Summary
Background
A signaling pathway is difficult, if not impossible, to elucidate in platelets using only in vivo studies. Likewise, the physiological significance of signaling information obtained exclusively from in vitro observations is unknown. Therefore, both in vitro and in vivo experiments are required to establish the physiological significance of a signaling pathway.
Objective
To evaluate the physiological significance of signaling data obtained from botrocetin (bt)/von Willebrand factor (VWF)-stimulated washed platelets.
Method
Stable thrombus formation in response to FeCl3-induced injury of the mouse carotid artery was used to evaluate the physiological significance of signaling data obtained from bt/VWF-stimulated washed platelets.
Results
Syk, PLCγ2, Gαq and P2Y12, but not LAT, were found either to be required for or to affect stable thrombus formation. Prior in vitro studies had demonstrated that LAT is not required for bt/VWF-induced platelet aggregation in the presence of exogenous fibrinogen. These data provide the first demonstration of the in vivo role for these signaling molecules in GPIb-dependent/initiated signal transduction and are consistent with the signaling pathway deduced from in vitro studies of bt/VWF-stimulated washed platelets using metabolic inhibitors and knockout mice.
Conclusion
The broad agreement between the in vitro and the in vivo results establish that bt/VWF stimulation of washed platelets can provide physiologically significant glycoprotein Ib-dependent/initiated signaling data.
Keywords: carotid artery, GPIb/IX/V, in vivo thrombosis
Introduction
Stable thrombus formation in response to FeCl3-induced arterial injury provides an endpoint for evaluating the physiological role of signaling molecules in hemostasis in vivo [1,2]. Stable thrombus formation in this system results from at least two processes: (i) glycoprotein Ibα (GPIb)-initiated signaling that activates αIIbβ3; and (ii) the consequences of subsequent αIIbβ3 and contact-dependent outside-in signaling. The GPIb signaling that activates αIIbβ3 is initiated by GPIb binding to subendothelial von Willebrand factor (VWF) exposed by damage to the endothelium. A diverse range of GPIb-dependent in vivo signaling events have been implicated as causing activation of αIIbβ3 [3]. Although the versatility of GPIb signaling in vitro is interesting, it raises the perplexing question of which of these mechanisms, if any, are utilized by GPIb in vivo for stable thrombus formation at sites of vascular injury [4]. This is an important concern because stable arterial thrombus formation associated with endothelial damage underlies many aspects of cardiovascular disease [5].
Stable thrombus formation on the subendothelial matrix is a complex process involving a variety of receptors, including GPIb-IX-V and αIIbβ3, and multiple signaling molecules [3,6]. Elucidation of the physiological significance of the platelet signaling that underlies this complex process requires a combination of in vitro and in vivo experimentation, particularly because platelets are anucleate. Elucidation of this signaling can be simplified by characterizing the two distinct component processes of stable thrombus formation independently of each other in vitro. Under appropriate conditions botrocetin (bt)/VWF induces a GPIb-dependent biphasic aggregation-like response [7]. The first phase is VWF-mediated platelet agglutination resulting from cross-linking of platelets by VWF binding to GPIbα on adjacent platelets. During agglutination, signaling initiated by GPIb elicits αIIbβ3 activation. The second phase of the biphasic response is platelet aggregation mediated by αIIbβ3. The αIIbβ3-initiated signaling that occurs during the second phase is aggregation dependent in vitro [7].
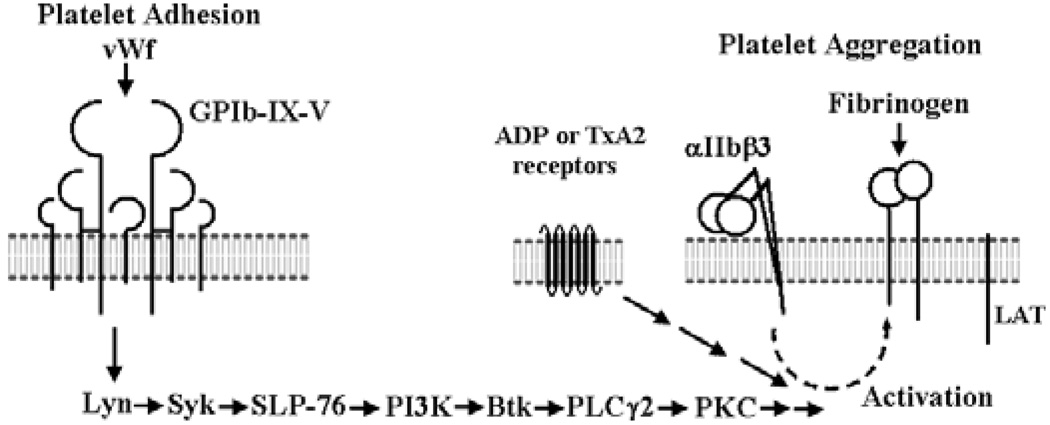
Results obtained using bt/VWF-stimulated washed mouse platelets demonstrate that the GPIb-initiated signaling that activates αIIbβ3 culminates in thromboxane A2(TxA2) production and TxA2-elicited signaling [7,8]. The TxA2-elicited signaling is required for αIIbβ3 activation [7,8]. The GPIb-elicited signaling that results in TxA2 production is initiated by Lyn, enhanced by Src, and propagated through Syk, Src homology 2 (SH2) domain-containing leukocyte protein76 (SLP-76), phosphatidylinositol 3-kinase(PI3K), Bruton tyrosine kinase (Btk), phospholipase Cγ2 (PLCγ2) and protein kinase C (PKC) [4,9] (Fig. 1). These signaling molecules were required for the first phase of the bt/VWF-induced response. Specifically, the GPIb/VWF-mediated platelet agglutination-initiated signaling utilized these signaling molecules to elicit the production of the TxA2 that is required for activation of αIIbβ3.
