Abstract
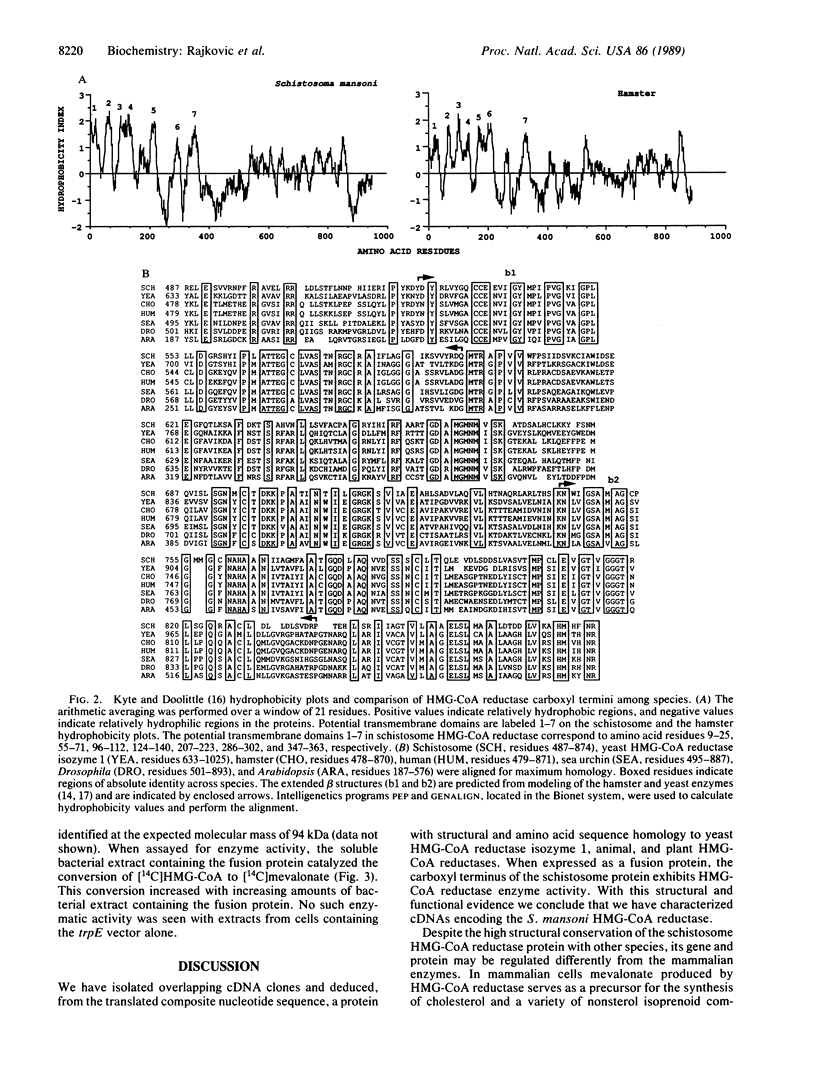
cDNA clones encoding the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase [(S)-mevalonate:NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34] from the human parasite Schistosoma mansoni have been isolated and characterized. The composite 3459 base pairs of cDNA sequence contains a 2844-base-pair open reading frame corresponding to a protein of 948 amino acids. The predicted S. mansoni HMG-CoA reductase protein contains a hydrophobic amino terminus consisting of seven potential transmembrane domains that are structurally conservative but are not identical in amino acid sequence with HMG-CoA reductases from other species. The hydrophilic carboxyl terminus of the S. mansoni HMG-CoA reductase protein, however, shares 48-52% sequence identity with the carboxyl termini of other HMG-CoA reductases in a region that contains the catalytic domain. When expressed as a fusion protein in Escherichia coli, the carboxyl-terminal domain of the schistosome protein exhibits HMG-CoA reductase enzyme activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson M. E., Thorsness M., Finer-Moore J., Stroud R. M., Rine J. Structural and functional conservation between yeast and human 3-hydroxy-3-methylglutaryl coenzyme A reductases, the rate-limiting enzyme of sterol biosynthesis. Mol Cell Biol. 1988 Sep;8(9):3797–3808. doi: 10.1128/mcb.8.9.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Chin D. J., Gil G., Russell D. W., Liscum L., Luskey K. L., Basu S. K., Okayama H., Berg P., Goldstein J. L., Brown M. S. Nucleotide sequence of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, a glycoprotein of endoplasmic reticulum. Nature. 1984 Apr 12;308(5960):613–617. doi: 10.1038/308613a0. [DOI] [PubMed] [Google Scholar]
- Davis R. E., Davis A. H., Carroll S. M., Rajkovic A., Rottman F. M. Tandemly repeated exons encode 81-base repeats in multiple, developmentally regulated Schistosoma mansoni transcripts. Mol Cell Biol. 1988 Nov;8(11):4745–4755. doi: 10.1128/mcb.8.11.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gertler F. B., Chiu C. Y., Richter-Mann L., Chin D. J. Developmental and metabolic regulation of the Drosophila melanogaster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol Cell Biol. 1988 Jul;8(7):2713–2721. doi: 10.1128/mcb.8.7.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Progress in understanding the LDL receptor and HMG-CoA reductase, two membrane proteins that regulate the plasma cholesterol. J Lipid Res. 1984 Dec 15;25(13):1450–1461. [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Klein P., Kanehisa M., DeLisi C. The detection and classification of membrane-spanning proteins. Biochim Biophys Acta. 1985 May 28;815(3):468–476. doi: 10.1016/0005-2736(85)90375-x. