073/pnas.86.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley D. C., Lipscomb W. N. Crystallographic determination of symmetry of aspartate transcarbamylase. Nature. 1968 Jun 22;218(5147):1119–1121. doi: 10.1038/2181119a0. [DOI] [PubMed] [Google Scholar]