Fig. 1.
The signaling pathway mobilized by GPIbα in response to botrocetin (bt)/ von Willebrand factor (VWF) treatment of suspension washed mouse platelets. The signaling pathway is not comprehensive, but provides background information for this in vivo study [4,7,9].
The second phase of the bt/VWF-induced biphasic aggregation response is dependent on αIIbβ3, signaling through the TxA2 receptor, Tp [10], the ADP receptor P2Y1, the heterotrimeric G protein, Gαq, the ADP receptor P2Y12 and presumably Gi [4,7]. Specifically, the VWF/GPIb-mediated agglutination initiates signaling that culminates in TxA2 production which enables a low level of ADP secretion in a Gαq-independent manner. The ADP secreted in response to agglutination apparently signals through P2Y1 and causes the secretion of more ADP in a Gαq-dependent manner. This secreted ADP signals via P2Y12 causing α-granule secretion [7]. Although the Fc receptor γ-chain (FcRγ-chain) and the linker for activation of T cells (LAT) were required for bt/VWF-induced aggregation of washed platelets in the absence of exogenous fibrinogen, these requirements were eliminated by the presence of exogenous fibrinogen [9]. FcRγ-chain and LAT were also not required for the GPIb-mediated signaling leading to αIIbβ3 activation [9,11]. Although much has been learned about GPIb signaling in vitro using this system [4,7,9], evaluation of the physiological significance of bt/VWF-elicited signaling requires in vivo analysis [4].
The physiological significance of bt/VWF facilitated, GPIb-elicited Btk and TxA2 signaling was shown by demonstrating the requirement for Btk and TxA2 signaling for stable thrombus formation in vivo using the FeCl3 carotid artery injury model [4]. This model was used because the damage elicited by FeCl3 treatment of mouse mesentery has been characterized [12,13] and because stable thrombus formation in this system is GPIb-dependent [4,14,15] and mediated primarily, though not exclusively, by VWF [16]. Thus, the FeCl3 carotid artery injury model is appropriate for evaluating the requirement for specific signaling molecules during GPIb-dependent stable thrombus formation in vivo. Although other in vivo thrombosis models may be GPIb-dependent, they were deemed inappropriate for this comparison because they are not known to be GPIb-dependent.
In this study, we tested the physiological significance of other signaling molecules found to be required for either GPIb-dependent TxA2 production, and therefore αIIbβ3 activation, or for the subsequent aggregation of bt/VWF-stimulated mouse platelets by asking if the same molecules that are required for bt/VWF-induced platelet aggregation are also required for GPIb-dependent stable thrombus formation in FeCl3-injured murine carotid arteries.
Materials and methods
Materials
Isofluorane was from NLS Animal Health (Oklahoma City, OK,USA). Cacodylate, paraformaldehyde, the glutaraldehyde solution, osmium tetroxide and uranyl acetate were from Electron Microscopy Sciences (Hatfield, PA, USA).
Animals
Mice deficient in PLCγ2 [17], Gαq [18], LAT [19], P2Y12 [20] or IL-4Rα/GPIbα-tg [21] were generated as described. The IL-4Rα/GPIbα-tg mice do not express murine GPIbα; instead they expressed the extracellular domain of the human interleukin-4 (IL-4) receptor fused with the transmembrane and cytoplasmic domains of human GPIbα [21]. Heterozygotes of this strain have been backcrossed with C57Bl/6 for 10 generations. Syk−/− chimeric mice were produced by fetal liver cell transplant as described [22]. The Syk chimaeras were made with Syk+/− mice backcrossed with C57/Bl6 mice for eight generations and their cells were transferred to inbred C57/Bl6 recipients. Wild-type littermate siblings were used as controls unless otherwise stated. All the other mouse strains with the exception of the P2Y12 strain have been inbred in our mouse facility for at least 5 years.
Ferric chloride-induced carotid artery injury
Carotid arteries were separated from other tissue in isofluorane-anesthetized mice. Injury was induced using 10% FeCl3 and blood flow monitored using a laser Doppler system as described [4]. Monitoring of carotid artery blood flow was initiated at the time of FeCl3 treatment. FeCl3 treatment consisted of placement of a 4 × 10-mm strip of Whatman No. 1 filter paper saturated with a 10% FeCl3 solution over the exposed adventitia of the carotid artery for 3 min. The treated tissue was then rinsed three times with a physiologic saline solution. Blood flow was continuously monitored for 45 min. Carotid artery blood flow of < 0.6 blood flow units (BFUs) per minute was scored as occlusion, allowing the time to first occlusion time to be determined. Statistical analyses were performed using Student’s t-test.