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Learned R. M., Fink G. R. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase from Arabidopsis thaliana is structurally distinct from the yeast and animal enzymes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2779–2783. doi: 10.1073/pnas.86.8.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum L., Finer-Moore J., Stroud R. M., Luskey K. L., Brown M. S., Goldstein J. L. Domain structure of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a glycoprotein of the endoplasmic reticulum. J Biol Chem. 1985 Jan 10;260(1):522–530. [PubMed] [Google Scholar]
- Luskey K. L., Stevens B. Human 3-hydroxy-3-methylglutaryl coenzyme A reductase. Conserved domains responsible for catalytic activity and sterol-regulated degradation. J Biol Chem. 1985 Aug 25;260(18):10271–10277. [PubMed] [Google Scholar]
- Lustigman S., Hamburger J. Schistosoma mansoni: radiometric assay of lectin binding specificities of the major egg glycoprotein and its carbohydrate-rich fragment. Exp Parasitol. 1985 Feb;59(1):59–67. doi: 10.1016/0014-4894(85)90057-8. [DOI] [PubMed] [Google Scholar]
- Meyer F., Meyer H., Bueding E. Lipid metabolism in the parasitic and free-living flatworms, Schistosoma mansoni and Dugesia dorotocephala. Biochim Biophys Acta. 1970 Jul 14;210(2):257–266. doi: 10.1016/0005-2760(70)90170-0. [DOI] [PubMed] [Google Scholar]
- Norden A. P., Strand M. Schistosoma mansoni, S. haematobium, and S. japonicum: identification of genus- and species-specific antigenic egg glycoproteins. Exp Parasitol. 1984 Dec;58(3):333–344. doi: 10.1016/0014-4894(84)90050-x. [DOI] [PubMed] [Google Scholar]
- Rumjanek F. D., Campos E. G., Afonso L. C. Evidence for the occurrence of LDL receptors in extracts of schistosomula of Schistosoma mansoni. Mol Biochem Parasitol. 1988 Mar;28(2):145–152. doi: 10.1016/0166-6851(88)90062-x. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Nordstrom J. L., Mitschelen J. J., Rodwell V. W., Schimke R. T. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974 Dec 29;370(2):369–377. doi: 10.1016/0005-2744(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veloso D., Cleland W. W., Porter J. W. pH properties and chemical mechanism of action of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochemistry. 1981 Feb 17;20(4):887–894. doi: 10.1021/bi00507a036. [DOI] [PubMed] [Google Scholar]
- Warren K. S. The pathology, pathobiology and pathogenesis of schistosomiasis. Nature. 1978 Jun 22;273(5664):609–612. doi: 10.1038/273609a0. [DOI] [PubMed] [Google Scholar]
- Woodward H. D., Allen J. M., Lennarz W. J. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase of the sea urchin embryo. Deduced structure and regulatory properties. J Biol Chem. 1988 Dec 5;263(34):18411–18418. [PubMed] [Google Scholar]