Histological and scanning electron microscope studies
For histological studies, the isolated vessel segment was fixed in 16% paraformaldehyde for 24 h at 4 °C. The tissue was processed at room temperature with increasing concentrations of ethanol (70% for 30 min, 95%, 3× for 30 min, and absolute ethanol, 3× for 30 min), cleared with xylene, 3× for30 min, infiltrated with paraffin for 1 h, and then embedded in paraffin blocks. Sections of the tissue (5 µm thickness) were stained with Diffquick reagent.
For preparation of the scanning electron microscope images, the carotid artery tissue was fixed by immersing the tissue in aqueous 0.1 m cacodylate, pH 7.35, containing 2.5% glutaraldehyde for at least 2 h at room temperature. The tissue was then rinsed three times for 5 min in aqueous 0.1 m cacodylate, pH 7.35, and fixed in 2% osmium tetroxide. After being sequentially rinsed with 0.1 m cacodylate and deionized water, the tissue was stained with 2% aqueous uranyl acetate for 2 h. The stained tissue was rinsed with deionized water and dehydrated with a graded series of ethyl alcohol (10% through absolute). The tissue was dried using a Critical Point Dryer in absolute ethyl alcohol and then in liquid carbon dioxide. The longitudinal incision segment of the carotid artery was sputter coated with gold-palladium, mounted and analyzed using a Philips Environmental Scanning Electron Microscope (SEM).
Results
GPIba is necessary for thrombus formation in response to arterial injury induced by FeCl3
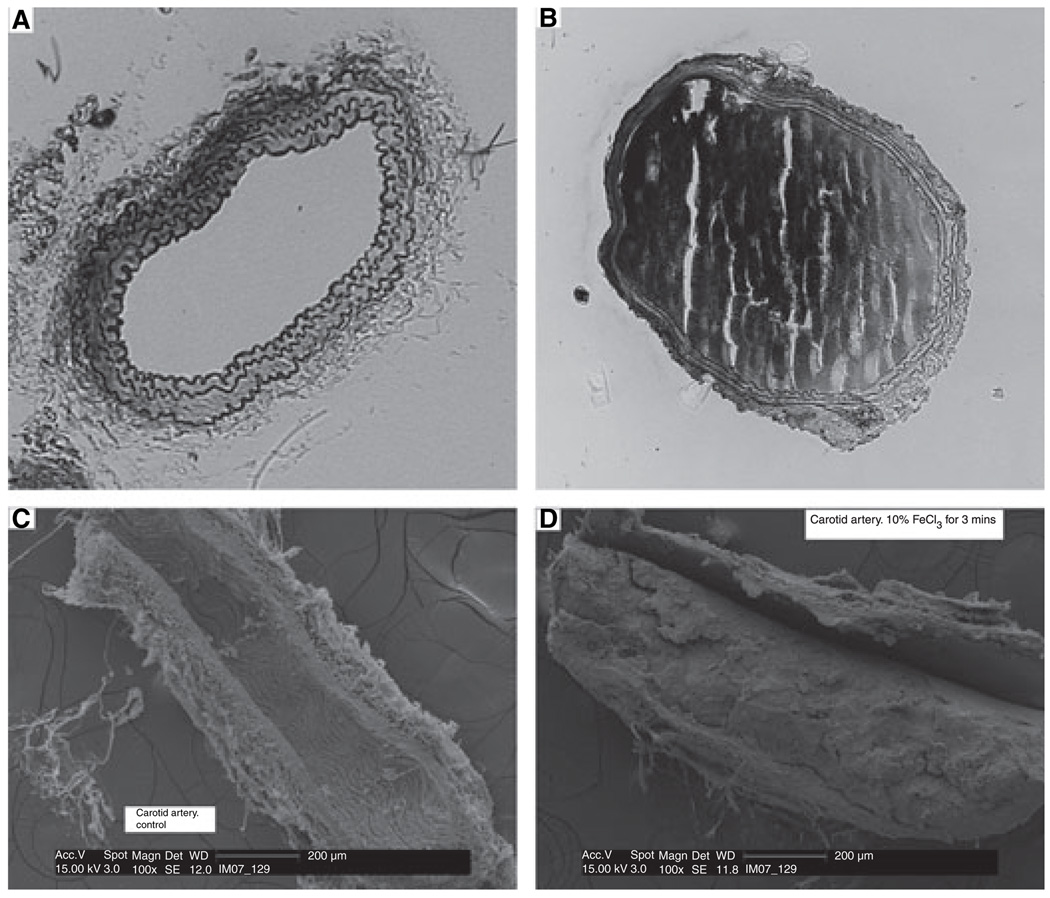
As shown by others [23], histological and SEM images of sections obtained from the occluded carotid arteries demonstrated that occlusion resulted from thrombus formation (Fig. 2). After total occlusion, if blood flow did not increase during the observation period, occlusion was scored as resulting from stable thrombus formation. It is also unlikely that the occlusion characterized here resulted from vasospasm because occlusion was GPIb-dependent (Table 1), and there is no obvious relationship between vasospasm and GPIb. If blood flow increased after occlusion occurred, it was assumed that embolization occurred.
Fig. 2.
FeCl3-treatment of carotid arteries causes stable thrombus formation. Light microscope images of sections of saline-treated (A) or FeCl3-treated (B) wild-type mouse carotid arteries. Scanning electron microscope (SEM) images of saline-treated (C) or FeCl3-treated (D) wild-type mouse carotid arteries.
Table 1.
GPIb is essential for stable thrombus formation in FeCl3-treated carotid arteries
| Type of mice | Time of occlusion (mean ± SD) | No. of mice |
|---|---|---|
| WT | 5.81 ± 1.37 | 8 |
| IL-4R | No occlusion at the end of 45 min* | 8 |
WT, wild type.
P < 0.0001.
Syk plays a role in stable thrombus formation in response to FeCl3-induced injury
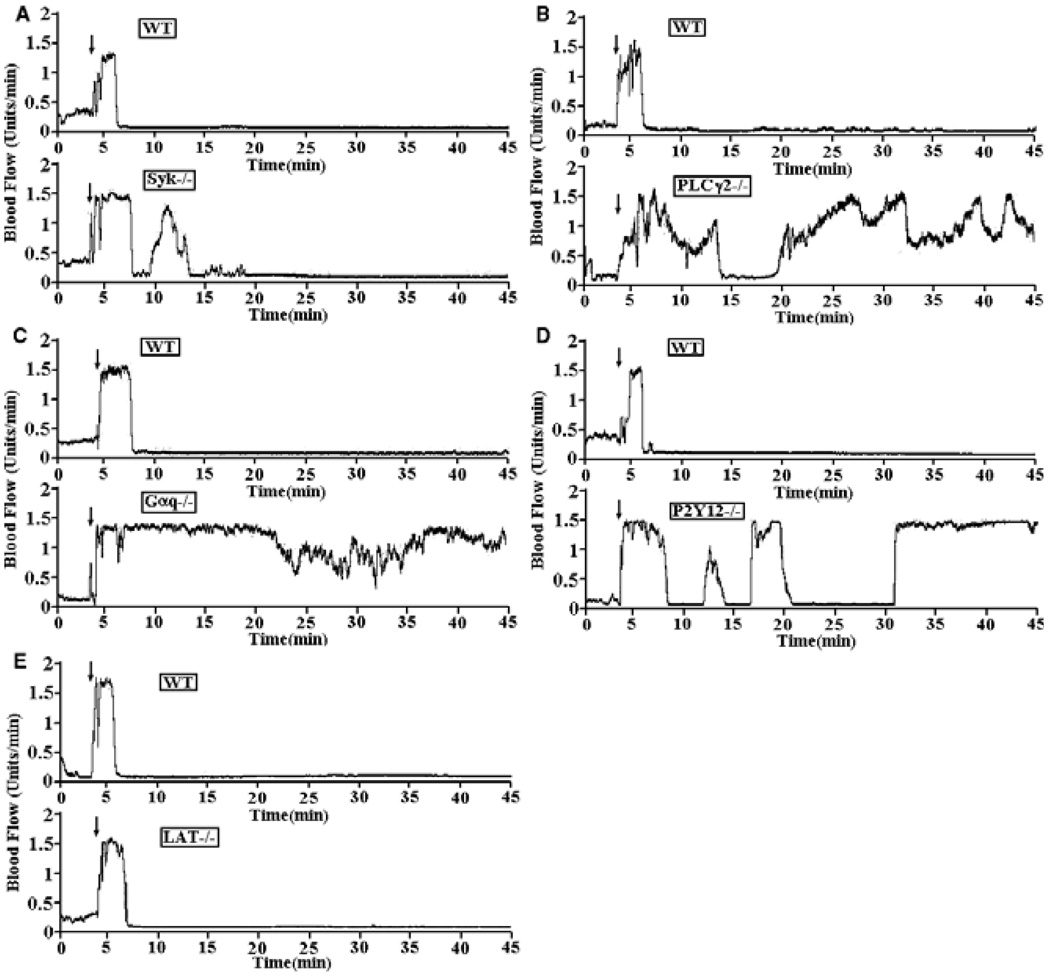
Syk is required for GPIb-dependent TxA2 production in bt/VWF-stimulated washed mouse platelets [9]. Therefore, we examined wild-type (either +/− or +/+ mice) and Syk-deficient chimeric mice in the FeCl3-induced carotid artery thrombosis model. The average time to first occlusion for the Syk−/− chimeric mice was 8.04 min, in contrast to 5.25 min (P < 0.01) in the control mice (Table 2, line A). Also, substantial embolization occurred in four out of five Syk−/− chimeras, but in only one of the four control mice, and that was minor. The injured carotid artery of only one of the five Syk−/− chimeras remained patent; none of the injured carotid arteries of the control chimeras remained patent (Fig. 3A). These results demonstrate that Syk plays a role in GPIb-dependent stable thrombus formation. The diminished, but distinct blood flow characteristic of the Syk−/− chimeras in response to arterial injury is discussed below.
Table 2.
Summary of the FeCl3 treatment data
| Type of mice | Time of occlusion (Mean ± SD) | No. of mice | |
|---|---|---|---|
| A | WT | 5.25 ± 0.65 | 4 |
| Syk−/− | One out of five CAs remained patent 8.04 ± 0.93* | 5 | |
| B | WT | 5.00 ± 0.79 | 5 |
| PLCγ2−/− | No occlusion at the end of 45 min 11.30 ± 4.82** | 5 | |
| C | WT | 5.95 ± 0.82 | 5 |
| Gαq−/− | No occlusion at the end of 45 min*** | 4 | |
| D | WT | 5.16 ± 0.79 | 5 |
| P2Y12−/− | No occlusion at the end of 45 min 17.40 ± 16.59**** | 5 | |
| E | WT | 5.63 ± 1.25 | 5 |
| LAT−/− | 5.75 ± 0.96 | 5 |
WT, wild type; LAT, linker for activation of T cells.
P < 0.01,
P < 0.05,
P < 0.0001,
P < 0.05.
Fig. 3.
Traces of blood flow in FeCl3-treated carotid arteries of wild type and knockout strains of mice. (A) The tracings in this figure are representative of blood flow in FeCl3-treated carotid arteries of wild-type and Syk−/− chimeric mice. Blood flow was monitored for 45 min, including a 3-min treatment starting at zero time done with a strip of filter paper soaked in 10% FeCl3. The arrow indicates the time of removal of the FeCl3 containing strip of filter paper. (B) Representative data of blood flow in FeCl3-treated exposed carotid arteries of control and PLCγ2−/− mice. Blood flow was monitored as described above. Representative data of blood flow in FeCl3-treated exposed carotid arteries of wild-type, Gαq−/− (C), P2Y12−/− (D) and LAT−/− (E) mice.
PLCγ2 is required for stable thrombus formation in response to arterial injury induced by FeCl3
PLCγ2 is required for GPIb-dependent signaling in general [24–26] and for TxA2 production in bt/VWF-stimulated washed mouse platelets in particular [9]. Accordingly, we found that PLCγ2 was required for stable thrombus formation in FeCl3-injured carotid arteries (Table 2, line B and Fig. 3B). The time to first partial occlusion in the PLCγ2−/− mice (11.30 ± 4.82, P < 0.05) was greater than the time to first occlusion in wild-type carotid arteries (5.00 ± 0.79), and in contrast to injured wild-type carotid arteries, none of which remained patent, all of the injured carotid arteries in the PLCγ2−/− mice remained patent. These results demonstrate that PLCγ2 is required for GPIb-dependent stable thrombus formation.
The physiological significance of Syk and PLCγ2 in FeCl3-induced stable thrombus formation was tested because those signaling molecules had been shown to be required for GPIb-induced αIIbβ3 activation in response to platelet treatment with bt/VWF [4,9], the first phase of bt/VWF-induced platelet aggregation.
Gαq, P2Y12, but not LAT, are required for stable thrombus formation in response to arterial injury induced by FeCl3
The second stage of thrombus formation was also investigated. We investigated the role of three signaling molecules that are required for bt/VWF-induced, GPIb-elicited αIIbβ3-dependent aggregation, but not for αIIbβ3 activation: Gαq, P2Y12 and LAT [7,9]. Gαq, P2Y12 and LAT are required in vitro in response to bt/VWF stimulation for the αIIbβ3 signaling that results in fibrinogen secretion and platelet aggregation [7,9]. Despite these similarities, there was an important difference in the behavior of the LAT−/− platelets relative to the Gαq−/− platelets and wild-type platelets treated with a P2Y12 antagonist, AR-C69931MX. Stimulation of all these platelets with bt/VWF resulted in agglutination and the agglutination driven levels of TxA2, but not aggregation and the aggregation driven levels of TxA2. However, aggregation of the LAT−/− washed platelets enabled by exogenous fibrinogen restored TxA2 production to almost 80% of the level induced by aggregation in wild-type platelets; in contrast, aggregation of washed Gαq−/− and the AR-C69931MX-treated platelets enabled by exogenous fibrinogen did not enhance TxA2 production [7,9]. Accordingly, both Gαq (Fig. 3C) and P2Y12 (Fig. 3D), but not LAT (Fig. 3E) were required for stable thrombus formation. FeCl3 treatment failed to cause occlusion of blood flow in Gαq−/−mice and blood flow remained patent. Likewise, stable occlusion of blood flow did not occur in the FeCl3-treated P2Y12−/− mice [Fig. 3D, mean time to first partial occlusion 17.40 ± 16.59, P < 0.0001 (Table 2)] and blood flow remained patent. These data demonstrate that Gαq and P2Y12, but not LAT, are required for GPIb-dependent stable thrombus formation in vivo in the FeCl3-induced arterial injury model and are consistent with the importance of TxA2-induced signaling in this process because in the absence of either of those signaling molecules, TxA2 is not produced and platelet aggregation does not occur [4,7].
Discussion
The results presented here demonstrate the requirement for a variety of signaling molecules for GPIb-dependent stable thrombus formation in response to FeCl3-treated mouse carotid arteries. Stable thrombus formation was shown to be GPIb-dependent by injecting VCL (a recombinant protein functionally equivalent to a monomeric formof the A1 domain of VWF [27], which contains the VWF GPIb binding site) into tail veins of mice containing FeCl3-treated carotid arteries [4]. In addition, two studies using mice that did not express the extracellular domain of GPIb (IL-4Rα/GPIbα-tg mice) have demonstrated that GPIb is essential for stable thrombus formation in FeCl3-treated carotid arteries [14] and mesenteric arterioles [15]. The data in Table 1 confirm that the FeCl3- induced platelet signaling characterized in our experiments was also GPIb dependent.
The results obtained using Syk−/− chimeric mice (Fig. 3A) demonstrate a requirement for Syk in stable thrombus formation in this system. The results were confounded by the findings that the injured carotid arteries in four of the five Syk deficient chimeras did not remain patent and that blood flow was greatly diminished in the other Syk-deficient chimera, presumably as a result of the presence of 1–2% remanant circulating wild-type platelets in the Syk−/− chimeras. That is, the circulating wild-type platelets in the Syk−/− chimeras would accumulate as thrombi at the sites of FeCl3-induced injury, but the limited availability of wild-type platelets would preclude extensive thrombus formation. Although only 1–2% of the platelets in each of the Syk−/− chimeric mice usually are wild type, each mouse could easily have 1–2 × 107 wild-type platelets in circulation (based on 1.0 × 109 platelets/mouse). The presence of a small percentage of wild-type platelets in the Syk−/− chimeric mice results from the method used to construct the chimeras. Although the chimeras were produced by infusing fetal Syk−/− stem cells into lethally irradiated Syk+/+ mice, the irradiation does not kill all the platelet generating stem cells in the recipient mice. However, other factors may be responsible for the response of the Syk−/− chimeric mice to FeCl3 injury. For example, Syk may not be the only non-receptor tyrosine kinase that can contribute to PLCγ2 activation in response to GPIb signaling in vivo as some level of PLCγ2 activation may be mediated directly by Src-family kinases. Further work is required to resolve this issue.
As expected, PLCγ2 was found to be required for stable thrombus formation in this system (Fig. 3B). Although all the carotid arteries of the PLCγ2−/− mice remained patent, blood flow was diminished relative to flow in the uninjured artery. Possibly another phospholipase C (PLC) isoform present in low amount in platelets [28] accounts for the formation of unstable thrombi in the PLCγ2-deficient mice and/or thrombin may compensate in part for the absence of PLCγ2 [13,29]. Nonetheless, the data presented here demonstrate that PLCγ2 is required for GPIb-dependent stable thrombus formation in vivo and thereby establish the physiological significance of a variety of in vitro data.
The role of PLCγ2 has also been studied in vivo using the laser-induced mesenteric arteriole injury model in mice [26]. Those results are not discussed here because their relevance to those reported here is not obvious. Unlike the FeCl3 carotid artery injury model, thrombus formation in the laser-induced injury model has not been shown to be GPIb-dependent and platelet activation in that system has been shown to be initiated by thrombin and independent of VWF even although VWF enhances the size of the thrombus [30].
Analyses of the results obtained using FeCl3-injured carotid arteries of Gαq−/− mice and P2Y12−/− mice revealed that both Gαq and P2Y12 are required for GPIb-dependent stable thrombus formation in vivo (Fig. 3C,D). It is also clear from these results that G13 cannot compensate for the absence of Gαq [31]. The requirement for P2Y12 for stable thrombus formation has been shown previously, but not with carotid arteries [32]. Results obtained in vitro demonstrated that Gαq, P2Y1 and P2Y12 are required for signaling events subsequent to activation of αIIbβ3, including α-granule secretion, but not for GPIb-induced αIIbβ3 activation [7]. Accordingly, bt/VWF stimulated Gαq−/− platelets aggregated in the presence, but not in the absence of exogenous fibrinogen [7]. Likewise, A3P5P (a selective P2Y1 antagonist)-treated and AR-C69931MX (a selective P2Y12 antagonist)-treated, bt/VWF-stimulated washed platelets aggregated in the presence, but not in the absence of exogenous fibrinogen [7]. This in vitro signaling work demonstrated that P2Y1 is required for α-granule secretion, presumably by supplying ADP for P2Y12/Gi-mediated signaling [7]. Previous work has demonstrated a P2Y1 requirement for stable thrombus formation in response to FeCl3-induced injury of the carotid artery [33]. Taken together, these results appear to demonstrate the importance of α-granule secretion in stabilizing thrombus formation as fibrinogen is not limiting in the KO mice after FeCl3-induced injury. Because Gαq, P2Y1 and P2Y12 are not required for αIIbβ3 activation in response to GPIb signaling, these molecules are likely utilized during αIIbβ3-dependent outside-in signaling for stabilization of the thrombus.
As with PLCγ2−/− mice, the requirement for Gαq for thrombus formation was evaluated in the mesenteric arteriole laser-induced injury model in mice [26]. Consistent with our data, the Gαq-deficient mice failed to form thrombi that were stable during the brief observation period even in response to severe laser-induced injury. So, stable thrombus formation in these very different arterial injury models requires Gαq. Presumably, the agreement between the results obtained using the Gαq−/− mice in the two systems reflects a common Gαq requirement for aggregation (thrombus formation) in both the VWF-independent (laser treatment) [30] and the GPIb-dependent (FeCl3 treatment) systems [4,14,15].
The data presented here do not provide any insight into which ITAM (immunoreceptor tyrosine-based activation motif) containing signaling molecule facilitates Syk activation [34].However, data published elsewhere have shown the FcRγ-chain is not required for GPIb-dependent activation of αIIbβ3 in mice [9,11]. Therefore, another ITAM containing signaling molecule apparently functions to facilitate activation of Syk.
In summary and conclusion, the data presented here demonstrate that all of the signaling molecules shown to be required in vitro for bt/VWF induced GPIb-dependent activation of αIIbβ3 and subsequent platelet aggregation [4,7,9] tested here were found to be required for GPIb-dependent stable thrombus formation in response to FeCl3 induced injury of mouse carotid arteries. These are the first results demonstrating that Syk, PLCγ2, Gαq and P2Y12 are required in vivo for GPIb-dependent stable thrombus formation in response to carotid artery injury induced by FeCl3. They also demonstrate that G13, although coupled [31] to Tp [10] (the TxA2 receptor), is unable to compensate for the absence of Gαq for the response to FeCl3-induced injury to mouse carotid arteries. Analyses of the in vitro and in vivo data reveals that α-granule secretionmay be required for stable thrombus formation in vivo and that Gi appears to be required for α-granule secretion. The high concordance between the in vitro and in vivo results suggests that these signaling molecules play similar roles in both models. It is unlikely that α2β1, rather than GPIb and αIIbβ3, initiated the activation of these signaling molecules because resting α2β1 does not support the adhesion of platelets to immobilized collagen [35] and therefore presumably cannot signal until activated. This is an important point because activation of α2β1 requires the activation of αIIbβ3 [36] and ADP [35,37]. If these in vitro observations are relevant to the in vivo situation characterized here, α2β1 signaling, if it occurs, is downstream of and dependent on GPIb and αIIbβ3 signaling. Finally, although the genetic deficiencies of knockout mice used here were shown to affect GPIb-dependent platelet signaling in vitro in the absence of vascular tissue, we cannot exclude the possibility those genetic deficiencies also affect vascular function. It seems unlikely that vascular deficiencies affected the results presented here because enhanced thrombosis was not observed and because the in vivo results were congruent with the platelet in vitro results. This latter point is significant because it is not uncommon that the results of in vitro studies based on the use of knockout mice differ substantially from those of the corresponding in vivo studies [38].
Acknowledgements
This work was supported in part by HL63216 and HL50545 from the National Heart Lung and Blood Institute, NSF DUE-9850780, the W. Harry Feinstone Center for Genomic Research, and the National Health and Medical Research Council of Australia. We thank Dr Jean Lachowicz and Lia Kwee Isaac of the Schering-Plough Research Institute for providing the P2Y12 breeding stock. We thank the University of Memphis Integrated Microscopy Center for the images presented Fig. 2.
Footnotes
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
References
- 1.Kurz KD, Main BW, Sandusky GE. Rat model of arterial thrombosis induced by ferric chloride. Thromb Res. 1990;60:269–280. doi: 10.1016/0049-3848(90)90106-m. [DOI] [PubMed] [Google Scholar]
- 2.Farrehi PM, Ozaki CK, Carmeliet P, Fay WP. Regulation of arterial thrombolysis by plasminogen activator inhibitor-1 in mice. Circulation. 1998;97:1002–1008. doi: 10.1161/01.cir.97.10.1002. [DOI] [PubMed] [Google Scholar]
- 3.Du X. Signaling and regulation of the platelet glycoprotein Ib-IX-V complex. Curr Opin Hematol. 2007;14:262–269. doi: 10.1097/MOH.0b013e3280dce51a. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Fitzgerald ME, Berndt MC, Jackson CW, Gartner TK. Bruton’s tyrosine kinase is essential for botrocetin/VWF- induced signaling and GPIb-dependent thrombus formation in-vivo. Blood. 2006;108:2596–2603. doi: 10.1182/blood-2006-01-011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Zanten GH, de Graaf S, Slootweg PJ, Heijnen HF, Connolly TM, de Groot PG, Sixma JJ. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93:615–632. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Pestina TI, Berndt MC, Steward SA, Jackson CW, Gartner TK. The roles of ADP and TxA2 in botrocetin/VWF-induced aggregation of washed platelets. J Thromb Haemost. 2004;2:2213–2222. doi: 10.1111/j.1538-7836.2004.01023.x. [DOI] [PubMed] [Google Scholar]
- 8.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Pestina TI, Berndt MC, Jackson CW, Gartner TK. Botrocetin/VWF-induced signaling through GPIb-IX-V produces TxA2 in an alphaIIbbeta3- and aggregation-independent manner. Blood. 2005;106:2750–2756. doi: 10.1182/blood-2005-04-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 2004;103:3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 12.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois C, Panicot-Dubois L, Merrill-Skoloff G, Furie B, Furie BC. Glycoprotein VI-dependent and -independent pathways of thrombus formation in-vivo. Blood. 2006;107:3902–3906. doi: 10.1182/blood-2005-09-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinides S, Ware J, Marchese P, Almus-Jacobs F, Loskutoff DJ, Ruggeri ZM. Distinct antithrombotic consequences of platelet glycoprotein Ibalpha and VI deficiency in a mouse model of arterial thrombosis. J Thromb Haemost. 2006;9:2014–2021. doi: 10.1111/j.1538-7836.2006.02086.x. [DOI] [PubMed] [Google Scholar]
- 15.Bergmeier W, Piffath CL, Goerge T, Cifuni SM, Ruggeri ZM, Ware J, Wagner DD. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci U S A. 2006;103:16900–16905. doi: 10.1073/pnas.0608207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95:9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D, Feng J, Wen R, Sangster MY, Parganas E, Hoffmeyer A, Jackson CW, Cleveland JL, Murray PJ, Ihle JN. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity. 2000;13:25–35. doi: 10.1016/s1074-7613(00)00005-4. [DOI] [PubMed] [Google Scholar]
- 18.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 19.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 20.Foster CJ, Prosser DM, Agans JM, Zhai Y, Smith MD, Lachowicz JE, Zhang FL, Gustafson E, Monsma FJ, Jr, Wiekowski MT, Abbondanzo SJ, Cook DN, Bayne ML, Lira SA, Chintala MS. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 22.Poole A, Gibbins JM, Turner M, van Vugt MJ, van deWinkel JG, Saito T, Tybulewicz VL, Watson SP. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jirousková M, Chereshnev I, Väänänen H, Degen JL, Coller BS. Antibody blockade or mutation of the fibrinogen gamma-chain C-terminus is more effective in inhibiting murine arterial thrombus formation than complete absence of fibrinogen. Blood. 2004;103:1995–2002. doi: 10.1182/blood-2003-10-3401. [DOI] [PubMed] [Google Scholar]
- 24.Mangin P, Yuan Y, Goncalves I, Eckly A, Freund M, Cazenave JP, Gachet C, Jackson SP, Lanza F. Signaling role for phospholipase C gamma 2 in platelet glycoprotein Ib alpha calcium flux and cytoskeletal reorganization. Involvement of a pathway distinct from FcR gamma chain and Fc gamma RIIA. J Biol Chem. 2003;278:32880–32891. doi: 10.1074/jbc.M302333200. [DOI] [PubMed] [Google Scholar]
- 25.Rathore V, Wang D, Newman DK, Newman PJ. Phospholipse Cγ2 contributes to stable thrombus formation on VWF. FEBS Lett. 2004;573:26–30. doi: 10.1016/j.febslet.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 26.Nonne C, Lenain N, Hechler B, Mangin P, Cazenave JP, Gachet C, Lanza F. Importance of platelet phospholipase Cgamma2 signaling in arterial thrombosis as a function of lesion severity. Arterioscler Thromb Vasc Biol. 2005;25:1293–1298. doi: 10.1161/01.ATV.0000163184.02484.69. [DOI] [PubMed] [Google Scholar]
- 27.Gralnick HR, Williams S, McKeown L, Kramer W, Krutzsch H, Gorecki M, Pinet A, Garfinkel LI. A monomeric von Willebrand factor fragment, Leu-504-Lys-728, inhibits von Willebrand factor interactions with glycoprotein Ib-IX [corrected]. 1992; 89:7880-4. Proc Natl Acad Sci USA. 1992;89:7880–7884. doi: 10.1073/pnas.89.17.7880. Erratum in: Proc Natl Acad Sci U S A 1993; 90: 3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki-Inoue K, Inoue O, Frampton J, Watson SP. Murine GPVI stimulates weak integrin activation in PLCgamma2−/− platelets: involvement of PLCgamma1 and PI3-kinase. Blood. 2003;102:1367–1373. doi: 10.1182/blood-2003-01-0029. [DOI] [PubMed] [Google Scholar]
- 29.Mangin P, Yap CL, Nonne C, Sturgeon SA, Goncalves I, Yuan Y, Schoenwaelder SM, Wright CE, Lanza F, Jackson SP. Thrombin overcomes the thrombosis defect associated with platelet GPVI/FcRgamma deficiency. Blood. 2006;107:4346–4353. doi: 10.1182/blood-2005-10-4244. [DOI] [PubMed] [Google Scholar]
- 30.Dubois C, Panicot-Dubois L, Gainor JF, Furie BC, Furie B. Thrombin-initiated platelet activation in vivo is vWF independent during thrombus formation in a laser injury model. J Clin Invest. 2007;117:953–960. doi: 10.1172/JCI30537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med. 2003;9:1418–1422. doi: 10.1038/nm943. [DOI] [PubMed] [Google Scholar]
- 32.Andre P, Delaney SM, LaRocca T, Vincent D, DeGuzman F, Jurek M, Koller B, Phillips DR, Conley PB. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112:398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahner BN, Dorsam RT, Mada SR, Kim S, Stalker TJ, Brass LF, Daniel JL, Kitamura D, Kunapuli SP. Hematopoietic lineage cell specific protein 1 (HS1) is a functionally important signaling molecule in platelet activation. Blood. 2007;110:2449–2456. doi: 10.1182/blood-2006-11-056069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abram CL, Lowell CA. The expanding role for ITAM-based signaling pathways in immune cells. Sci STKE. 2007;377:re2. doi: 10.1126/stke.3772007re2. Review. [DOI] [PubMed] [Google Scholar]
- 35.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–780. [Google Scholar]
- 36.Van de Walle GR, Schoolmeester A, Iserbyt BF, Cosemans JM, Heemskerk JW, Hoylaerts MF, Nurden A, Vanhoorelbeke K, Deckmyn H. Activation of alphaIIbbeta3 is a sufficient but also an imperative prerequisite for activation of alpha2beta1 on platelets. Blood. 2007;109:595–602. doi: 10.1182/blood-2005-11-011775. [DOI] [PubMed] [Google Scholar]
- 37.Jung SM, Moroi M. Signal-transducing mechanisms involved in activation of the platelet collagen receptor integrin alpha(2)beta(1) J Biol Chem. 2000;275:8016–8026. doi: 10.1074/jbc.275.11.8016. [DOI] [PubMed] [Google Scholar]
- 38.Dumont JE, Dremier S, Pirson I, Maenhaut C. Cross signaling, cell specificity, and physiology. Am J Physiol Cell Physiol. 2002;283:C2–C28. doi: 10.1152/ajpcell.00581.2001. [DOI] [PubMed] [Google Scholar]